Development of a Circular Oriented Bioprocess for Microbial Oil Production Using Diversified Mixed Confectionery Side-Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Raw Materials
2.3. Solid-State Fermentation and Enzymatic Hydrolytic Experiments
2.4. Microbial Oil Fermentations
2.5. Analytical Methods
3. Results and Discussion
3.1. Enzymatic Hydrolysis of Different Mixed Confectionery Waste Streams
3.2. Shake Flask Fermentations for Microbial Oil Production
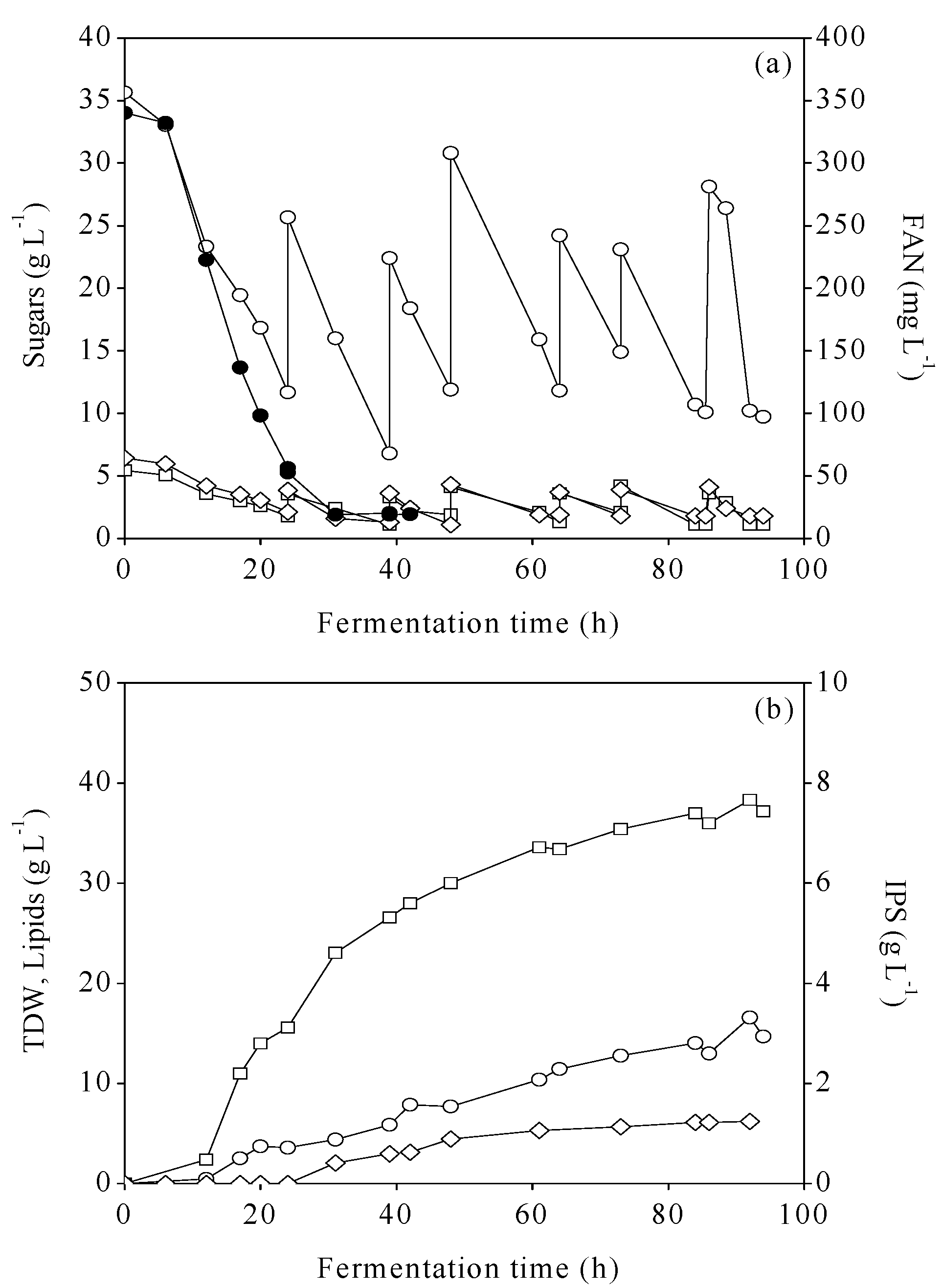
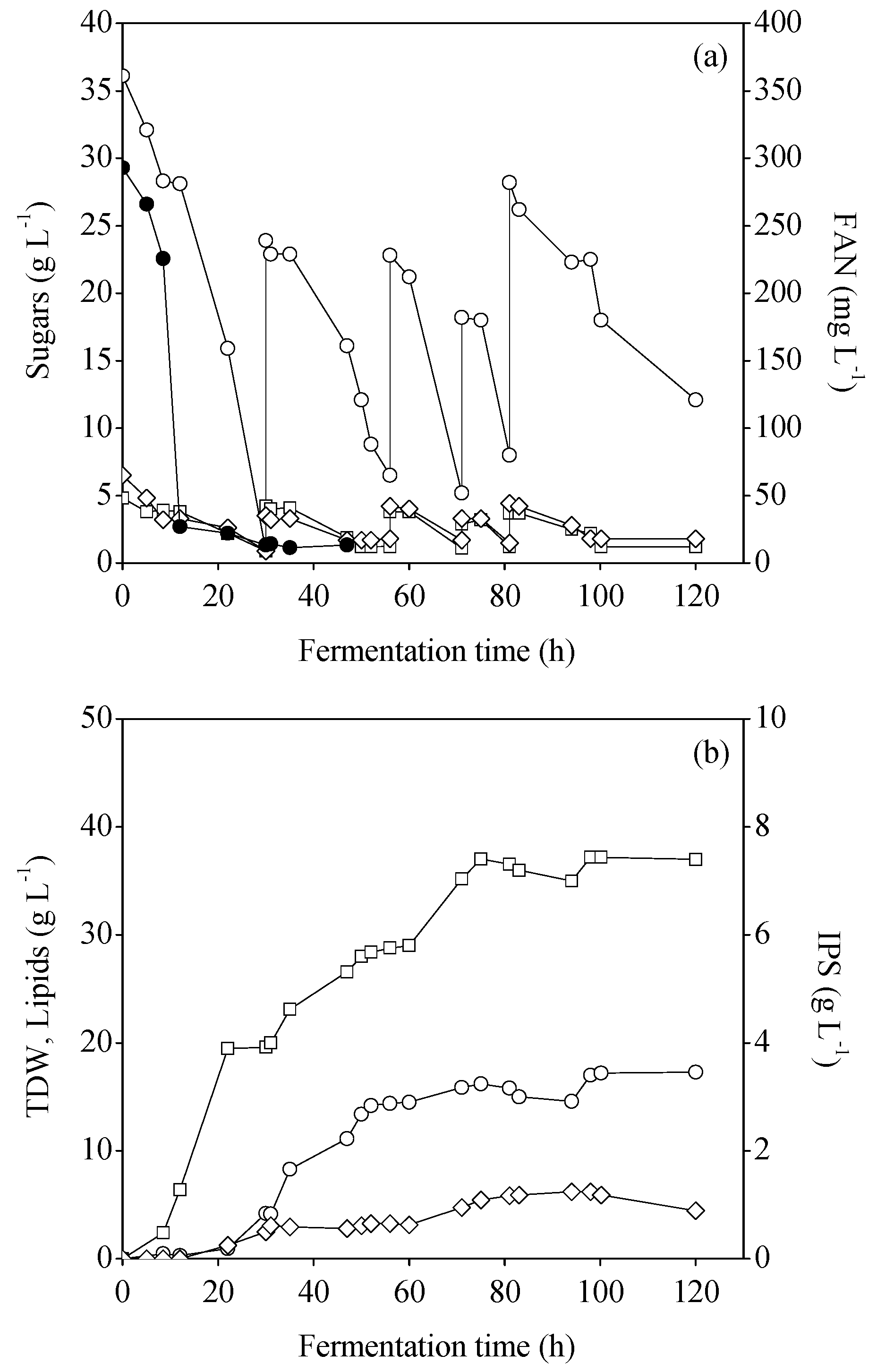
3.3. Fed-Batch Bioreactor Cultures Using MFI and MCWS Hydrolysates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production Strategies and Applications of Microbial Single Cell Oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, G.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. J. Chem. Techol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D. Exploitation of genus Rhodosporidium for microbial lipid production. World J. Microbiol. Biotechnol. 2017, 33, 54. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Nicaud, J.M.; Ledesma-Amaro, R. The Engineering Potential of Rhodosporidium toruloides as a Workhorse for Biotechnological Applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef]
- Leiva-Candia, D.E.; Tsakona, S.; Kopsahelis, N.; García, I.L.; Papanikolaou, S.; Dorado, M.P.; Koutinas, A.A. Biorefining of by-product streams from sunflower-based biodiesel production plants for integrated synthesis of microbial oil and value-added co-products. Bioresour. Technol. 2015, 190, 57–65. [Google Scholar] [CrossRef]
- Tsakona, S.; Skiadaresis, A.G.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Valorisation of side streams from wheat milling and confectionery industries for consolidated production and extraction of microbial lipids. Food Chem. 2016, 198, 85–92. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess development for biolubricant production using microbial oil derived via fermentation from confectionery industry waste. Bioresour. Technol. 2018, 267, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Mallouchos, A.; Efthymiou, M.-N.; Gardeli, C.; Kopsahelis, N.; Aguieiras, E.C.G.; Freire, D.M.G.; Papanikolaou, S.; Koutinas, A.A. Production of wax esters via microbial oil synthesis from food industry waste and by-product streams. Bioresour. Technol. 2017, 245, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Boviatsi, E.; Papadaki, A.; Efthymiou, M.-N.; Nychas, G.-J.E.; Papanikolaou, S.; da Silva, J.A.C.; Freire, D.M.G.; Koutinas, A. Valorisation of sugarcane molasses for the production of microbial lipids via fermentation of two Rhodosporidium strains for enzymatic synthesis of polyol esters. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Chen, L.; Zong, M. Production of microbial oil with high oleic acid content by Trichosporon capitatum. Appl. Energ. 2011, 88, 138–142. [Google Scholar] [CrossRef]
- Pulidindi, K.; Chakraborty, S. Tall Oil Fatty Acid Market Size By Product (Oleic Acid, Linoleic Acid), By Application (Dimer Acids, Alkyd Resins, Fatty Acid Esters), By End-Use (Soap &Detergents, Coatings, Lubricants, Plastics, Fuel Additives, Metal Working Fluid), Industry Analysis Report, Regional Outlook (U.S., Canada, Germany, UK, France, Spain, Italy, China, India, Japan, Australia, Indonesia, Malaysia, Brazil, Mexico, South Africa, GCC), Application Growth Potential, Price Trends, Competitive Market Share & Forecast, 2017–2024; Global Market Insights: Selbyville, DE, USA, 2017. [Google Scholar]
- Mert, B.; Demirkesen, I. Evaluation of highly unsaturated oleogels as shortening replacer in a short dough product. LWT - Food Sci. Technol. 2016, 68, 477–484. [Google Scholar] [CrossRef]
- Food and Drug Administration. Final determination regarding partially hydrogenated oils. Notification; declaratory order; extension of compliance date. Fed. Regist. 2018, 83, 23358–23359. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Liang, Y.; Sarkany, N.; Cui, Y.; Blackburn, J.W. Batch stage study of lipid production from crude glycerol derived from yellow grease or animal fats through microalgal fermentation. Bioresour. Technol. 2010, 101, 6745–6750. [Google Scholar] [CrossRef]
- Blandino, A.; Iqbalsyah, T.; Pandiella, S.; Cantero, D.; Webb, C. Polygalacturonase production by Aspergillus awamori on wheat in solid-state fermentation. Appl. Microbiol. Biotechnol. 2002, 58, 164–169. [Google Scholar] [CrossRef]
- Díaz, A.B.; Alvarado, O.; de Ory, I.; Caro, I.; Blandino, A. Valorization of grape pomace and orange peels: Improved production of hydrolytic enzymes for the clarification of orange juice. Food Bioprod. Process. 2013, 91, 580–586. [Google Scholar] [CrossRef]
- Smaali, I.; Soussi, A.; Bouallagui, H.; Chaira, N.; Hamdi, M.; Marzouki, M.N. Production of high-fructose syrup from date by-products in a packed bed bioreactor using a novel thermostable invertase from Aspergillus awamori. Biocatal. Biotransformation 2011, 29, 253–261. [Google Scholar] [CrossRef]
- Bertolin, T.E.; Schmidell, W.; Maiorano, A.E.; Casara, J.; Costa, J.A.V. Influence of Carbon, Nitrogen and Phosphorous Sources on Glucoamylase Production by Aspergillus awamori in Solid State Fermentation. Z. Naturforsch. C 2003, 58, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Botella, C.; De Ory, I.; Webb, C.; Cantero, D.; Blandino, A. Hydrolytic enzyme production by Aspergillus awamori on grape pomace. Biochem. Eng. J. 2005, 26, 100–106. [Google Scholar] [CrossRef]
- McGhee, J.E.; Silmaim, R.; Bagley, E.B. Production of α-galactosidase from Aspergillus awamori: Properties and action on para-nitrophenyl α-D-galactopyranoside and galacto-Oligosaccharides of soy milk. J. Am. Oil Chem. Soc. 1978, 55, 244–247. [Google Scholar] [CrossRef]
- Ali, U.F.; El-Gindy, A.A.; Ali, U.F.; Ibrahim, Z.M.; Isaac, G.S. A Cost-effective Medium for Enhanced Production of Extracellular α-galactosidase in Solid Substrate Cultures of Aspergillus awamori and A. carbonarius. Aust. J. Basic Appl. Sci. 2008, 2, 880–899. [Google Scholar]
- Matsakas, L.; Bonturi, N.; Miranda, E.; Rova, U.; Christakopoulos, P. High concentrations of dried sorghum stalks as a biomass feedstock for single cell oil production by Rhodosporidium toruloides. Biotechnol. Biofuels 2015, 8, 6. [Google Scholar] [CrossRef]
- Martins, V.; Dias, C.; Caldeira, J.; Duarte, L.C.; Reis, A.; Lopes da Silva, T. Carob pulp syrup: A potential Mediterranean carbon source for carotenoids production by Rhodosporidium toruloides NCYC 921. Bioresour. Technol. Reports 2018, 3, 177–184. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides. Bioprocess. Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Sabra, W.; Maheshwari, G.; Zeng, A.P. Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell. Fact. 2015, 14. [Google Scholar] [CrossRef]
- Tchakouteu, S.S.; Kopsahelis, N.; Chatzifragkou, A.; Kalantzi, O.; Stoforos, N.G.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Rhodosporidium toruloides cultivated in NaCl-enriched glucose-based media: Adaptation dynamics and lipid production. Eng. Life Sci. 2016, 17, 237–248. [Google Scholar] [CrossRef]
- Slininger, P.J.; Dien, B.S.; Kurtzman, C.P.; Moser, B.R.; Bakota, E.L.; Thompson, S.R.; O’Bryan, P.J.; Cotta, M.A.; Balan, V.; Jin, M.; et al. Comparative Lipid Production by Oleaginous Yeasts in Hydrolyzates of Lignocellulosic Biomass and Process Strategy for High Titers. Biotechnol. Bioeng. 2016, 113, 1676–1690. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Bai, F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.; Wang, F.; Li, Y.; Jiang, X.; Ye, M.; Zhao, Z.K.; Zou, H. Comparative proteomic analysis of Rhodosporidium toruloides during lipid accumulation. Yeast 2009, 26, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, S.; Liu, H.; Shen, H.; Lin, X.; Yang, F.; Zhou, Y.J.; Jin, G.; Ye, M.; Zou, H.; et al. A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides. Nat. Commun. 2012, 3, 1112. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, M.T.; Nuñez, S.; Garelli, F.; Voget, C.; De Battista, H. Comprehensive analysis of a metabolic model for lipid production in Rhodosporidium toruloides. J. Biotechnol. 2018, 280, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, R.R.; Sabra, W.; Zeng, A.P. Glucose-mediated regulation of glycerol uptake in Rhodosporidium toruloides: Insights through transcriptomic analysis on dual substrate fermentation. Eng. Life Sci. 2017, 17, 282–291. [Google Scholar] [CrossRef]
- Wiebe, M.G.; Koivuranta, K.; Penttilä, M.; Ruohonen, L. Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol. 2012, 12. [Google Scholar] [CrossRef]
- Easterling, E.R.; French, W.T.; Hernandez, R.; Licha, M. The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis. Bioresour. Technol. 2009, 100, 356–361. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, P.; Liu, Y.; Tang, H.; Chen, Y.; Zhang, L. Identification and characterization of a type-2 diacylglycerol acyltransferase (DGAT2) from Rhodosporidium diobovatum. Antonie Van Leeuwenhoek 2014, 106, 1127–1137. [Google Scholar] [CrossRef]
- Fillet, S.; Ronchel, C.; Callejo, C.; Fajardo, M.-J.; Moralejo, H.; Adrio, J.L. Engineering Rhodosporidium toruloides for the production of very long-chain monounsaturated fatty acid-rich oils. Appl. Microbiol. Biotechnol. 2017, 101, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Van Gerpen, J.; Shanks, B.; Pruszko, R.; Clements, D.; Knothe, G. Biodiesel Production Technology: August 2002--January 2004; National Renewable Energy Laboratory: Golden, CO, USA, 2004.
- Ratledge, C. Single cell oils—have they a biotechnological future? Trends Biotechnol. 1993, 11, 278–284. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sust. Energ. Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive oil composition. In Olive Oil, 2nd ed.; Boskou, D., Ed.; Elsevier Inc. AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. [Google Scholar] [CrossRef]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced lipid production by Rhodosporidium toruloides using different fed-batch feeding strategies with lignocellulosic hydrolysate as the sole carbon source. Biotechnol. Biofuel. 2016, 9. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Synergistic effect of fermentable and non-fermentable carbon sources enhances TAG accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 188, 136–144. [Google Scholar] [CrossRef]
- Zeng, Y.; Xie, T.; Li, P.; Jian, B.; Li, X.; Xie, Y.; Zhang, Y. Enhanced lipid production and nutrient utilization of food waste hydrolysate by mixed culture of oleaginous yeast Rhodosporidium toruloides and oleaginous microalgae Chlorella vulgaris. Renew. Energ. 2018, 126, 915–923. [Google Scholar] [CrossRef]
- Minkevich, I.; Dedyukhina, E.G.; Chistyakova, T.I. The effect of lipid content on the elemental composition and energy capacity of yeast biomass. Appl. Microbiol. Biotechnol. 2010, 88, 799–806. [Google Scholar] [CrossRef]
- Signori, L.; Ami, D.; Posteri, R.; Giuzzi, A.; Mereghetti, P.; Porro, D.; Branduardi, P. Assessing an effective feeding strategy to optimize crude glycerol utilization as sustainable carbon source for lipid accumulation in oleaginous yeasts. Microb. Cell Factor. 2016, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Leiva-Candia, D.E.; Pinzi, S.; Redel-Macías, M.D.; Koutinas, A.; Webb, C.; Dorado, M.P. The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Louhasakul, Y. Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour Technol. 2013, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, B.; Sridharan, S.; Sowmya, V.; Yuvaraj, D.; Praveenkumar, R. Microbial oil—A plausible alternate resource for food and fuel application. Bioresour. Technol. 2017, 233, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, Μ.C.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A. Development of Microbial Oil Wax-Based Oleogel with Potential Application in Food Formulations. Food Bioprocess. Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]

| Waste Stream Concentration | Composition | MFI | MCWS | MWS |
|---|---|---|---|---|
| 50 g·L−1 | Starch | 95.6 ± 0.42 | 96.9 ± 0.89 | 93.6 ± 0.89 |
| Sucrose | 91.9 ± 1.48 | 90.9 ± 1.96 | 92.1 ± 0.67 | |
| Lactose | 89.2 ± 0.92 | 72.3 ± 2.75 | 88.9 ± 1.28 | |
| 100 g·L−1 | Starch | 93.4 ± 1.06 | 94.5 ± 0.85 | 91.3 ± 1.62 |
| Sucrose | 83.9 ± 2.26 | 88.9 ± 1.02 | 83.6 ± 1.34 | |
| Lactose | 81.8 ± 3.54 | 71.3 ± 0.98 | 78.1 ± 0.71 | |
| 150 g·L−1 | Starch | 91 ± 1.63 | 93.6 ± 0.56 | 91 ± 0.99 |
| Sucrose | 79.4 ± 2.19 | 84.2 ± 1.93 | 78.4 ± 1.59 | |
| Lactose | 75.1 ± 2.83 | 70.1 ± 1.63 | 73.8 ± 2.59 |
| Substrate | Fermentation Time (h) | Consumed Substrate (g·L−1) | TDW (g·L−1) | MO (g·L−1) | Oil Content (%, w/w) |
|---|---|---|---|---|---|
| Glucose | 141 | 30 ± 0.3 | 9.4 ± 0.1 | 3.7 ± 0.3 | 39.3 ± 2.4 |
| Sucrose | 147 | 30 ± 0.2 | 10.5 ± 0.3 | 4.6 ± 0.3 | 43.8 ± 1.5 |
| Fructose | 147 | 29.4 ± 0.9 | 9.7 ± 0.4 | 3.5 ± 0.1 | 36.1 ± 0.9 |
| Galactose | 147 | 29.6 ± 0.6 | 9.1 ± 0.1 | 3.6 ± 0.1 | 39.6 ± 1.9 |
| Fermentation Time (h) | C14:0 | C16:0 | Δ9 C16:1 | C18:0 | Δ9 C18:1 | Δ9,12 C18:2 | Δ9,12,15 C18:3 |
|---|---|---|---|---|---|---|---|
| Glucose | |||||||
| 60 | 1.8 | 34 | 0.3 | 7.6 | 43.2 | 8.4 | 4.13 |
| 92 | 1.3 | 27.4 | 0.8 | 8 | 46.9 | 9.5 | 3.5 |
| 140 | 0.9 | 24.5 | 0.8 | 5.9 | 51.5 | 12.3 | 4 |
| Sucrose | |||||||
| 23 | 1.9 | 34.7 | - | 7.9 | 42.8 | 8.6 | 4.1 |
| 103 | 1.2 | 27.1 | 0.8 | 8.5 | 48.9 | 9.7 | 3.7 |
| 147 | 0.9 | 24.9 | 0.8 | 6.1 | 50.7 | 12.4 | 4.1 |
| Fructose | |||||||
| 45 | 1.6 | 28.1 | 1.1 | 7.5 | 46.8 | 10.9 | 2.7 |
| 103 | 1.4 | 27.9 | 0.7 | 7.9 | 48.7 | 9.5 | 3.6 |
| 147 | 0.9 | 24.5 | 0.9 | 5.7 | 51.5 | 12.3 | 3.7 |
| Galactose | |||||||
| 24 | 1.2 | 26.2 | 0.9 | 6.8 | 45.3 | 11.1 | 2.7 |
| 105 | 1.6 | 27.5 | 0.9 | 7.5 | 49.7 | 8.9 | 3.8 |
| 140 | 0.9 | 24.9 | 0.9 | 6.8 | 50.3 | 11.3 | 4.6 |
| Oil Source | Fatty Acids (%) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | Δ9 C16:1 | C18:0 | Δ9 C18:1 | Δ9,12 C18:2 | Δ9,12,15 C18:3 | ||
| Soybean | – | 6–10 | 0.1 | 2–5 | 20–24.9 | 50–60 | 4.3–11 | [43,44,45,46,47] |
| Rapeseed | – | 2.8–14 | 0.9–2 | 13.6–64.1 | 11.8–26 | 7.5–13.2 | [43,44,45,46,47] | |
| Cottonseed | – | 27–28.7 | – | 0.9–2 | 13–18 | 51–58 | 8 | [43,44,45,46,47] |
| Sunflower | – | 4.6–6.4 | 0.1 | 2.9–3.7 | 17–62.8 | 27.5–74 | 0.1–0.2 | [43,44,45,46,47] |
| Palm oil | 0.7 | 36.7–44 | 0.1 | 5–6.6 | 3–46.1 | 8.6–11 | 0.3 | [43,44,45,46,47] |
| Olive oil | <0.1 | 7.5–20 | 0.3–3.5 | 0.5–5 | 55–83 | 3.5–21.0 | ≤1.0 | [48] |
| Microbial lipids from bioreactor cultures | ||||||||
| FRWa | 1.5 | 28.7 | 0.6 | 7.5 | 50.3 | 9.5 | 1.4 | [10] |
| MFI | 1.4 | 10.3 | 0.7 | 14.5 | 61.2 | 5.3 | 0.4 | This study |
| MCWS | 0.9 | 15.2 | 0.9 | 13.8 | 61.7 | 6.1 | 0.1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakona, S.; Papadaki, A.; Kopsahelis, N.; Kachrimanidou, V.; Papanikolaou, S.; Koutinas, A. Development of a Circular Oriented Bioprocess for Microbial Oil Production Using Diversified Mixed Confectionery Side-Streams. Foods 2019, 8, 300. https://doi.org/10.3390/foods8080300
Tsakona S, Papadaki A, Kopsahelis N, Kachrimanidou V, Papanikolaou S, Koutinas A. Development of a Circular Oriented Bioprocess for Microbial Oil Production Using Diversified Mixed Confectionery Side-Streams. Foods. 2019; 8(8):300. https://doi.org/10.3390/foods8080300
Chicago/Turabian StyleTsakona, Sofia, Aikaterini Papadaki, Nikolaos Kopsahelis, Vasiliki Kachrimanidou, Seraphim Papanikolaou, and Apostolis Koutinas. 2019. "Development of a Circular Oriented Bioprocess for Microbial Oil Production Using Diversified Mixed Confectionery Side-Streams" Foods 8, no. 8: 300. https://doi.org/10.3390/foods8080300





