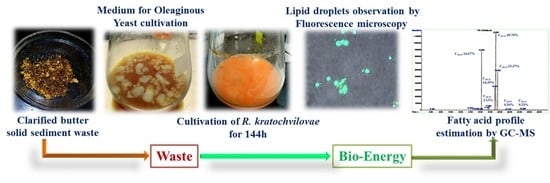
Utilization of Clarified Butter Sediment Waste as a Feedstock for Cost-Effective Production of Biodiesel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Yeast Strain and Preparation of Seed Culture
2.3. Preparation of CBM and Yeast Cultivation
2.4. Determination of Cell Dry Weight and Lipid Content
2.5. Determination of TAG Synthesis in R. kratochvilovae HIMPA1 Cells
2.6. Estimation of the Fatty Acid Profile
2.7. Theoretical Analysis of the Biodiesel’s Properties
- Saponification value (SV; mg KOH) = ∑ 560 (% FC)/M
- Iodine value (IV; gI2/100 g) = ∑ 254 DB × % FC/M
- Cetane number (CN) = 46.3 + 5.458/SV − (0.255 × IV)
- Degree of unsaturation (DU; % weight) = MUFA + (2 × PUFA)
- Long chain saturation factor (LCSF; % wt.) = (0.1 × C16) + (0.5 × C18) + (1 × C20) + (1.5 × C22) + (2 × C24)
- Cold filter plugging point ((CFPP); °C) = (3.417 × LCSF) − 16.477
- Higher heating value (HHV; MJ/Kg) = 49.43 − 0.041 (SV) − 0.015 (IV)
- Kinematic viscosity (ln KV at 40 °C in mm2/s) = −12.503 + 2.496 × ln (∑M) − 0.178 × ∑DB
- Density = 0.8463 + 4.9/∑M + 0.0118 × ∑DB
- Oxidative stability (OS; h) = 117.9295/(wt. % C18:2 + wt. % C18:3) + 2.5905
2.8. Statistical Analysis
3. Results and Discussion
3.1. Batch Cultivation of the Oleaginous Yeast in CBM
3.2. Detection of TAG by Fluorescence Microscopy in R. kratochvilovae Cultivated in CBM
3.3. Fatty Acid Profile of R. kratochvilovae Cultivated in CBM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amorim, W.S.; Valduga, I.B.; Ribeiro, J.M.P.; Williamson, V.G.; Krauser, G.E.; Magtoto, M.K.; de Andrade Guerra, J.B.S.O. The nexus between water, energy, and food in the context of the global risks: An analysis of the interactions between food, water, and energy security. Environ. Impact Assess. Rev. 2018, 72, 1–11. [Google Scholar] [CrossRef]
- Lin, L.; Gettelman, A.; Fu, Q.; Xu, Y. Simulated differences in 21st century aridity due to different scenarios of greenhouse gases and aerosols. Clim. Chang. 2018, 146, 407–422. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Jenniches, S. Assessing the regional economic impacts of renewable energy sources—A literature review. Renew. Sustain. Energy Rev. 2018, 93, 35–51. [Google Scholar] [CrossRef]
- Saidur, R.; Boroumandjazi, G.; Mekhilef, S.; Mohammed, H.A. A review on exergy analysis of biomass based fuels. Renew. Sustain. Energy Rev. 2012, 16, 1217–1222. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Fernández Martínez, R.; Lorza, R.L.; Gómez, F.S.; González, E.P.V. Optimizing biodiesel production fromwaste cooking oil using genetic algorithm-based support vector machines. Energies 2018, 11, 2995. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; Lorza, R.L.; Escribano-Garcia, R.; Gómez, F.S.; González, E.P.V. An improvement in biodiesel production from waste cooking oil by applying thought multi-response surface methodology using desirability functions. Energies 2017, 10, 130. [Google Scholar] [CrossRef]
- Su, C.H.; Nguyen, H.C.; Pham, U.K.; Nguyen, M.L.; Juan, H.Y. Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil. Energies 2018, 11, 2562. [Google Scholar] [CrossRef]
- Daraei, M.; Thorin, E.; Avelin, A.; Dotzauer, E. Potential biofuel production in a fossil fuel free transportation system: A scenario for the County of Västmanland in Sweden. Energy Procedia 2019, 158, 1330–1336. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Ban, J.; Arellano, J.L.; Alawami, A.; Aguilera, R.F.; Tallett, M. 2016 World Oil Outlook, 10th ed.; Griffin, J., Fantini, A.-M., Eds.; OPEC’s Economic Commission Board: Vienna, Austria, 2016; ISBN 978-3-9503936-2-0. [Google Scholar]
- Ragauskas, A.J. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Aggelis, G. Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour. Technol. 2002, 82, 43–49. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef]
- Xue, F.; Miao, J.; Zhang, X.; Luo, H.; Tan, T. Studies on lipid production by Rhodotorula glutinis fermentation using monosodium glutamate wastewater as culture medium. Bioresour. Technol. 2008, 99, 5923–5927. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pruthi, V.; Pruthi, P.A. Synchronized nutrient stress conditions trigger the diversion of CDP-DG pathway of phospholipids synthesis towards de novo TAG synthesis in oleaginous yeast escalating biodiesel production. Energy 2017, 139, 962–974. [Google Scholar] [CrossRef] [Green Version]
- Beopoulos, A.; Chardot, T.; Nicaud, J.-M.M. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef] [Green Version]
- Thevenieau, F.; Beopoulos, A.; Desfougeres, T.; Sabirova, J.; Albertin, K.; Zinjarde, S.; Nicaud, J. Uptake and Assimilation of Hydrophobic Substrates by the Oleaginous Yeast Yarrowia lipolytica. Handb. Hydrocarb. Lipid Microbiol. 2010, 1, 1513–1661. [Google Scholar] [CrossRef]
- Käppeli, O.; Walther, P.; Mueller, M.; Fiechter, A. Structure of the cell surface of the yeast Candida tropicalis and its relation to hydrocarbon transport. Arch. Microbiol. 1984, 138, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Carsanba, E.; Papanikolaou, S.; Erten, H. Production of oils and fats by oleaginous microorganisms with an emphasis given to the potential of the nonconventional yeast Yarrowia lipolytica. Crit. Rev. Biotechnol. 2018, 38, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Aggelis, G.; Papadiotis, G.; Komaitis, M. Microbial fatty acid specificity. Folia Microbiol. (Praha). 1997, 42, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Modeling lipid accumulation and degradation in Yarrowia lipolytica cultivated on industrial fats. Curr. Microbiol. 2003, 46, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, M.C.; Carman, G.M. Isolation of a Bioemulsifier from Candida-Lipolytica. Appl. Environ. Microbiol. 1984, 48, 747–750. [Google Scholar] [PubMed]
- Martínez, E.J.; Raghavan, V.; González-Andrés, F.; Gómez, X. New biofuel alternatives: Integrating waste management and single cell oil production. Int. J. Mol. Sci. 2015, 16, 9385–9405. [Google Scholar] [CrossRef] [PubMed]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Kumar, M.; Pandya, H.B.; Dodiya, K.K.; Bhatt, R. Advancement in industrial method of ghee making process at Sarvottam Dairy, Bhavnagar, Gujarat (India). Int. J. Sci. Environ. 2017, 6, 1727–1736. [Google Scholar]
- Khanam, R.; Prasuna, R.G. Comparison of extraction methods and solvents for total phenolics from dairy waste. Asian J. Dairy Food Res. 2017, 36, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Sahasrabudhe, J.; Palshikar, S.; Goja, A.; Kulkarni, C. Use of Ghee Residue as a Substrate for Microbial Lipase Production. Int. J. Sci. Technol. Res. 2012, 1, 61–64. [Google Scholar]
- Khanam, R.; Prasuna, R.G.; Akbar, S. Exploitation of Industrial Waste Ghee Residue in Laccases Production by Pycnoporus cinnabarinus strain SYBC-L14. Int. J. Plant Anim. Environ. Sci. 2012, 2, 1–10. [Google Scholar]
- Chkraboi, B.K. Industrial Ghee Production TrendS and Innovation. Indian Dairym. 1980, 32, 737–742. [Google Scholar]
- Khanam, R.; Prasuna, R.G.; Akbar, S. Evaluation of Total Phenolic Content in Ghee Residue: Contribution to Higher Laccase Production. Microbiol. J. 2013, 3, 12–20. [Google Scholar] [CrossRef]
- Poopathi, S.; Abibidha, S. The use of clarified butter sediment waste from dairy industries for the production of mosquitocidal bacteria. Int. J. Dairy Technol. 2012, 65, 152–157. [Google Scholar] [CrossRef]
- Patel, A.; Pravez, M.; Deeba, F.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Boosting accumulation of neutral lipids in Rhodosporidium kratochvilovae HIMPA1 grown on hemp (Cannabis sativa Linn) seed aqueous extract as feedstock for biodiesel production. Bioresour. Technol. 2014, 165, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Synergistic effect of fermentable and non-fermentable carbon sources enhances TAG accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 188, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer: A User-Friendly Software for Predicting the Properties of Prospective Biodiesel. Biofuel Res. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.-M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef]
- Thevenieau, F.; Le Dall, M.T.; Nthangeni, B.; Mauersberger, S.; Marchal, R.; Nicaud, J.M. Characterization of Yarrowia lipolytica mutants affected in hydrophobic substrate utilization. Fungal Genet. Biol. 2007, 44, 531–542. [Google Scholar] [CrossRef]
- Garti, N.; Yaghmur, A.; Leser, M.E.; Clement, V.; Watzke, H.J. Improved oil solubilization in oil/water food grade microemulsions in the presence of polyols and ethanol. J. Agric. Food Chem. 2001, 49, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Luo, M.T.; Chen, X.F.; Qi, G.X.; Xiong, L.; Lin, X.Q.; Wang, C.; Li, H.L.; Chen, X. De Combined ‘de novo’ and ‘ex novo’ lipid fermentation in a mix-medium of corncob acid hydrolysate and soybean oil by Trichosporon dermatis. Biotechnol. Biofuels 2017, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L. A comparative study on de novo and ex novo lipid fermentation by oleaginous yeast using glucose and sonicated waste cooking oil. Ultrason. Sonochem. 2019, 52, 364–374. [Google Scholar] [CrossRef]
- Mlícková, K.; Roux, E.; Athenstaedt, K.; D’Andrea, S.; Daum, G.; Chardot, T.; Nicaud, J. Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2004, 70, 3918–3924. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological conversion of waste cooking olive oil into lipid-rich biomass using Aspergillus and Penicillium strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Tachapattaweawrakul, T.; Bourtoom, T.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Aggelis, G.; Komaitis, M.; Papanikolaou, S.; Papadopoulos, G.; Papadopoulos, G. A mathematical model for the study of lipid accumulation in oleaginous microorganisms. I. Lipid accumulation during growth of Mucor circinelloides CBS 172-27 on a vegetable oil. Grasas y Aceites 1995, 46, 169–1873. [Google Scholar] [CrossRef]
- Boyle, N.R.; Morgan, J.A. Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC Syst. Biol. 2009, 3, 1–14. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Shen, H.; Wang, Q.; Zhao, Z.K. Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour. Technol. 2011, 102, 1803–1807. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Jin, G.; Zhao, X.; Zhao, Z.K. Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour. Technol. 2010, 101, 6124–6129. [Google Scholar] [CrossRef]
- Jiru, T.M.; Steyn, L.; Pohl, C.; Abate, D. Production of single cell oil from cane molasses by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 as a biodiesel feedstock. Chem. Cent. J. 2018, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [Google Scholar] [PubMed]
- Papanikolaou, S. Oleaginous Yeasts: Biochemical Events Related with Lipid Synthesis and Potential Biotechnological Applications. Ferment. Technol. 2012, 1, e103. [Google Scholar] [CrossRef]
- Guo, Y.; Cordes, K.R.; Farese, R.V.; Walther, T.C. Lipid droplets at a glance. J. Cell Sci. 2009, 122, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Kit, Y.Y.; Nicaud, J.M.; Le Dall, M.T.; Sears, S.K.; Vali, H.; Chan, H.; Rachubinski, R.A.; Titorenko, V.I. Peroxisome division in the yeast Yarrowia lipolytica is regulated by a signal from inside the peroxisome. J. Cell Biol. 2003, 162, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.W.; Hu, C.Y.; Liang, W.S. A cost efficient way to obtain lipid accumulation in the oleaginous yeast Rhodotorula glutinis using supplemental waste cooking oils (WCO). J. Taiwan Inst. Chem. Eng. 2019, 97, 80–87. [Google Scholar] [CrossRef]
- Papanikolaou, I.; Chevalot, M.; Komai, S. Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl. Microbiol. Biotechnol. 2002, 58, 308–312. [Google Scholar] [CrossRef]
- Bati, N.; Hammond, E.G.; Glatz, B.A. Biomodification of fats and oils: Trials with Candida lipolytica. J. Am. Oil Chem. Soc. 1984, 61, 1743–1746. [Google Scholar] [CrossRef]
- Katre, G.; Ajmera, N.; Zinjarde, S.; RaviKumar, A. Mutants of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil as a biofactory for biodiesel production. Microb. Cell Fact. 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Parton, R.G. Lipid droplets: A unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Ploegh, H.L. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature 2007, 448, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Kohlwein, S.D.; Veenhuis, M.; van der Klei, I.J. Lipid droplets and peroxisomes: Key players in cellular lipid homeostasis or a matter of fat—Store ’em up or burn ’em down. Genetics 2013, 193, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A. Biological treatment of pulp and paper industry effluent by oleaginous yeast integrated with production of biodiesel as sustainable transportation fuel. J. Clean. Prod. 2017, 142, 2858–2864. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A review of the effect of the composition of biodiesel on NOx emission, oxidative stability and cold flow properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Energy Rev. 2016, 56, 1129–1138. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.D.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Oliveira, L.E.; Da Silva, M.L.C.P. Relationship between cetane number and calorific value of biodiesel from Tilapia visceral oil blends with mineral diesel. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’13), Bilbao, Spain, 20–23 March 2013. [Google Scholar]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Suh, H.K.; Lee, C.S. A review on atomization and exhaust emissions of a biodiesel-fueled compression ignition engine. Renew. Sustain. Energy Rev. 2016, 58, 1601–1620. [Google Scholar] [CrossRef]






| Oleaginous Yeast | Hydrophobic Substrate | Cell Dry Weight (g/L) | Lipid Concentration (g/L) | Lipid Content (% w/w) | References |
|---|---|---|---|---|---|
| Trichosporon dermatis | Soybean oil (20 g/L) | 26.7 | 11.6 | 43.4 | [43] |
| Cryptococcus. curvatus | Sonicated waste cooking oil (20 g/L) | 18.62 | 13.06 | 70.13 | [44] |
| Rhodotorula glutinis | Waste cooking oils + crude glycerol | 25.5 | 46 ± 5% | [59] | |
| Yarrowia lipolytica | Industrial saturated fats (stearin) | 12.5 | 6.8 | 54 | [60] |
| Candida lipolytica 1094 | Corn oil (18 g/L) | 10.1 | 55 | [61] | |
| Yarrowia lipolytica mutant strains (YlB6, YlC7, and YlE1) | Waste cooking oil (100 g/L) | 10.86, 7.1, and 5.84 | 5.97, 4.28, and 3.91 | 55, 60, and 67 | [62] |
| Rhodosporidium kratochvilovae HIMPA1 | CBM | 15.52 | 10.98 | 70.74 | This Study |
| Biodiesel Properties | CBM | GSM [18] | Standard Biodiesel Parameters | |
|---|---|---|---|---|
| ASTM D6751-02 | EN 14214 | |||
| Degree of unsaturation | 83.09 | 27.61 | - | - |
| Saponification value (mg/g) | 199.78 | 160.26 | 0.50 min | 0.50 min |
| Iodine value (mgI2/100 g) | 76.97 | 23.71 | - | 120 (max) |
| Cetane number | 56.30 | 74.30 | 47 min | 51 min |
| Long chain saturated factor | 9.91 | 14.13 | - | - |
| Cold filter plugging point | 14.66 | 31.83 | - | - |
| Cloud point (°C) | 3.77 | 13.30 | - | - |
| Pour point (°C) | −2.72 | 7.625 | - | - |
| Oxidation stability (h) | 9.70 | 55.47 | 3 min | 6 min |
| Higher heating value (MJ/kg) | 40.08 | 42.50 | - | - |
| Kinematic viscosity (mm2/s) | 3.96 | 4.52 | 1.9–6.0 | 3.5–5 |
| Density (g/cm3) | 0.87 | 0.87 | - | 0.86–0.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Sartaj, K.; Pruthi, P.A.; Pruthi, V.; Matsakas, L. Utilization of Clarified Butter Sediment Waste as a Feedstock for Cost-Effective Production of Biodiesel. Foods 2019, 8, 234. https://doi.org/10.3390/foods8070234
Patel A, Sartaj K, Pruthi PA, Pruthi V, Matsakas L. Utilization of Clarified Butter Sediment Waste as a Feedstock for Cost-Effective Production of Biodiesel. Foods. 2019; 8(7):234. https://doi.org/10.3390/foods8070234
Chicago/Turabian StylePatel, Alok, Km Sartaj, Parul A. Pruthi, Vikas Pruthi, and Leonidas Matsakas. 2019. "Utilization of Clarified Butter Sediment Waste as a Feedstock for Cost-Effective Production of Biodiesel" Foods 8, no. 7: 234. https://doi.org/10.3390/foods8070234