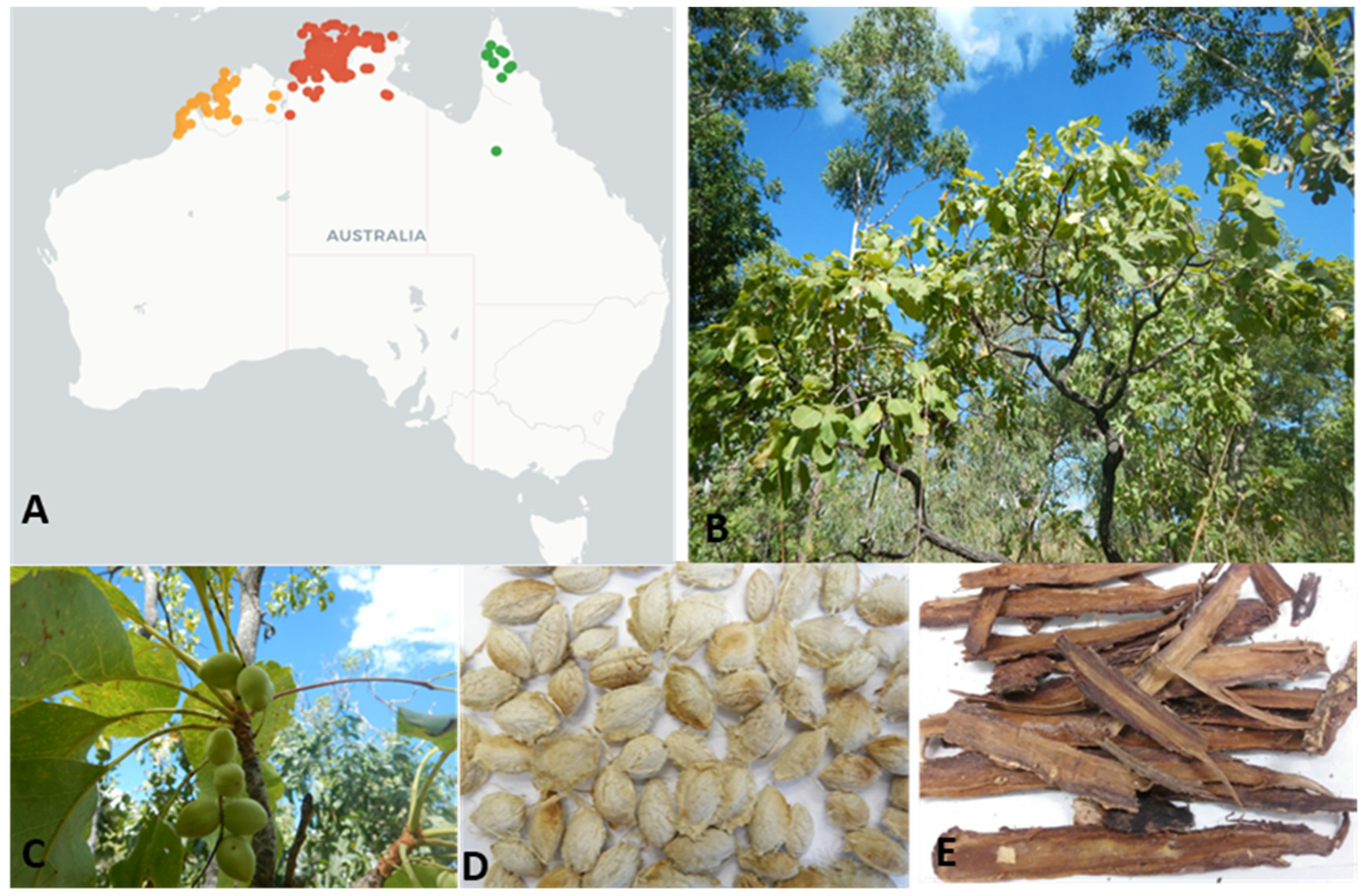
3.5.1. Disc Diffusion Assay
Plant derived antimicrobials can effectively reduce or inhibit pathogenic and spoilage microorganisms and have the potential to be an alternative to synthetic antimicrobials [
6]. The use of natural antimicrobial agents in food processing to extend the shelf-life of food products is well documented [
6]. Consumer concern over synthetic preservatives in food products has contributed to the search for preservatives from natural sources. The antimicrobial activities of extracts from
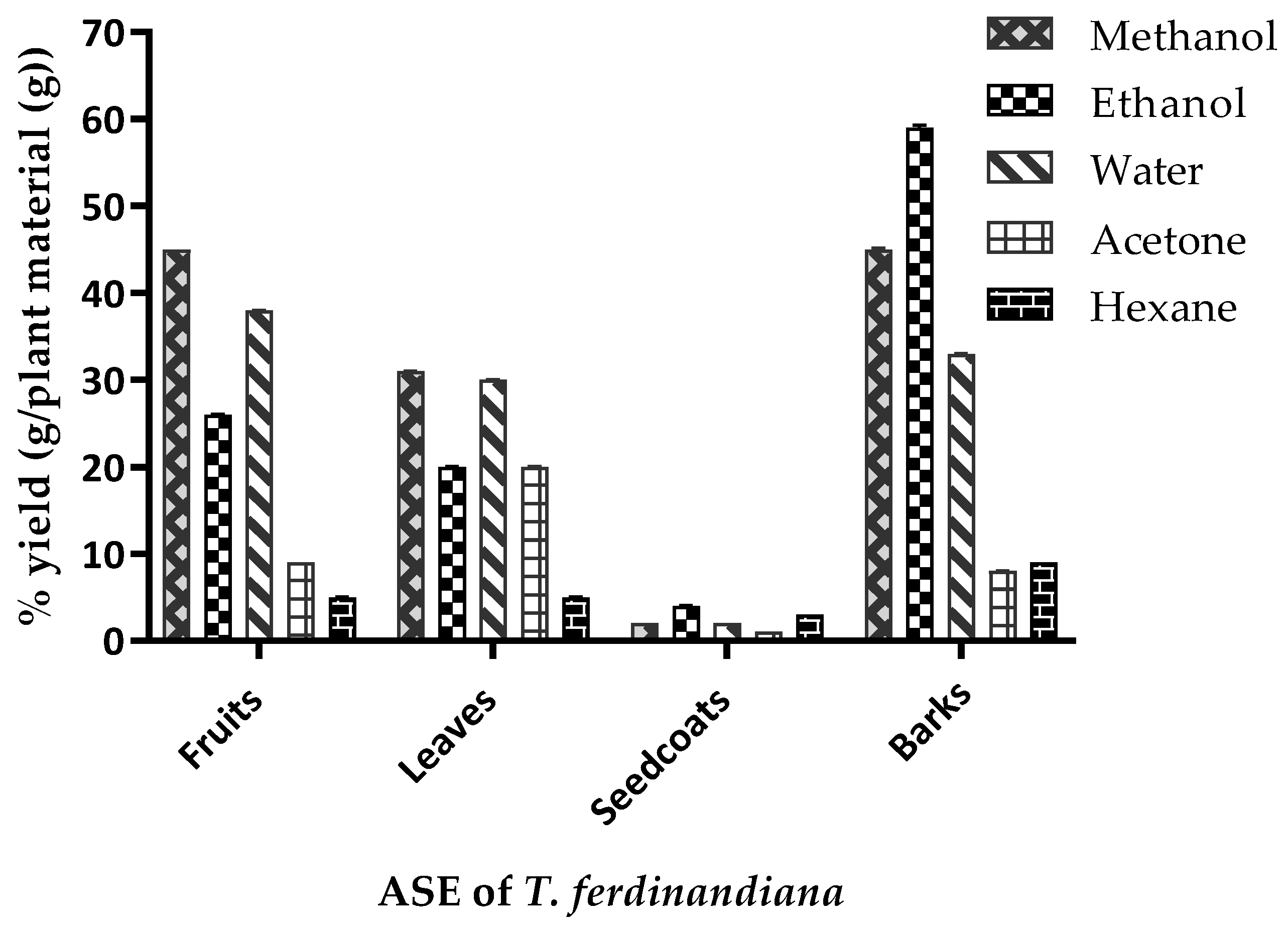
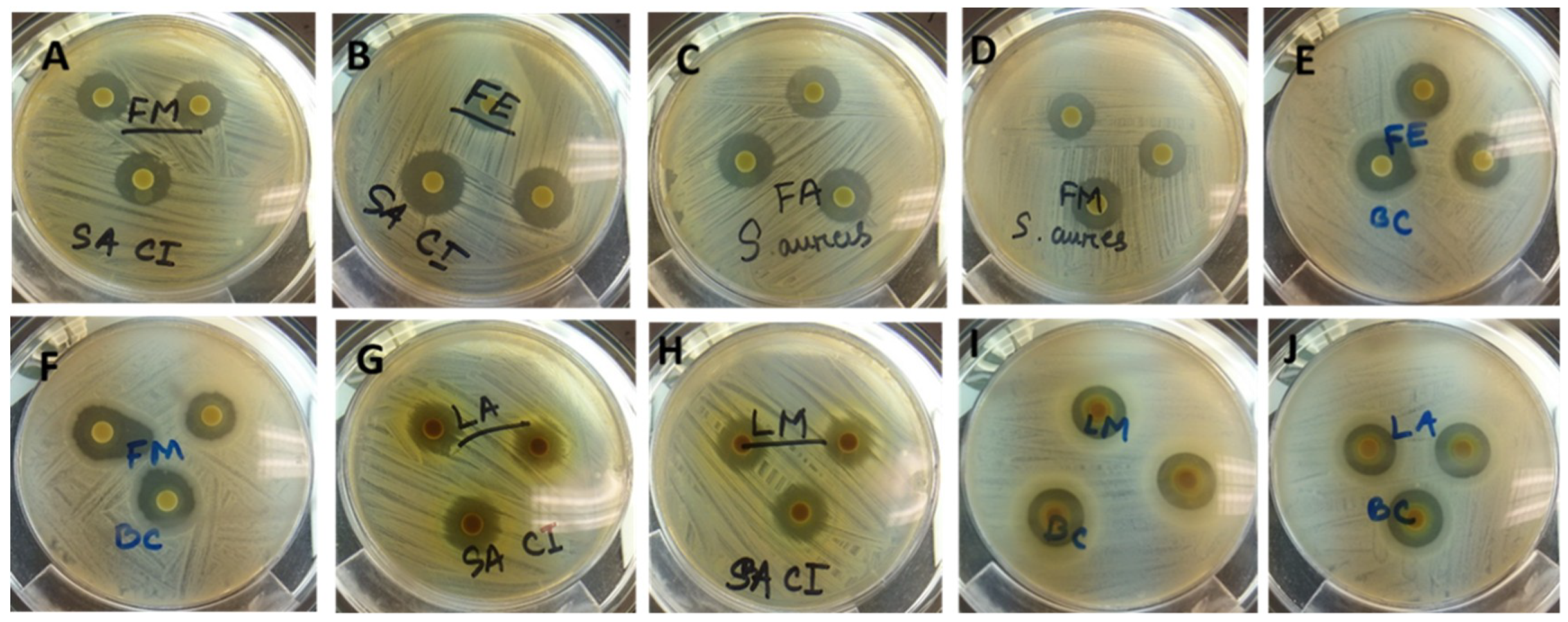
T. ferdinandiana tissues prepared with different solvents were determined against different microorganisms, with the inhibition zone measured in mm, and presented in
Table 5 and illustrated in
Figure 3. Overall, methanol extracts were found to be the most effective against the organisms tested and showed a broad spectrum of antimicrobial activity against the tested bacteria. The antimicrobial activity of the methanol extracts was similar to the acetone extracts, whilst water extracts from fruit, leaves, and bark were found to be active against
S. aureus, MRSA,
P. aeruginosa CI, and
B. cereus. Fruit and leaf extracts were found to have similar zones of inhibition against the tested organisms, with MRSA,
L. monocytogenes, and
B. cereus the most sensitive bacteria among those tested.
S. aureus was inhibited less compared to MRSA. Seedcoat extracts were found to be the least active against the microorganisms tested.
Herbal remedies formulated from whole plants are gaining more interest, as they are safer than synthetic options. The antimicrobial activity from
T. ferdinandiana extracts against different microbial strains supports the scientific rationality of using plants/plant tissue in traditional medicine [
42]. The inhibition of the growth of six bacterial strains by the fruit and leaf extracts could be due to the presence of antioxidant phytochemicals, mainly polyphenols, in the extracts. The
T. ferdinandiana results support several other studies, showing the antimicrobial activity of plant extracts due to the presence of polyphenolic compounds in the extracts [
26,
43]. Polyphenols, particularly tannins and flavonols, are known to possess antimicrobial activity and can suppress the growth of microorganisms by various mechanisms, such as the inhibition of biofilm formation, host-ligand adhesion reduction, and the neutralization of bacterial toxins [
44].
In the present study, we found that
T. ferdinandiana tissue extracts are high in TPC and tannins. Other species of Terminalia plants, such as
Terminalia arjuna,
Terminalia bellerica,
Terminalia chebula,
Terminalia sambesiaca,
Terminalia Kaiserana and
Terminalia sericia, are also high in tannins and other polyphenols [
45,
46,
47]. Previous reports on the antimicrobial properties of Terminalia plants were supported by the presence of a vast range of phytochemicals, including polyphenols and tannins [
48,
49,
50]. Tannins inhibit bacterial growth by binding to bacterial enzymes and interfering with phosphorylation, and sometimes forming complexes with transition metal ions, which are important for bacterial growth [
51].
3.5.3. Scanning Electron Microscopy
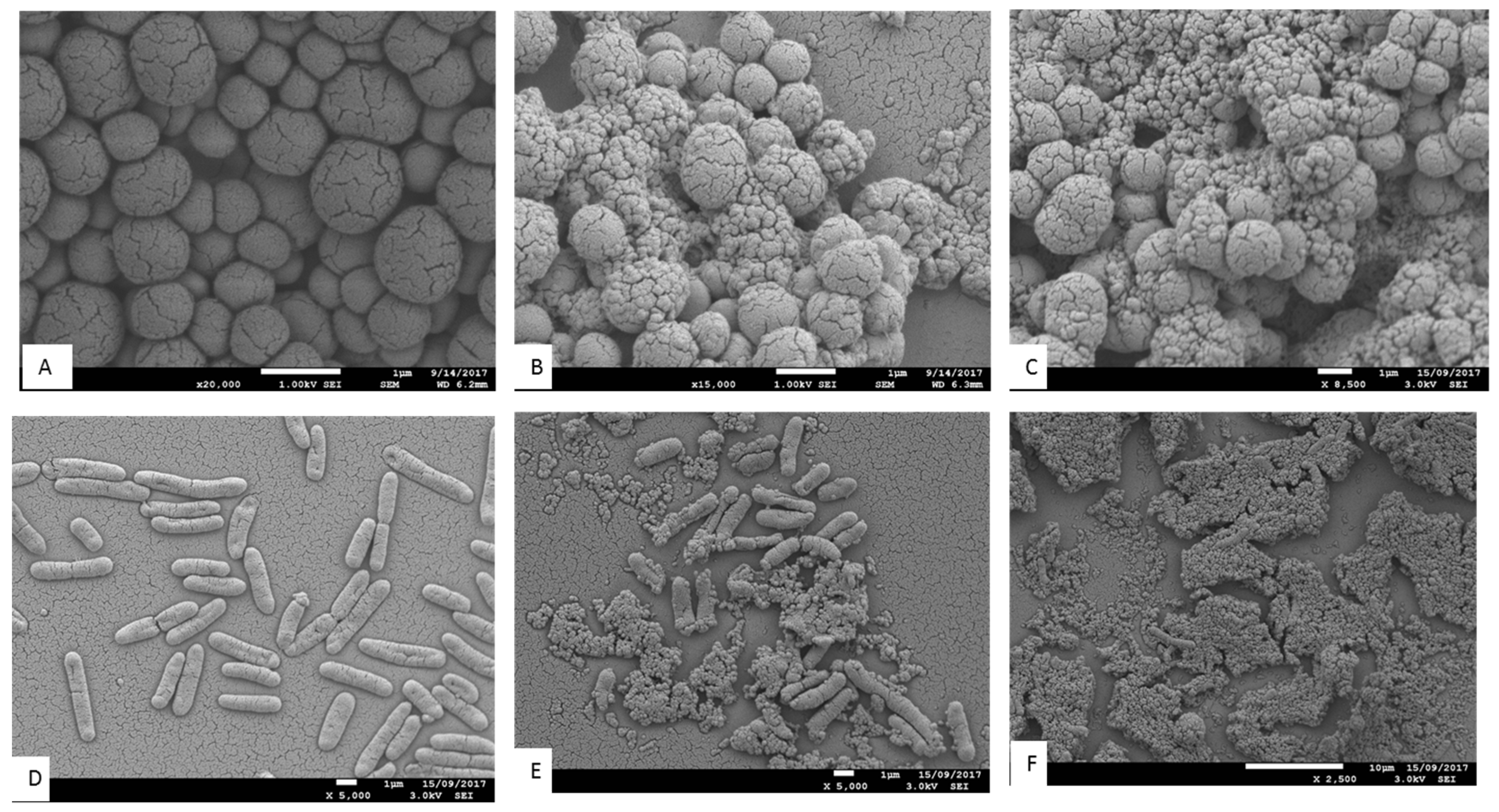
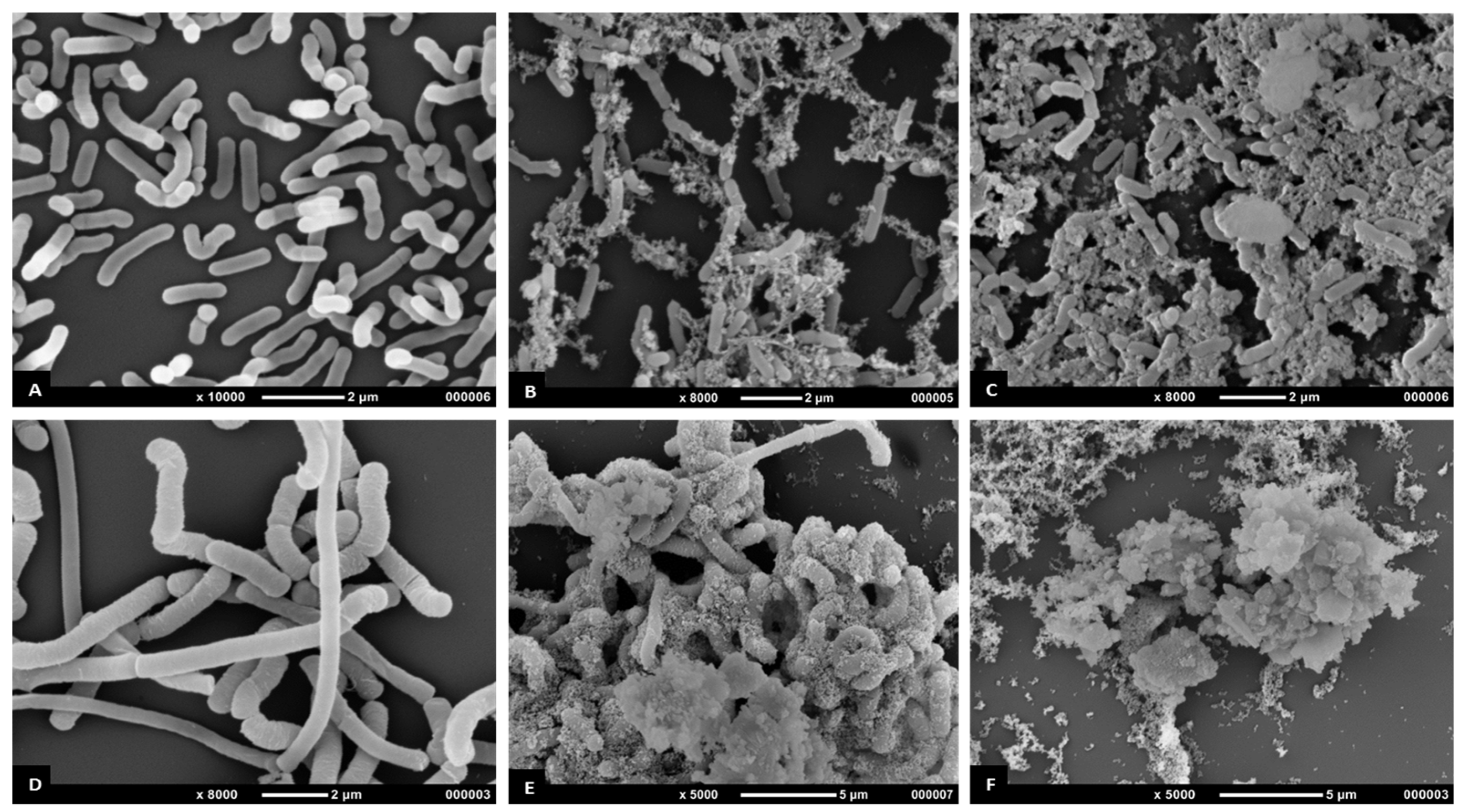
The antimicrobial effects of
T. ferdinandiana fruit and leaf extracts on the morphology of MRSA,
B. cereus,
L. monocytogenes, and
P. aeruginosa CI cells were determined by scanning electron microscopy, as illustrated in
Figure 4 and
Figure 5. All bacterial cells treated with the extracts at the MIC were damaged compared to the control cells (20%
v/
v ethanol). The control cells had a smooth surface, with the outer layer of the bacteria relatively intact (
Figure 4A,D and
Figure 5A,D). By contrast, the damaging effects of the fruit and leaf extracts on bacterial cell walls were evident compared to the appearance of the control cells (
Figure 4B,C,E,F and
Figure 5B,C,E,F). Almost all the bacterial cells treated with the fruit and leaf extracts showed the disintegration of the outermost layer and, in some cases, the outermost layer had disappeared (
Figure 4F and
Figure 5E,F).
The antimicrobial mechanisms or exact target sites for natural antimicrobials have not been identified yet and warrant further investigation [
6]. However, it is thought that terpenoids and phenolics are involved in membrane disruption, phenolic acids and flavonoids cause metal chelation, coumarin interferes with the genetic material, and alkaloids inhibit the growth of microorganisms [
52]. Phytochemicals are also reported to be involved in membrane disruption and, in turn, cause leakage of cellular content [
53]. It was observed that plant phytochemicals interfere with active transport mechanisms and possibly dissipate cellular energy in adenosine triphosphate (ATP) form [
54].
In
Figure 5B,C, some of the extract-treated
L. monocytogenes cells underwent splitting, a change in cell morphology due to deep wrinkling and distortion. Therefore, it is postulated that fruit and leave methanol extracts have antimicrobial activity against
L. monocytogenes. Antioxidative polyphenols might have been involved in causing lesions in the cytoplasmic membrane, which in turn may have caused leakage of intracellular contents, impairment of microbial enzymes, and potentially cell death [
53]. This evidence suggests that
T. ferdinandiana fruits extracts may effectively inhibit
L. monocytogenes in food products.
To visualize the effects of
T. ferdinandiana fruit and leaf methanol extracts, SEM images of
B. cereus cells treated with MIC doses of extracts were taken and are presented in
Figure 5. The fruit and leaf extracts altered the cell morphology (
Figure 5E,F) in comparison to controls (
Figure 5D). The control bacterial cells appeared whole and distinct from one another, whilst the bacterial cells treated with both the fruit and leaf extracts were deformed. In particular, the cell wall of
B. cereus treated with leaf extracts appeared to be degraded (
Figure 5F).
A change in cell morphology was observed in
P. aeruginosa clinical isolates incubated with
T. ferdinandiana fruit and leaf extracts, as shown in
Figure 4E and 4F. The cell surface morphology of
P. aeruginosa control cells was intact and smooth (
Figure 4D) compared to cells incubated with the MIC of
T. ferdinandiana fruit extracts, which changed to granular with the appearance of blisters (
Figure 4E). Treatment with leaf extracts was even more pronounced, as evidenced by the loss of cellular orientation (
Figure 4F). These results suggest that
T. ferdinandiana leaf extracts are more active than fruit extracts in promoting
P. aeruginosa cell death caused by cell membrane disintegration and cell atrophy, indicating that the active compounds present in
T. ferdinandiana leaf extracts may act on the cell membrane or extracellular proteins, resulting in the inhibition of bacterial cell growth.
Scanning electron microscopy images of MRSA (
Figure 4B,C) treated with
T. ferdinandiana extracts also showed partial disintegration of the bacterial cell surfaces and reduced residual cellular content. Cell surfaces also appeared rougher after
T. ferdinandiana extract treatment. The potent antimicrobial activity observed in the
T. ferdinandiana extracts in the present study can therefore be attributed to the presence of numerous phytochemicals in the plant, especially ascorbic and ellagic acid, as previously reported [
15]. In the presence of
T. ferdinandiana extracts, bacterial cells grew as isolated colonies, compared to control cells. The antimicrobial activity of plants is mostly attributed to their principal phenolic components, which exhibit significant bactericidal activity against MRSA. A reaction between phenolic compounds and bacterial membrane proteins was suggested to be involved in their antimicrobial action, which can weaken the cell wall or damage the cytoplasmic membrane directly [
55].
These results indicate that antimicrobial compounds are contained in
T. ferdinandiana leaves and fruit and act by damaging bacterial cell walls or inducing cell lysis. It is possible that the antimicrobial compounds present in
T. ferdinandiana extracts readily enter the cells through these lesions, whilst also facilitating the leakage of cell contents. That is, when microbial cell walls or membranes become compromised, possibly by interacting with phenolic compounds, low molecular weight substances, such as K
+ and PO
43−, tend to leach out first, followed by the loss of other intracellular molecules, such as proteins, DNA, RNA, and other higher molecular weight materials [
56]. These antimicrobial compounds may even react with bacterial DNA, ultimately resulting in cell death. Some researchers have reported that bioactive compounds derived from plants have antimicrobial effects on cells through reduced oxygen uptake, reduced cellular growth, inhibition of lipid, protein, and nucleic acid synthesis, changes in the lipid profile of the cell membrane, and inhibition of microbial cell wall synthesis. Cox et al. [
57] reported that slight changes in the structural integrity of cell membranes can affect cell metabolism and lead to cell death.
A wide variety of phenolic compounds, including tannins, gallic acid, ellagic acid, corilagin, geraniin, tannic acid, punicalagin, castalagin, and punicalin, have been reported to be present in the
Terminalia genus [
58]. Antimicrobial activity of these compounds has also been reported against a number of microorganisms, such as MRSA,
S. aureus,
P. aeruginosa, Genus
vibrio,
Escherichia coli,
Candida Albicans, and
Aspergillus fumigatus [
59]. Previous reports on the phytochemicals present in
T. ferdinandiana include gallic acid, apionic acid, gluconolactone, chebulic acid, ferulic acid, exifone, corilagin, punicalin, castalagin, and chebulagic acid [
14,
60]. High levels of ellagic acid and ascorbic acid have also been reported in
T. ferdinandiana [
15].
T. ferdinandiana fruit is currently marketed commercially as a functional ingredient in the form of a freeze-dried powder in the food industry, however, other tissues such as leaves have not yet been considered as functional (food) ingredients.