Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germplasm Evaluated
2.2. Sample Preparation
2.3. Evaluation of Polyphenols, Flavonoids, Anthocyanins, and Antioxidant Activity
2.4. Statistical Analysis
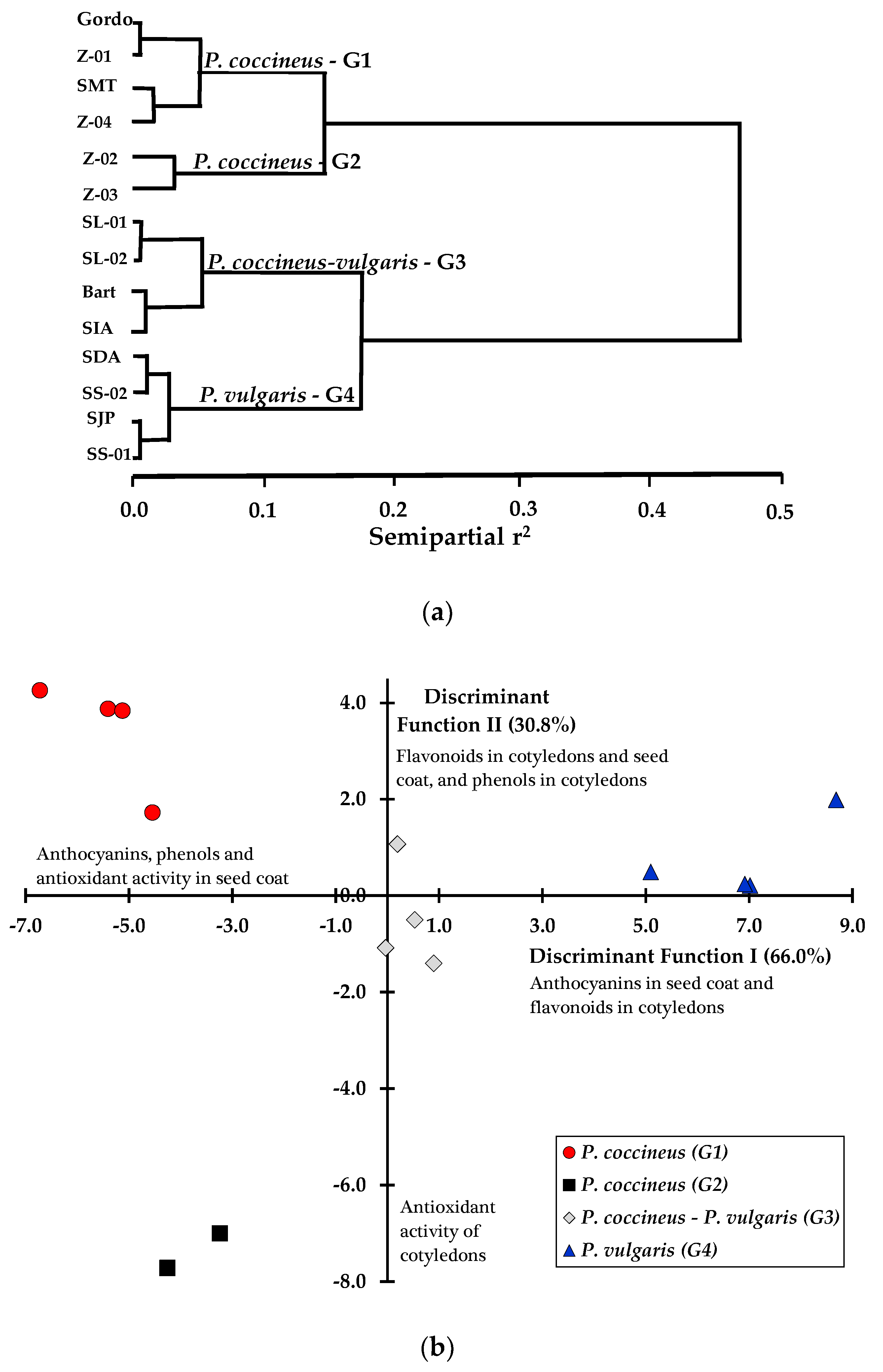
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delgado-Salinas, A.; Bibler, R.; Lavin, M. Phylogeny of the genus Phaseolus (Leguminosae): A recent diversification in an ancient landscape. Syst. Bot. 2006, 31, 779–791. [Google Scholar] [CrossRef]
- Freytag, G.F.; Debouck, D.G. Taxonomy, Distribution, and Ecology of the Genus Phaseolus (Leguminosae-Papilionodeae) in North America, Mexico and Central America; Botanical Miscellany No. 23, SIDA; Botanical Research Institute of Texas: Forth Worth, TX, USA, 2002; p. 298. [Google Scholar]
- Maréchal, R. Etude taxonomique d’un groupe complexe d’espèces des genres Phaseolus et Vigna (Papilionaceae) sur la base de données morphologiques et polliniques, traitées par l’analyse informatique. Boissiera 1978, 28, 1–273. [Google Scholar]
- Boege, E. El patrimonio biocultural de los pueblos indígenas de Mexico. In Hacia la Conservación in situ de la Biodiversidad y Agrobiodiversidad en los Territorios Indígenas; Instituto Nacional de Antropología e Historia y Comisión Nacional para el Desarrollo de los Pueblos Indígenas: Ciudad de Mexico, Mexico, 2010; p. 341. [Google Scholar]
- Chávez-Servia, J.L.; Heredia-García, E.; Mayek-Pérez, N.; Aquino-Bolaños, E.N.; Hernández-Delgado, S.; Carrillo-Rodríguez, J.C.; Gill-Langarica, H.R.; Vera-Guzmán, A.M. Diversity of common bean (Phaseolus vulgaris L.) landraces and the nutritional value of their grains. In Grain Legumes; Goyal, A.K., Ed.; InTech: Rijeka, Croatia, 2016; pp. 1–33. [Google Scholar]
- Lin, L.-Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Gálvez-Ranilla, L.; Genovese, M.I.; Lajolo, F.M. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Nie, S.; Hu, J.; Liu, Z.; Xie, M. Antioxidant activities and anthocyanins composition of seed coats from twenty-six kidney bean cultivars. J. Funct. Food 2016, 26, 622–631. [Google Scholar] [CrossRef]
- De Mejía, E.G.; Guzmán-Maldonado, S.H.; Acosta-Gallegos, J.A.; Reynoso-Camacho, R.; Ramírez-Rodríguez, E.; Pons-Hernández, J.L.; González-Chavira, M.M.; Castellanos, J.Z.; Kelly, J.D. Effect of cultivar and growing locations on the trypsin inhibitors, tannins, and lectins on common beans (Phaseolus vulgaris L.) grown in the semiarid highlands of Mexico. J. Agric. Food Chem. 2003, 51, 5962–5966. [Google Scholar] [CrossRef]
- Quiroz-Sodi, M.; Mendoza-Díaz, S.; Hernández-Sandoval, L.; Carrillo-Ángeles, I. Characterization of the secondary metabolites in the seeds of nine native bean varieties (Phaseolus vulgaris and P. coccineus) from Queretaro, Mexico. Bot. Sci. 2018, 96, 650–661. [Google Scholar] [CrossRef]
- Mosisa, M.T. Effect of processing on proximate and mineral composition of black climbing (P. coccineus L.) bean flour. Afr. J. Food Sci. 2017, 11, 74–81. [Google Scholar]
- Giupponi, L.; Tamburini, A.; Glorgi, A. Prospect for broader cultivation and commercialization of copafam, a local variety of Phaseolus coccineus L., in the Brescia Pre-Alps. Mt. Res. Dev. 2018, 38, 24–34. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, Y.; Okuno, R.; Kameda, K.; Isobe, M.; Kondo, T. Structural analysis and measurement of anthocyanins from colored seed coats of Vigna, Phaseolus, and Glycine legumes. Biosci. Biotech. Biochem. 1996, 60, 589–593. [Google Scholar] [CrossRef]
- Macz-Pop, G.; González-Paramás, A.M.; Pérez-Alonso, J.J.; Rivas-Gonzalo, J.C. New flavonol-anthocyanin condensed pigments and anthocyanins composition in Guatemalan beans (Phaseolus spp.). J. Agric. Food Chem. 2006, 54, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Soleri, D.; Worthington, M.; Aragón-Cuevas, F.; Smith, S.E.; Gepts, P. Farmers’ varietal identification in a reference sample of local Phaseolus species in the Sierra Juárez, Oaxaca, Mexico. Econ. Bot. 2013, 67, 283–298. [Google Scholar] [CrossRef]
- Vargas-Vázquez, M.L.; Muruaga-Martínez, J.S.; Mayek-Pérez, N.; Pérez-Guerrero, A.; Ramírez-Sánchez, S.E. Characterization of the runner bean (Phaseolus coccineus L.) of the trans-Mexican Neovolcanic belt and Sierra Madre oriental. Rev. Mex. Cienc. Agric. 2014, 5, 191–200. [Google Scholar]
- Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C.; Vera Guzmán, A.M.; Aquino-Bolaños, E.N.; Hernández-Delgado, S.; Mayek-Pérez, N.; Lobato-Ortiz, R. Traditional family production and nutritional-nutraceutical value of common beans (Phaseolus vulgaris L.) in Southeast Mexico. In Phaseolus vulgaris: Cultivars, Production and Uses; Campos-Vega, R., Bassinello, P.Z., Ooma, B.D., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2018; pp. 167–198. [Google Scholar]
- Gutiérrez, J.P.; Rivera-Dommarco, J.; Shamah-Levy, T.; Villalpando-Hernández, S.; Franco, A.; Cuevas-Nasu, L.; Romero-Martínez, M.; Hernández-Ávila, M. Encuesta Nacional de Salud y Nutrición 2012. In Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2012; p. 196. [Google Scholar]
- Aguirre-Arenas, J.; Escobar-Pérez, M.; Chávez-Villasana, A. Evaluation of the food consumption and nutrition in four rural communities. Salud Publica en Mexico 1998, 40, 398–407. [Google Scholar]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and woman. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, I.; Olson, B.; Backus, R.; Richter, B.D.; Davis, P.A.; Schneeman, B.O. Beans as a source of dietary fiber increase cholecystokinin and apolipoprotein b48 response to test meals in men. J. Nutr. 2001, 131, 1485–1490. [Google Scholar] [CrossRef]
- Beninger, C.W.; Hosfield, G.L. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 2003, 51, 7879–7883. [Google Scholar] [CrossRef]
- Choung, M.G.; Choi, B.R.; An, Y.N.; Chu, Y.H.; Cho, Y.S. Anthocyanin profile of Korean cultivated kidney bean (Phaseolus vulgaris L). J. Agric. Food Chem. 2003, 51, 7040–7043. [Google Scholar] [CrossRef]
- Lazze, M.C.; Pizzala, R.; Savio, M.; Stivala, L.A.; Prosperi, E.; Bianchi, L. Anthocyanins protect against DNA damage induced by tert-butylhydroperoxide in rat smooth muscle and hepatoma cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 535, 103–115. [Google Scholar] [CrossRef]
- Huber, K.; Brigide, P.; Bretas, E.B.; Canniatti-Brazaca, S.G. Phenolic acid, flavonoids and antioxidant activity of common brown beans (Phaseolus vulgaris L.) before and after cooking. J. Nutr. Food Sci. 2016, 6, 5. [Google Scholar] [CrossRef]
- Labuda, H. Runner bean (Phaseolus coccineus L.)—Biology and use. Acta Sci. Pol. Hortorum Cultus 2010, 9, 117–132. [Google Scholar]
- Ayala-Garay, O.J.; Pichardo-González, J.M.; Estrada-Gómez, J.A.; Carrillo-Salazar, J.A.; Hernández-Livera, A. Yield and seed quality of Ayocote bean at the Valley of Mexico. Agric. Tecn. Mex. 2006, 32, 313–321. [Google Scholar]
- Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chavez-Servia, J.L.; Carrillo-Rodríguez, J.C.; Vera-Guzmán, A.M.; Heredia-García, E. Anthocyanins, polyphenols, flavonoids and antioxidant activity in common bean (Phaseolus vulgaris L.) landraces. Emir. J. Food Agric. 2016, 28, 581–588. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoids contents in mulberry and their scavenging effects in superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 2, F1.2.1–CF1.2.13. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Statistical Analysis System (SAS). SAS/STAT User’s Guides; Release 9.0; SAS Institute Inc.: Cary, NC, USA, 2002; p. 4424. [Google Scholar]
- Bitocchi, E.; Nanni, L.; Bellucci, E.; Rossi, M.; Giardini, A.; Zeuli, P.S.; Logozzo, G.; Stougaard, J.; McClean, P.; Attene, G.; et al. Mesoamerican origin of the common beans (Phaseolus vulgaris L.) is revealed by sequence data. Proc. Natl. Acad. Sci. USA 2012, 109, E788–E796. [Google Scholar] [CrossRef]
- Guerra-García, A.; Suárez-Atilano, M.; Mastretta-Yanez, A.; Delgado-Salinas, A.; Piñeiro, D. Domestication genomics of the open-pollinated scarlet runner bean (Phaseolus coccineus L.). Front. Plant Sci. 2017, 8, 1891. [Google Scholar] [CrossRef]
- Zeven, A.C.; Mohamed, H.H.; Waninge, J.; Veurink, H. Phenotypic variation within landrace of runner bean (Phaseolus coccineus L.). Euphytica 1993, 68, 155–166. [Google Scholar] [CrossRef]
- Vargas-Vázquez, M.L.; Muruaga-Martínez, J.S.; Pérez-Herrera, P.; Gil-Langarica, H.R.; Esquivel-Esquivel, G.; Martínez-Damian, M.A.; Rosales-Serna, R.; Mayek-Pérez, N. Morphoagronomic characterization of the INIFAP core collection of the cultivated form of common bean. Agrociencia 2008, 42, 787–797. [Google Scholar]
- Sinkovič, L.; Pipan, B.; Sinkovič, E.; Meglic, V. Morphological seed characterization of common (Phaseolus vulgaris L.) and runner (Phaseolus coccineus L.) bean germplasm: A Slovenian gene bank example. BioMed Res. Intern. 2019, 2019, 6376948. [Google Scholar] [CrossRef] [PubMed]
- Palmero, D.; Iglesias, C.; de Cara, M.; Tello, J.C.; Camacho, F. Diversity and health traits of local landraces of runner bean (Phaseolus coccineus L.) from Spain. J. Food Agric. Environ. 2011, 9, 290–295. [Google Scholar]
- Onylagha, J.C.; Islam, S. Flavonoids and other polyphenols of the cultivated species of the genus Phaseolus. Int. J. Agric. Biol. 2009, 11, 231–234. [Google Scholar]
- Chávez-Mendoza, C.; Hernández-Figueroa, K.; Sánchez, E. Antioxidant capacity and phytonutrient content in the seed coat and cotyledon of common beans (Phaseolus vulgaris L.) from various regions in Mexico. Antioxidants 2019, 8, 1–19. [Google Scholar]
- Ombra, M.N.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxid. Med. Cell. Long. 2016, 2016, 1398298. [Google Scholar] [CrossRef]
- Sicard, D.; Nanni, L.; Porfiri, O.; Bulfon, D.; Papa, R. Genetic diversity of Phaseolus vulgaris L. and P. coccineus L. landraces in central Italy. Plant Breed. 2005, 124, 464–472. [Google Scholar] [CrossRef]
| ID-Accession | Community of Origin (North Latitude; West Longitude; Altitude in Masl 1) | Bean Color | Pictures of Grain Characteristic |
|---|---|---|---|
| Phaseolus coccineus | |||
| Gordo | Coatepec, Veracruz (19° 27′ 19″; 96° 57′ 31″; 1192) | Light brown |  |
| SMT | San Miguel Tlanichico, Zaachila, Oaxaca (16° 55′; 96° 48′; 1520) | Gray-black |  |
| SL-01 | Santa Lucía Miahuatlán, Oaxaca (16° 11′; 96° 37′; 2000) | Black |  |
| SL-02 | Santa Lucía Miahuatlán, Oaxaca (16° 11′; 96° 37′; 2000) | Brown-red |  |
| Z-01 | Villa de Zaachila, Oaxaca (16° 56′; 96° 45′; 1520) | Red-purple |  |
| Z-02 | Villa de Zaachila, Oaxaca (16° 56′; 96° 45′; 1520) | Gray-black |  |
| Z-03 | Villa de Zaachila, Oaxaca (16° 56′; 96° 45′; 1520) | Gray-black |  |
| Z-04 | Villa de Zaachila, Oaxaca (16° 56′; 96° 45′; 1520) | Gray-black |  |
| Phaseolus vulgaris | |||
| Bart | San Bartolomé Quialana, Oaxaca (16° 54′; 96° 30; 1780) | Black |  |
| SDA | Santo Domingo Amatlan, Oaxaca (16° 18′; 96° 26′; 1540) | Black |  |
| SIA | San Idelfonso Amatlán, Oaxaca (16° 20′; 96° 29′; 1540) | Black |  |
| SJP | San José del Peñasco, Oaxaca (16° 18′; 96° 30′; 1589) | Black |  |
| SS-01 | San Sebastián Abasolo, Oaxaca (17° 00′; 96° 35′; 1550) | Black |  |
| SS-02 | San Sebastián Abasolo, Oaxaca (17° 00′; 96° 35′; 1550) | Black |  |
| Sources of Variation | Seed Coat | Cotyledons | |||||
|---|---|---|---|---|---|---|---|
| Total Polyphenols | Flavon. | Anthocyan. | Antioxidant Activity | Total Polyphenols | Flavon. | Antioxidant Activity | |
| Species | 31853.3 ** (84.1) 2 | 236.6 ** (65.8) | 467.3 ** (98.3) | 1563069 ** (85.4) | 0.599 ** (79.7) | 0.040 ** (64.5) | 40.43 ** (77.9) |
| Accessions (species) 1 | 5999.7 ** (15.8) | 122.8 ** (34.2) | 8.1 ** (1.7) | 267007 ** (14.6) | 0.152 ** (20.2) | 0.021 ** (33.9) | 11.43 ** (22.0) |
| Error | 1.94 (<0.01) | 0.05 (<0.01) | 0.19 (<0.01) | 261.6 (<0.01) | <0.001 (0.1) | <0.001 (1.6) | 0.07 (0.1) |
| Coef. of variation (%) | 1.1 | 1.5 | 13.6 | 1.4 | 1.2 | 6.6 | 2.9 |
| ID-Accession | Seed Coat | Cotyledons | |||||
|---|---|---|---|---|---|---|---|
| Total Polyphenols 1 | Flavon. 2 | Anthocy. 3 | Antiox. Activity 4 | Total Polyphenols 1 | Flavon. 2 | Antiox. Activity 4 | |
| P. coccineus (Pc) | |||||||
| Gordo | 152.4 d 5 | 22.0 c | 0.15 g | 1169.4 d | 2.41 b | 0.30 d | 8.11 f |
| SMT | 218.0 a | 26.3 a | 0.40 fg | 1789.2 a | 2.49 a | 0.40 b | 11.28 b |
| SL-01 | 78.3 j | 12.7 fg | 1.12 fg | 888.1 ef | 2.18 e | 0.30 d | 8.40 ef |
| SL-02 | 95.5 i | 12.1 h | 0.30 fg | 813.2 g | 2.28 d | 0.40 b | 7.18 g |
| Z-01 | 126.5 f | 22.7 b | 0.52 fg | 1261.0 c | 2.16 e | 0.32 cd | 9.08 d |
| Z-02 | 136.6 e | 6.3 k | 1.37 f | 1297.4 c | 2.08 f | 0.20 e | 9.96 c |
| Z-03 | 194.9 b | 9.8 j | 0.75 fg | 1536.4 b | 2.52 a | 0.27 d | 13.52 a |
| Z-04 | 188.8 c | 20.2 d | 0.67 fg | 1498.0 b | 2.37 bc | 0.40 b | 10.00 c |
| Mean of Pc | 148.9 A 6 | 16.5 A | 0.65 B | 1281.6 A | 2.31 A | 0.32 B | 9.69 A |
| P. vulgaris (Pv) | |||||||
| Bart | 81.7 j | 10.6 i | 5.12 d | 874.2 f | 1.86 g | 0.30 d | 7.41 g |
| SDA | 94.7 i | 13.7 e | 8.25 b | 903.6 ef | 2.31 cd | 0.50 a | 8.44 def |
| SIA | 94.4 i | 13.2 ef | 3.72 e | 905.2 ef | 1.81 g | 0.30 d | 6.00 h |
| SJP | 100.7 h | 11.0 i | 6.47 c | 926.1 e | 2.02 f | 0.40 b | 8.28 f |
| SS-01 | 109.3 g | 13.1 ef | 5.77 cd | 922.9 e | 2.32 cd | 0.40 b | 8.98 de |
| SS-02 | 123.3 f | 12.5 gh | 9.65 a | 1132.0 d | 2.28 d | 0.37 bc | 8.74 def |
| Mean of Pv | 100.7 B | 12.4 B | 6.30 A | 944.0 B | 2.10 B | 0.37 A | 7.98 B |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capistrán-Carabarin, A.; Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces. Foods 2019, 8, 295. https://doi.org/10.3390/foods8080295
Capistrán-Carabarin A, Aquino-Bolaños EN, García-Díaz YD, Chávez-Servia JL, Vera-Guzmán AM, Carrillo-Rodríguez JC. Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces. Foods. 2019; 8(8):295. https://doi.org/10.3390/foods8080295
Chicago/Turabian StyleCapistrán-Carabarin, Arelly, Elia Nora Aquino-Bolaños, Yatzil Denih García-Díaz, José Luis Chávez-Servia, Araceli Minerva Vera-Guzmán, and José Cruz Carrillo-Rodríguez. 2019. "Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces" Foods 8, no. 8: 295. https://doi.org/10.3390/foods8080295





