1. Introduction
Avocado (
Persea americana Mill) is a fruit with high-calorie content, which is considered as a functional food due to lipid fraction. It is one of the ancient fruits known have survived from the Aztec times and has been used as vegetable butter since then [
1]. Avocados grow at tropical and subtropical regions and belong to the family of
Lauraceae, which has about 150 species all with a similar lipid profile to olive oil [
2]. The main reason avocados have attracted the attention of researchers and consumers is because of their functional compounds, which provide additional properties than just oil [
3]. The fruit flesh consists of 30% oil. Typical avocado oil has about 75% monounsaturated fat that comprises oleic and palmitoleic acids, where 25% is saturated and polyunsaturated fatty acids [
2]. The avocado oil has a characteristic flavor and a high smoke point that is above 250 °C. The oil has a green color and avocado flavor with mushroom flavor notes. This makes it worth promoting sensory aspects in the future. More importantly, the main functional component is the α-tocopherol, which is an antioxidant chemical that is usually around 79 to 190 mg/kg in avocado oil [
1]. In the literature, the avocado has been investigated as fruit and source of vegetable oil, and studies are mostly focusing on the characterization of the oil, physicochemical properties, fatty acid composition, and comparative studies with olive oil [
4,
5,
6].
To obtain the maximum functional components, the fruit needs to be carefully processed into the oil with extraction methods such the soxhlet extraction, pressing, and ultrasonic extraction [
7]. Not only for avocado but also for all the vegetable–fruit oils, quality parameters are crucial for legislation as well as consumers safety. While extracting the oil from the source, heat is generally applied for conventional extraction methods. When applying heat, many desirable technological advantages occur, as well as undesirable reactions. Oils are sensitive to heat and undergo quality loss caused by chemical instability [
7,
8]. The most critical chemical change that represents quality loss and deterioration in the oil is lipid oxidation. This well-studied field of oil occurs due to the chain reactions that result in the formation of various oxidation products that are not suitable for consumption. The oxidation procedure is the result of heat as well as environmental conditions, such as oxygen. Effects of the lipid oxidation can be analyzed from the oxidized product; however, we can also express those effects by mathematical relationships. Most often, the cause of the oxidation is the temperature, especially while storing the oil/fat, and dependence of the temperature can be expressed best by the Arrhenius model [
9]. However, a researcher should always keep in mind that this mathematical model can only be applied to a simple food system since the oxidative reactions are product dependent and often more complex [
10]. To apply the best fitting mathematical relationship to oil, it is necessary to obtain information about the chemical structure, as well as manufacturing and processing conditions. Kinetic data helps us to understand the oxidation reaction to calculate the best conditions to obtain better quality with minimum deterioration. Additionally, using kinetic data, we can predict the oxidative stability changes under various temperature applications, and storage and transporting periods [
7].
Oil pressing/extraction technology is facing changes in the main manufacturing processes due to the expected trend of cold pressing for better flavor, color, and higher functional nutrient content. Apart from the mentioned advantages, cold pressing leads to a lower oxidation probability. For other fruits and seeds such as olive oil, cold-pressing has become the conventional method.
Production volume of the avocado cold press oil is 2000 tonnes/year globally [
11]. Despite the increasing demand on the unsaturated functional oils, there is a limited number of studies on the characterization of the avocado oil and the effect of production technologies on the quality. The main reason for the cold pressing demand is due to easy and low-cost manufacturing as well as nutritional protection. On the other hand, non-thermal applications were found to be beneficial for the physical and chemical characteristics of the oil but disadvantaged in terms of extraction efficiency [
12].
This paper presents innovative information about the mathematical relationships of the kinetics of avocado oil as well as including some characteristic information about the oil source. The main objective of this study was to develop mathematical models to describe the reaction rate as a function of temperature for avocado oil to explore the shelf life and storage conditions, to provide insight into the new generation functional oils and also to provide a step for future studies that are essential for avocado oil. On the other hand, antioxidant properties and some characteristic properties were determined, which might be either missing in the literature or need confirmation.
3. Results and Discussion
The main aim of this study was to characterize and investigate the kinetic analysis of avocado oil. The rancimat method was used as a reflection of the expected shelf life determination as well as determining the kinetic behavior of the avocado oil. Rancimat measurements for the oxidation induction period (OIT, time in hours) were done isothermally at five different temperatures (100 °C, 110 °C, 120 °C, 130 °C, and 140 °C) and those values were selected according to the study done previously [
25]. OIT of the avocado oil can be seen in
Table 1. Results illustrate that OIT values are decreasing by the increasing temperature. This result has been previously validated [
26] According to the experiments and calculation, a linear regression model was obtained, which was used for calculating the expected shelf life of the avocado oil.
As mentioned earlier, the
k value (reaction rate constant) was determined by the reciprocal OIT for each temperature and represents lipid oxidation of avocado oil, which can be seen for different temperature operations in
Table 1. By using the
k value rate of lipid oxidation as a function of temperature, a direct relationship can be observed.
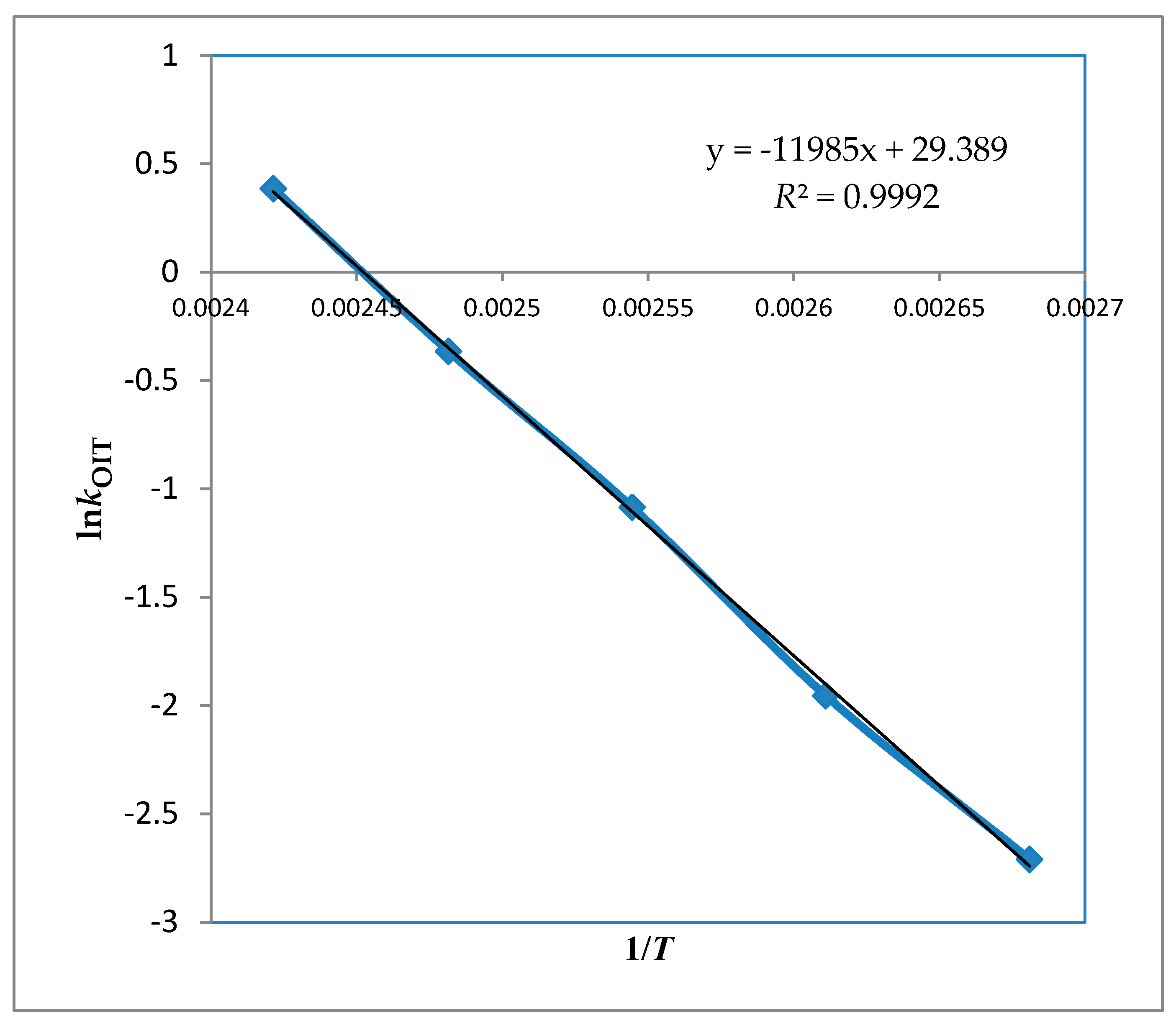
As shown in
Figure 1, ln (
k) vs. 1/
T was obtained from the measurements. Those values were used to obtain activation energy and pre-exponential factor. The slope was used to calculate
Ea while intercept used for
A (frequency factor) as explained in (
R2 > 0.99). More specifically,
k and
T values have a semi-logarithmic relationship with a linear dependency with good correlation of determination (
R2 > 0.99). Duncan’s multiple range tests were calculated for OIT assay and statistically significant (
p < 0.05) difference was found at all temperatures. The results indicate that the reaction rate constant increase with increasing temperature, which means that the oil oxidation is faster at higher temperatures as authors expected. Noteworthy, the kinetic rate constant prediction, especially at lower temperatures, has some limitations which cause some uncertainties and errors. Those limitations are linked with the test. Specifically, the oil follows different pathways of lipid oxidation at lower and higher temperatures depending on the metal ions and antioxidants activity. On the other hand, the degree of oxygen solubility is different in varying temperatures, where literature reporting to see an increase of 25% for each 10 °C.
Calculated Arrhenius activation energies (99.6 ± 2 kJ·mol
−1) and pre-exponential factor (5.8 × 10
12 h
−1) are listed in
Table 2. According to the literature, the stability of avocado oil was similar to that of olive oil because of similar lipid profile and stability results, which means our results are in accordance with literature findings [
11]. Another supporting data for the similarity of avocado oil and olive oil was investigated on different kinds of olive oils under rancimat conditions and illustrated that the activation energies for the oxidation of the olive oils were between 97.7 kJ·mol
−1 and 101.9 kJ·mol
−1, which is very similar to the findings in this study [
25]. A previous investigation showed that the kinetics of different type of vegetable oils, as well as
Ea values of selected vegetable oils, was found to be between 79 mol
−1 and 104 kJ·mol
−1 which have a different fatty acid profile [
7]. Alternative oils, such as vegetable oils and olive oil, were found to have similar activation energies. The oils which have lower activation energy are expected to require a higher temperature to induce a certain change in the rate of oxidation. Therefore according to our findings, avocado oil is stable compared to other oil sources as well as olive oil.
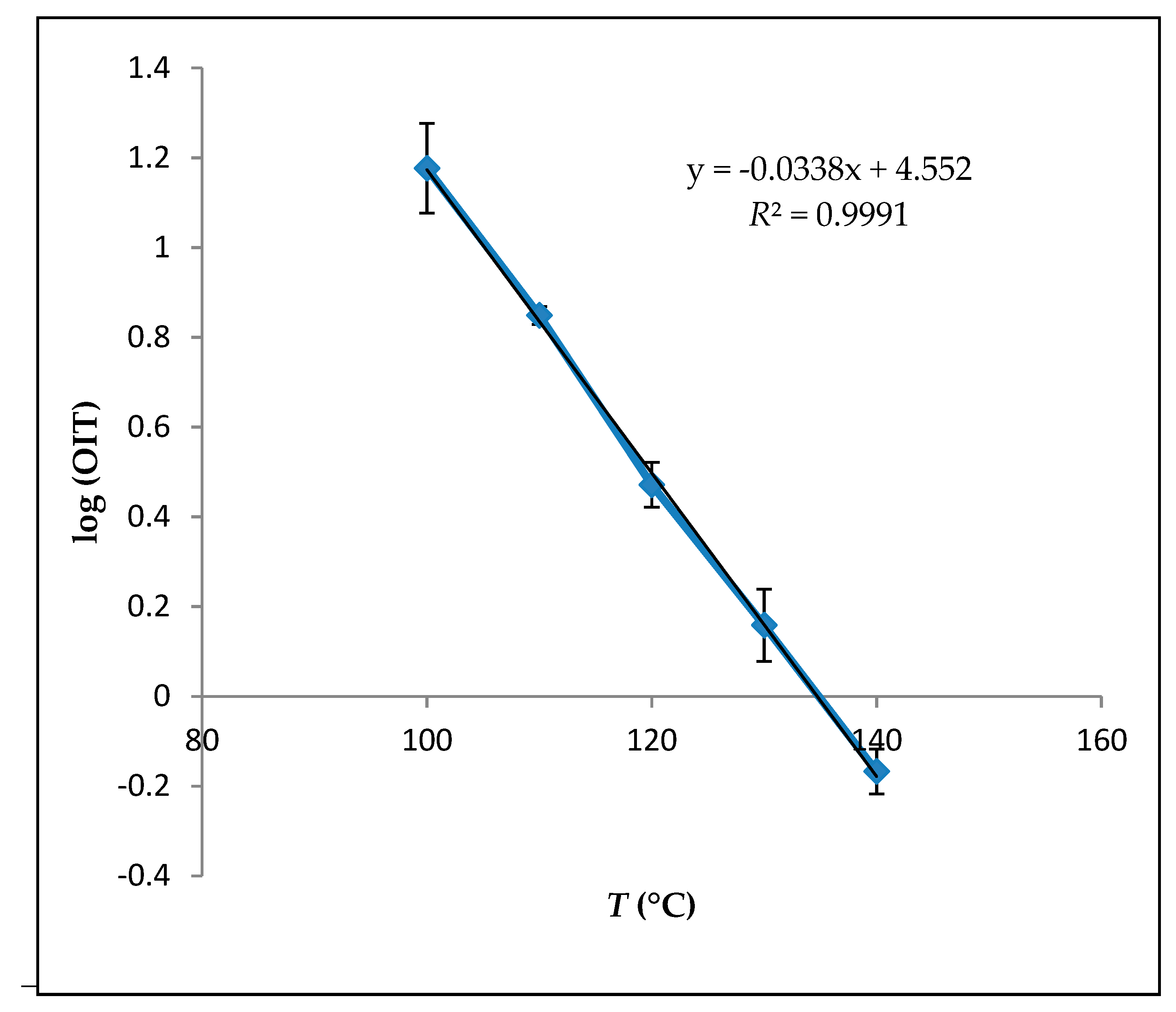
Kinetic properties calculations were based on the measurements done by the Rancimat method to investigate the expected shelf life of avocado oil. A linear relationship between the natural logarithm of the OIT and the temperature was used to calculate the predicted shelf life of avocado oil at 25 °C, as shown in
Figure 2.
It can be observed that avocado oil obeys the Arrhenius relationship over the temperature range of 100 °C to 140 °C (
R2 > 0.99). The data calculated from this relationship are shown in
Table 3. According to those calculations, the temperature coefficient of avocado oil was −3.4 × 10
−2 °C
−1 (logOIT = a(
T) + b). Kinetic behavior of the avocado oil has not been studied previously according to our literature knowledge. Therefore, the kinetic behavior of the avocado oil is still missing, and this study will trigger the further research ideas of the avocado oil. Previously other oil sources, such as soybean oil, were tested, and the temperature coefficient was found as −3.12 × 10
−2 °C
−1 [
27]. Another study on the vegetable oil temperature coefficient was calculated as −2.78 × 10
−2 and −3.15 × 10
−2 °C
−1 (mean value −3.01 × 10
−2 °C
−1) [
28]. Even though those findings are not necessarily comparable with the avocado oil, they seem to be fitting with our findings and calculations as a general profile.
Noteworthy, rancimat tests of the ambient storage were declared to lead either over prediction or under prediction of the actual shelf life depending on the type of oil [
27]. Additionally, the lipid oxidation at low and high temperatures may go through different reaction pathways, depending on the reactivity of metal ions and antioxidants at different temperatures [
19]. Therefore, the rancimat method can be claimed as a remarkable kind of a test as a fast shelf life prediction, but it would be suitable to compare fast and regular shelf life tests in future researches.
According to our calculations, avocado oil has 210 days of shelf life according to the model. The usual shelf life of the vegetable oils was presented to be between 12 and 15 months at 25 °C when good manufacturing and good storing conditions are followed [
26]. Additionally, it is also necessary to highlight that once the oxygen interaction initiates, shelf life starts to decrease. However, the addition of the alternative antioxidants, minor protective/antioxidative components may increase the shelf life, which is still in need of further investigation to obtain the best component and best practice.
Results of the physical and physicochemical properties, specifically density, refractive index, and free fatty acid measurements are shown in
Table 4. The density of the avocado oil was 0.91 g/mL (±0.0001). Meanwhile, the refractive index value was 1.4680 (±0.0002). For the density and refractive index values, literature has reported similar results [
6,
29]. Those properties are similar to those of olive oil. In the literature, virgin olive oil was presented to have 0.8639 g/mL density while the refractive index was between 1.480 and 1.465 according to varying wavelength values of experiments [
30].
The free fatty acid value was found to be 1.065% (±0.040) oleic acid. An earlier study illustrated that free fatty acid value of the virgin olive oil and extra virgin olive oil was measured as 0.26% and 0.36%, respectively [
31]. It can be seen that the acid value of the avocado oil is higher than the olive oil. Those values imply that avocado oil has similar characteristic properties to olive oil, which has also been supported by previous researches [
11,
32,
33]. Noteworthy, oil processing technique is critical and varies the free fatty acid values [
12]. Therefore, the effect of different oil pressing techniques on the free fatty acid value can be a further research question.
Results of fatty acid compositions of avocado oil were illustrated in
Table 5. The profile shows that obtained fatty acids are mostly unsaturated except for the palmitic acid. Specifically, avocado oil contains (in descending order) oleic acid (18:1 ω-9), palmitic acid (16:0), linoleic acid (18:3 ω-3), and palmitoleic acid (16:1). The high content of unsaturated fatty acid gives a unique property to avocado oil with delivering functionality to the product. Obtained fatty acid profile agrees with previous reports [
34,
35,
36,
37]. However, the differences in the cultivars of the fruit might change the concentration of the fatty acids presented, but it is likely to have a similar order of the fatty acid concentration [
38].
Due to the potential impact on human health and critical importance for the daily diet, the total phenolic content of the avocado oil was measured. The daily intake of the total phenolic content was estimated to be sourced by the most common 34 fruits and vegetables where avocado was one of them [
39]. Therefore, in this perspective, avocado is one of our phenolic source where it worth doing examination about its total phenolic content and the methods to increase the usage of it. In our study, the total phenolic content was found as 25.73 (±2.1) mg GAE per g of oil (
Table 6). The previous study done by [
39] presented that avocado fruit contains 33.62 mg GAE per g of oil. With a similar approach [
40] different cultivars of avocados found that they between 6 and 49 mg GAE per g of oil. The total phenolic content can get affected by the maturity state of the avocado, and the more mature fruit was found to have higher phenolic content [
41]. On the other hand, olive oil has shown similar results to those of avocado oil, and this finding supports the idea of avocado oil being an alternative oil [
42,
43].
Total antioxidant capacity varies from one kind of fruit/seed to another. Additionally, the total antioxidant capacity is easily affected by the processing methods as well as the different parts of the fruits [
43]. It should be highlighted that for functional oil (which is likely to contain antioxidants) resistance to oxidation stress is critical. There are several methods for measuring the total antioxidant capacity, and these methods generate different radical/target groups [
44,
45]. In this study, the DPPH method was used to evaluate the antioxidant capacity, which is also a very common method for the assessment of antioxidants [
46,
47]. According to the analysis done, the antioxidant capacity of avocado oil was found to be 32.4 mg/mL (±1.3) as IC
50 value, required to lower the initial DPPH concentration by 50% (
Table 6). A previous research illustrated the antioxidant capacities of the extra virgin olive oil, olive oil, corn oil, sunflower oil, and soybean oil as 15 mg/mL, 22 mg/mL, 52 mg/mL, 48 mg/mL, 45 mg/mL, respectively [
47]. The results showed that the radical scavenging capacity of avocado oil is higher than those of corn, sunflower, and soybean oil.
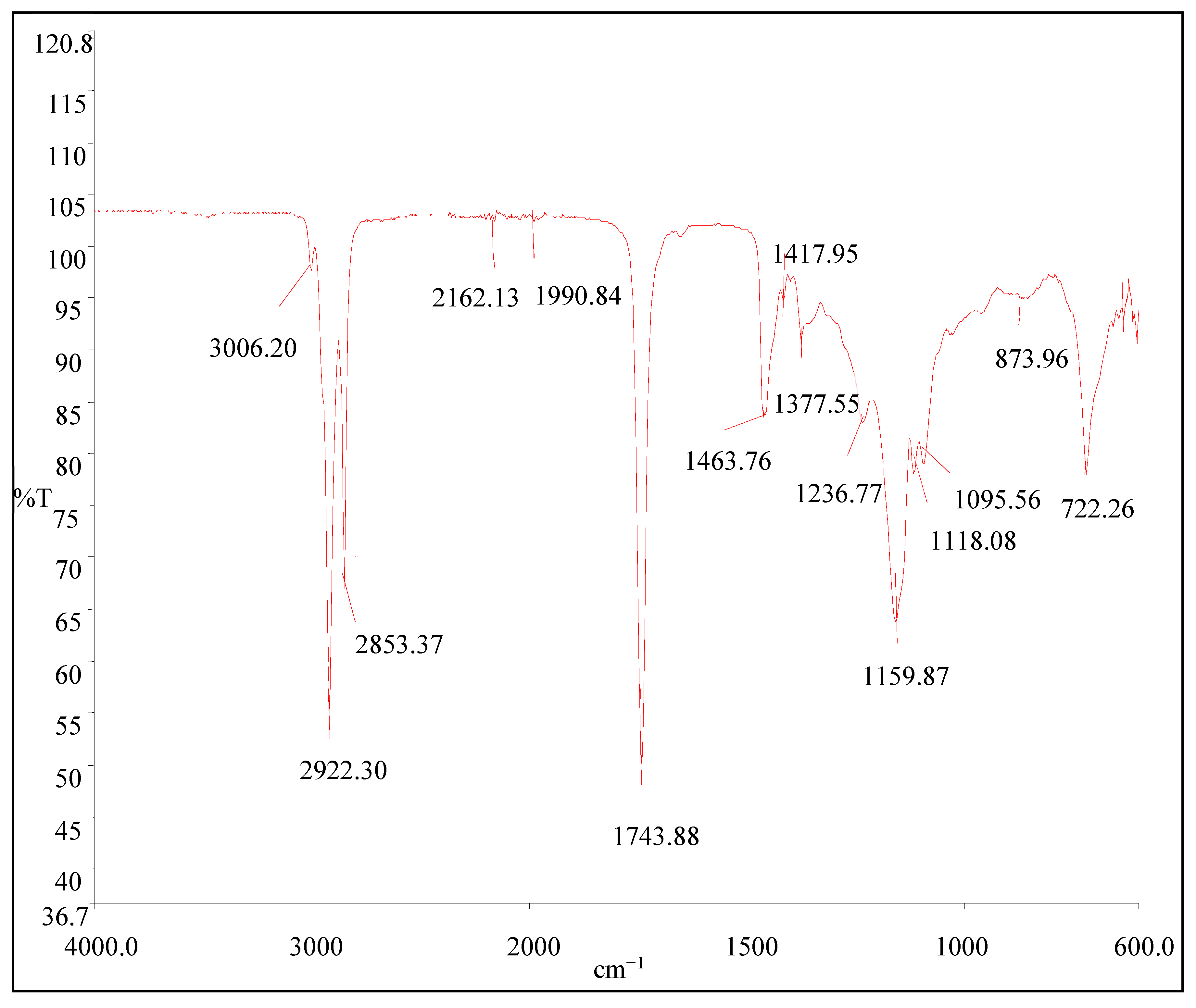
FTIR results are shown in
Figure 3. The spectral regions were chosen for developing the regressions with including the fingerprint regions were selected according to the previous researcher's observations [
48]. The spectra in the FTIR region have well-resolved bands that can be assigned to presented functional groups of the avocado oil. The spectra are dominated by some peaks at 3006, 2922, 2853, 2162, 1991, 1744, 1464, 1418, 1378, 1237, 1160, 1118, 1096, 874, and 722 cm
−1. According to that, the absorbance results for the region of 3006 to 2853 cm
−1 are due to the bands of CH
2 stretching vibrations, asymmetric and symmetric, respectively. The high value of the frequency of this band indicates its richness in polyunsaturated acyl groups. According to the literature, only linseed oil shows a band frequency as high as this; for example, olive oil shows values near 3005.4, rapeseed oil near 3007.5, and corn oil near 3008.8 cm
−1 [
49]. So, the frequency value of these bands in avocado oil is similar to those of olive oil. The major peak at 1744 cm
−1 arises from C=O stretching vibrations of aldehydes and ketones where the peaks at 1464, 1418, and 1378 cm
−1 arise from CH
2 and CH
3 scissoring vibration of ethers. Meanwhile, 1237, 1160, 1118, and 1096 cm
−1 peaks are associated with C–O stretching vibration. The last peak at 722 cm
−1 associates with the CH
2 rocking mode. Those observations are supported by the results of the other researchers performed with oils [
48,
50].