Assessment of Virgin Olive Oil Adulteration by a Rapid Luminescent Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Samples
2.2. Instrumentation
2.3. Data Analysis
3. Results and Discussion
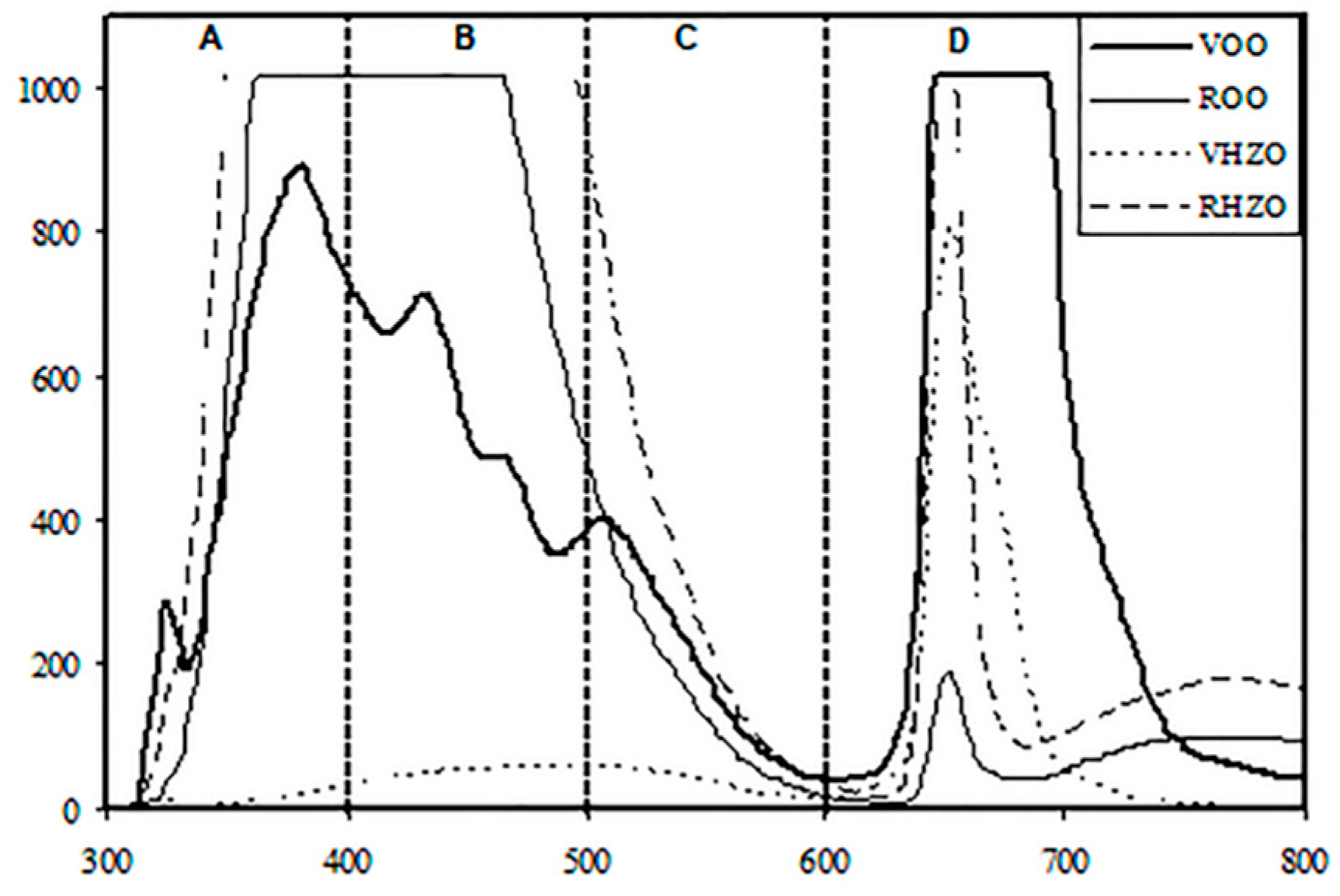
3.1. Spectral Characterization of Oil Samples
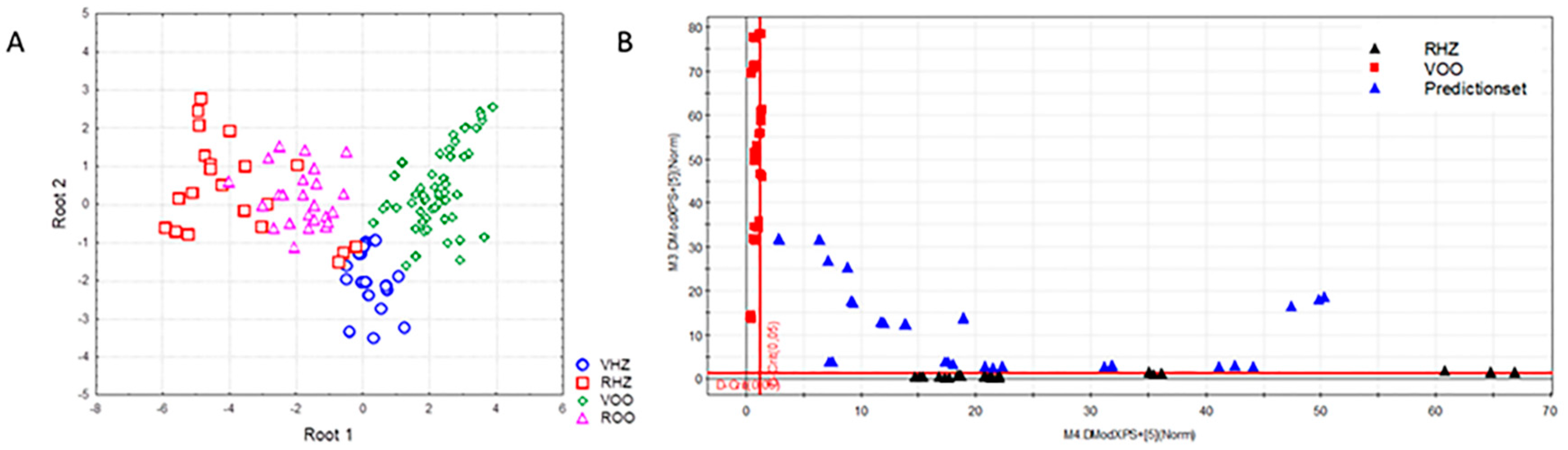
3.2. Classification of Oils
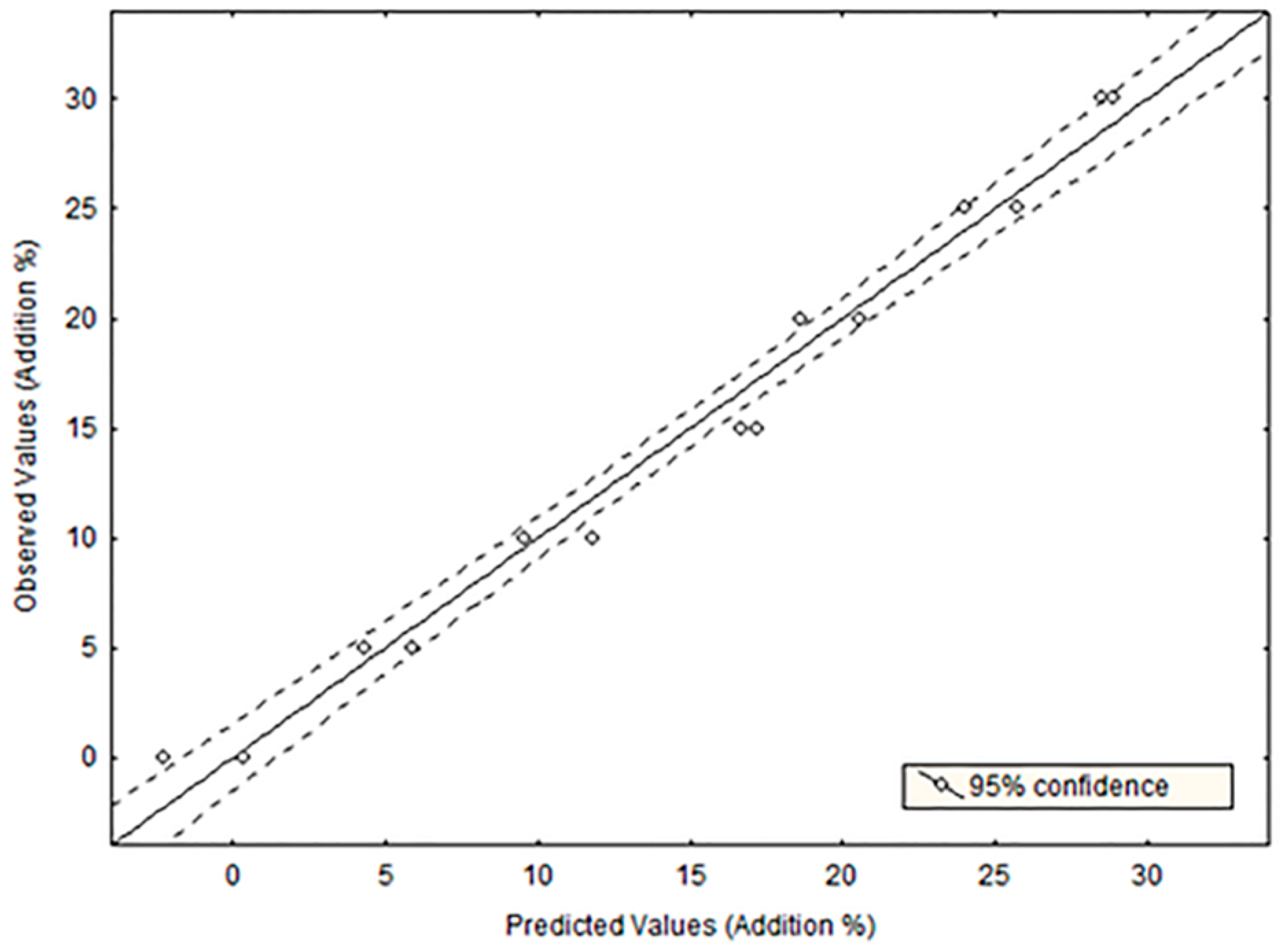
3.3. Detection of Virgin Olive Oil Adulteration by Regression Analysis
3.4. Method Performance: Comparison with Traditional Approaches
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alba, J. Elaboración de aceite de oliva virgen. In El cultivo del olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Ediciones Mundi-Prensa: Madrid, Spain, 2001. [Google Scholar]
- Aparicio, R. Authentication. In Handbook of Olive Oil: Analysis and Properties; Harwood, J.L., Aparicio, R., Eds.; Aspen Publishers: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Aparicio, R.; Morales, M.T.; Alonso, V. Authentication of European virgin olive oils by their chemical compounds, sensory attributes, and consumers’ attitudes. J. Agric. Food Chem. 1997, 45, 1076–1083. [Google Scholar] [CrossRef]
- Zabaras, D. Olive oil adulteration with hazelnut oil and analytical approaches for its detection. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2010; pp. 441–450. [Google Scholar]
- Oliveri, P. Class-modeling in food analytical chemistry: Development, sampling, optimization and validation issues—A tutorial. Anal. Chim. Acta 2017, 982, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Goh, J.Y.; Tay, M.; Lau, H.F.; Li, S.F.Y. Characterization of oils and fats by 1H NMR and GC/MS fingerprinting: Classification, prediction and detection of adulteration. Food Chem. 2013, 138, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, H.; Zribi, A.; Makni, J.; Rebai, A.; Abdelhedi, R.; Bouaziz, M. Detection of Chemlali extra-virgin olive oil adulteration mixed with soybean oil, corn oil, and sunflower oil by using GC and HPLC. J. Agric. Food Chem. 2014, 62, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, H.; Zribi, A.; Bouaziz, M. Extra-virgin olive oil and cheap vegetable oils: Distinction and detection of adulteration as determined by GC and chemometrics. Food Anal. Meth. 2016, 9, 712–723. [Google Scholar] [CrossRef]
- van Wetten, I.A.; van Herwaarden, A.W.; Splinter, R.; Boerrigter, R.; van Ruth, S.M. Detection of sunflower oil in extra virgin olive oil by fast differential scanning calorimetry. Termochim. Acta 2015, 603, 237–243. [Google Scholar] [CrossRef]
- Apetrei, C.; Rodriguez-Mendez, M.L.; de Saja, J.A. Modified carbon electrodes for discrimination of vegetable oils. Sens. Actuators B-Chem. 2005, 111–112, 403–409. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Detection of olive oil adulteration using a voltammetric e-tongue. Comput. Electron. Agric. 2014, 108, 148–154. [Google Scholar] [CrossRef]
- Apetrei, C.; Gutierez, F.; Rodriguez-Mendez, M.L.; de Saja, J.A. Novel method based on carbon paste electrodes for the evaluation of bitterness in extra virgin olive oils. Sens. Actuat. B-Chem. 2007, 121, 567–575. [Google Scholar] [CrossRef]
- Tsopelas, F.; Konstantopoulos, D.; Tsantili-Kakoulidou, A. Voltammetric fingerprinting of oils and its combination with chemometrics for the detection of extra virgin olive oil adulteration. Anal. Chim. Acta 2018, 1015, 8–19. [Google Scholar] [CrossRef]
- Wójcicki, K.; Khmelinskii, I.; Sikorski, M.; Caponio, F.; Paradiso, V.M.; Summo, C.; Paqualone, A.; Sikorska, E. Spectroscopic techniques and chemometrics in analysis of blends of extra virgin with refined and mild deodorized olive oils. Eur. J. Lipid Sci. Technol. 2015, 117, 92–102. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Muñoz-Pérez, M.E.; Arce, L. Detection of adulteration in extra virgin olive oils by using UV-IMS and chemometric analysis. Food Control. 2018, 85, 292–299. [Google Scholar] [CrossRef]
- Dankowska, A.; Małecka, M. Application of synchronous fluorescence spectroscopy for determination of extra virgin olive oil adulteration. Eur. J. Lipid Sci. Technol. 2009, 111, 1233–1239. [Google Scholar] [CrossRef]
- Nunes, C.A. Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Res. Int. 2014, 60, 255–261. [Google Scholar] [CrossRef]
- Sun, X.D.; Lin, W.Q.; Li, X.H.; Shen, Q.; Luo, H.Y. Detection and quantification of extra virgin olive oil adulteration with edible oils by FT-IR spectroscopy and chemometrics. Anal. Methods 2015, 7, 3939–3945. [Google Scholar] [CrossRef]
- Jiménez-Sanchidrián, C.; Ruiz, J.R. Use of Raman spectroscopy for analyzing edible vegetable oils. Appl. Spectrosc. Rev. 2016, 51, 417–430. [Google Scholar] [CrossRef]
- Sayago, A.; Morales, M.T.; Aparicio, R. Detection of hazelnut oil in virgin olive oil by a spectrofluriometric method. Eur. Food Res. Tech. 2004, 218, 480–483. [Google Scholar] [CrossRef]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Rapid synchronous fluorescence method for virgin olive oil adulteration assessment. Food Chem. 2007, 105, 369–375. [Google Scholar] [CrossRef]
- Kyriakidis, N.B.; Skarkalis, P. Fluorescence spectra measurement of olive oil and other vegetable oils. J. Aoac. Int. 2000, 83, 1435. [Google Scholar]
- Mínguez-Mosquera, M.I.; Gandul-Rojas, B.; Gallardo-Guerrero, L. Rapid method of quantification of chlorophylls and carotenoids in virgin olive oil by high-performance liquid chromatography. J. Agric. Food Chem. 1992, 40, 60–63. [Google Scholar] [CrossRef]
- Avramidou, E.V.; Doulis, A.G.; Petrakis, P.V. Chemometrical and molecular methods in olive oil analysis: A review. J. Food Process. Preserv. 2018, 42, e13770. [Google Scholar] [CrossRef]
- Sayago, A.; González-Domínguez, R.; Urbano, J.; Fernández-Recamales, Á. Combination of vintage and new-fashioned analytical approaches for varietal and geographical traceability of olive oils. LWT Food Sci. Technol. 2019, 111, 99–104. [Google Scholar] [CrossRef]
- Sayago, A.; González-Domínguez, R.; Beltrán, R.; Fernández-Recamales, Á. Combination of complementary data mining methods for geographical characterization of extra virgin olive oils based on mineral composition. Food Chem. 2018, 261, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Ruiz del Castillo, M.L.; Herraiz, M.; Blanch, G.P. Study of the adulteration of olive oil with hazelnut oil by on-line coupled high performance liquid chromatographic and gas chromatographic analysis of filbertone. Food Chem. 2006, 97, 742–749. [Google Scholar] [CrossRef]
- Zabaras, D.; Gordon, M.H. Detection of pressed hazelnut oil in virgin olive oil by analysis of polar components: Improvement and validation of the method. Food Chem. 2004, 84, 475–483. [Google Scholar] [CrossRef]
- Cercaci, L.; Rodriguez-Estrada, M.T.; Lercker, G. Solid-phase extraction-thin-layer chromatography-gas chromatography method for the detection of hazelnut oil in olive oils by determination of esterified sterols. J. Cromatogr. A. 2003, 985, 211–220. [Google Scholar] [CrossRef]
- Chen, H.; Angiulic, M.; Ferrari, C.; Tombari, E.; Salvetti, G.; Bramanti, E. Tocopherol speciation as first screening for the assessment of extra virgin olive oil quality by reversed-phase high-performance liquid chromatography/fluorescence detector. Food Chem. 2011, 125, 1423–1429. [Google Scholar] [CrossRef]
- Uncu, A.T.; Uncu, A.O.; Frary, A.; Doganlar, S. Barcode DNA length polymorphisms vs fatty acid profiling for adulteration detection in olive oil. Food Chem. 2017, 221, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ceglie, C.; Calvano, C.D.; Zambonin, C.G. Determination of hidden hazelnut oil proteins in extra virgin olive oil by cold acetone precipitation followed by in-solution tryptic digestion and MALDI-TOF-MS analysis. J. Agric. Food Chem. 2014, 62, 9401–9409. [Google Scholar] [CrossRef]
- Calvano, C.D.; Ceglie, C.D.; D’Accolti, L.; Zambonin, C.G. MALDI-TOF mass spectrometry detection of extra-virgin olive oil adulteration with hazelnut oil by analysis of phospholipids using an ionic liquid as matrix and extraction solvent. Food Chem. 2012, 134, 1192–1198. [Google Scholar] [CrossRef]
- Vigli, G.; Philippidis, A.; Spyros, A.; Dais, P. Classification of edible oils by employing 31P and 1H NMR spectroscopy in combination with multivariate statistical analysis. A proposal for the detection of seed oil adulteration in virgin olive oils. J. Agric. Food Chem. 2003, 51, 5715–5722. [Google Scholar] [CrossRef] [PubMed]
- Gouilleux, B.; Marchand, J.; Charrier, B.; Remaud, G.S.; Giraudeau, P. High-throughput authentication of edible oils with benchtop Ultrafast 2D NMR. Food Chem. 2018, 244, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ozen, B.F.; Mauer, L.J. Detection of hazelnut oil adulteration using FT-IR spectroscopy. J. Agric. Food Chem. 2002, 50, 3898–3901. [Google Scholar] [CrossRef] [PubMed]
- Baeten, V.; Fernández Pierna, J.A.; Dardenne, P.; Meurens, M.; García-González, D.L.; Aparicio-Ruiz, R. Detection of the presence of hazelnut oil in olive oil by FT-raman and FT-MIR spectroscopy. J. Agric. Food Chem. 2005, 53, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Vietina, M.; Agrimonti, C.; Marmiroli, N. Detection of plant oil DNA using high resolution melting (HRM) post PCR analysis: A tool for disclosure of olive oil adulteration. Food Chem. 2013, 141, 3820–3826. [Google Scholar] [CrossRef] [PubMed]

| Spectral Regions | |||||
|---|---|---|---|---|---|
| 300–400 nm | 400–500 nm | 500–600 nm | 600–800 nm | ||
| r | SD | 3.72 | 1.84 | 2.86 | 2.77 |
| RSD (%) | 1.3 | 0.43 | 1.81 | 1.21 | |
| R | SD | 14.96 | 22.27 | 17.33 | 6.07 |
| RSD (%) | 10.69 | 6.01 | 7.63 | 3.34 | |
| Method | Lowest Level of Adulteration Detected (w/w) | Reference |
|---|---|---|
| Chromatographic methods | ||
| LC/GC (filbertone) | 20–25% | [27] |
| LC (Maillard products) | 5% | [28] |
| GC (phytosterols) | >30% | [29] |
| LC (tocopherols) | 3% | [30] |
| GC (fatty acids) | Non detectable | [31] |
| Mass spectrometric methods | ||
| MALDI-TOF-MS (proteins) | 20% | [32] |
| MALDI-TOF-MS (phospholipids) | 1% | [33] |
| Spectroscopic methods | ||
| 1H/31P NMR | 5% | [34] |
| 2D NMR | 6.27% | [35] |
| FTIR | 25% | [36] |
| FT-Raman, FT-MIR | 8% | [37] |
| Genetic methods | ||
| PCR-CE | 5% | [31] |
| PCR-HRM | 10% | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Domínguez, R.; Sayago, A.; Morales, M.T.; Fernández-Recamales, Á. Assessment of Virgin Olive Oil Adulteration by a Rapid Luminescent Method. Foods 2019, 8, 287. https://doi.org/10.3390/foods8080287
González-Domínguez R, Sayago A, Morales MT, Fernández-Recamales Á. Assessment of Virgin Olive Oil Adulteration by a Rapid Luminescent Method. Foods. 2019; 8(8):287. https://doi.org/10.3390/foods8080287
Chicago/Turabian StyleGonzález-Domínguez, Raúl, Ana Sayago, María Teresa Morales, and Ángeles Fernández-Recamales. 2019. "Assessment of Virgin Olive Oil Adulteration by a Rapid Luminescent Method" Foods 8, no. 8: 287. https://doi.org/10.3390/foods8080287





