Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain)
Abstract
:1. Introduction
2. Material and methods
2.1. Geographical Origin of Honey Samples and Honey Characterization
2.2. Melissopalynological Analysis
2.3. Physicochemical Analysis
2.3.1. pH and Electrical Conductivity
2.3.2. Determination of Total Polyphenol and Flavonoid Content
2.4. Color Measurements
2.4.1. Pfund Scale
2.4.2. CIELab Coordinates
2.5. Multivariate Analysis
3. Results and Discussion
3.1. Botanical Origin and Pollen Spectra of Samples
3.2. Physicochemical Characteristics of Samples
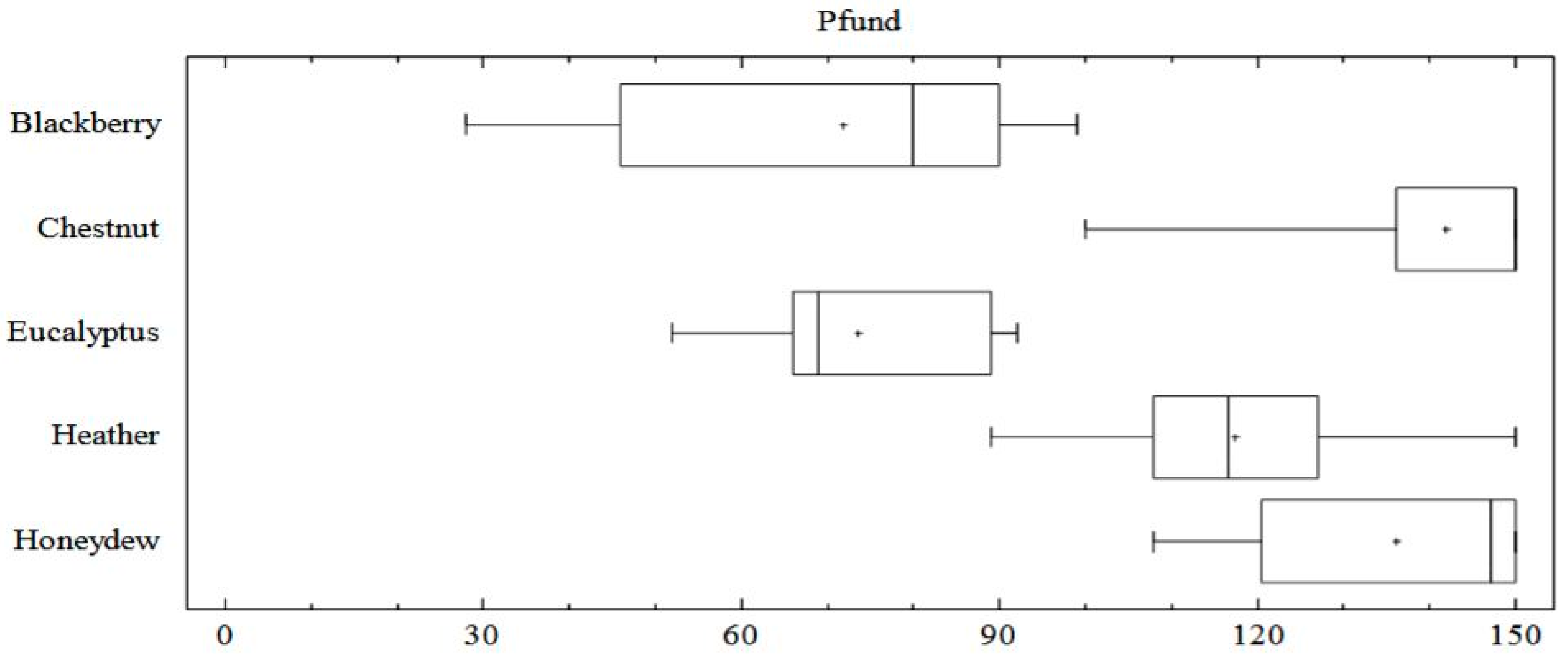
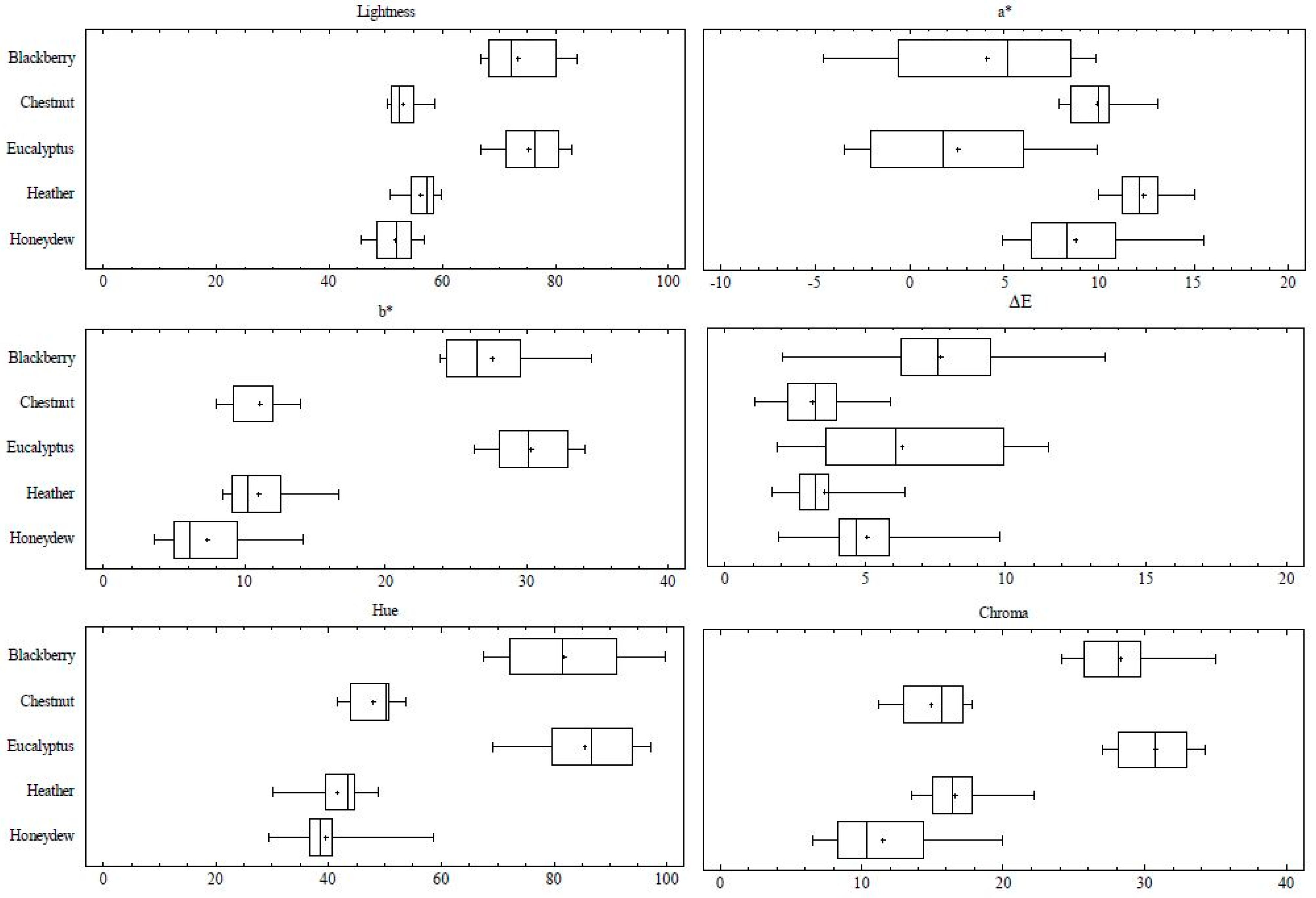
3.3. Typification of Chromaticity Coordinates and Pfund Scale by Honey Type
3.4. Influence of Botanical Origin and Physicochemical Parameters on the Color Honey Using Chemometric Techniques
3.4.1. Relationships among the Studied Variables
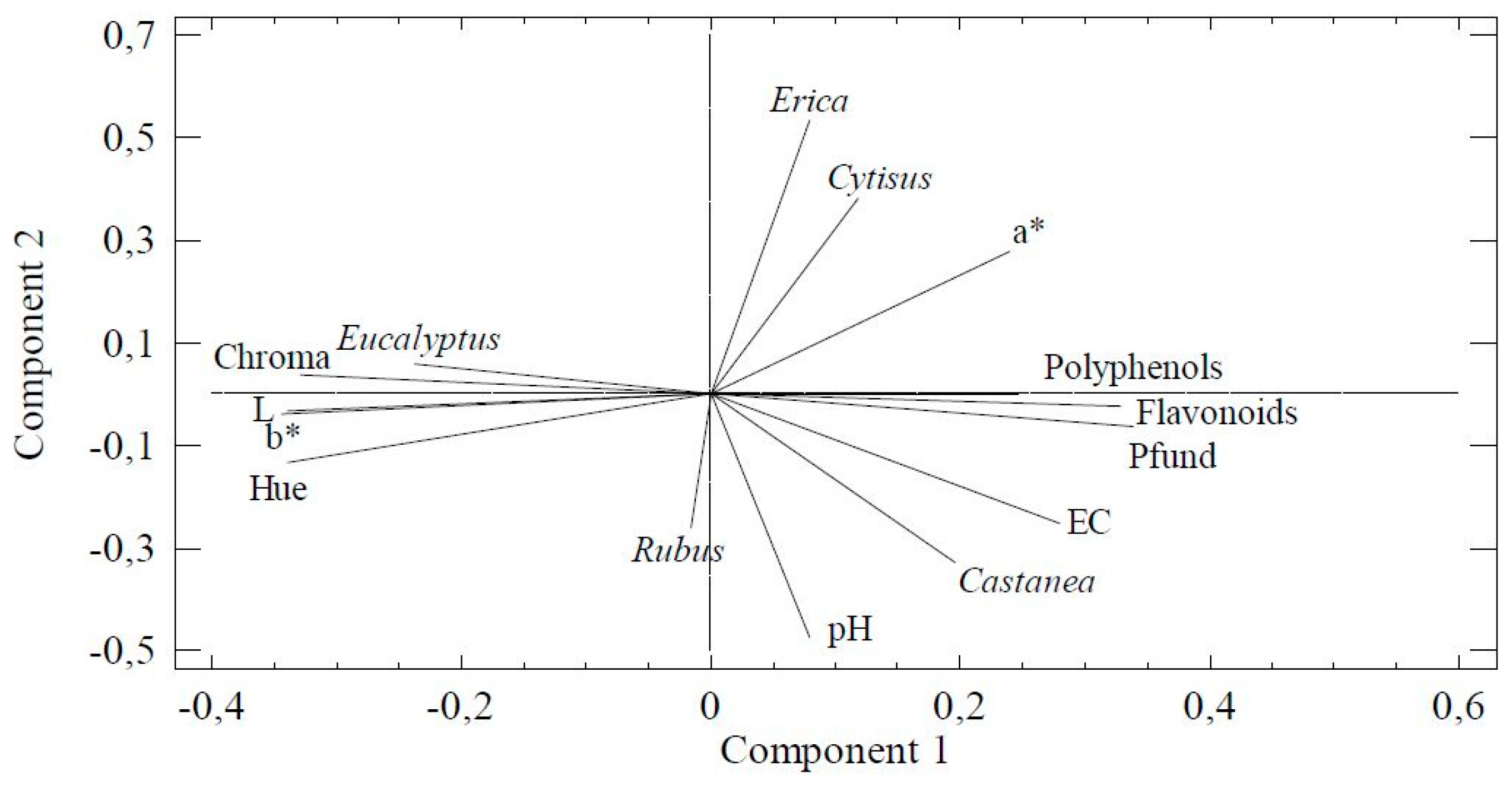
3.4.2. Principal Component Analysis
3.4.3. Multiple Regression Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Maia, M.; Karabagias, V.K.; Gatzias, I.; Badeka, A.V. Characterization of Eucalyptus, Chestnut and Heather Honeys from Portugal Using Multi-Parameter Analysis and Chemo-Calculus. Foods 2018, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Kaskoniene, V.; Venskutonis, P.R.; Ceksterytè, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. LWT-Food Sci. Technol. 2010, 43, 801–807. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bosco, L.B.; da Luz, C.F.P. Pollen analysis of Atlantic forest honey from the Vale do Ribeira Region, state of São Paulo, Brazil. Grana 2018, 57, 144–157. [Google Scholar] [CrossRef]
- Delmoro, J.; Muñoz, D.; Nadal, V.; Clementz, A.; Pranzetti, V. El color en los alimentos: Determinación de color en mieles. Invenio 2010, 25, 145–152. [Google Scholar]
- Szabó, R.T.; Mézes, M.; Szalai, T.; Zajácz, E.; Kovács-Weber, M. Colour identification of honey and methodical development of its instrumental measuring. Columella J. Agric. Environ. Sci. 2016, 3, 29–36. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerkovic, I.; Sarais, G.; Congiu, F.; Marijanovic, Z.; Kus, P.M. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L*C*hab° chromaticity coordinates. Food Chem. 2014, 145, 284–291. [Google Scholar] [CrossRef]
- Gámbaro, A.; Ares, G.; Giménez, A.; Pahor, S. Preference mapping of color of Uruguayan honeys. J. Sens. Stud. 2007, 22, 507–519. [Google Scholar] [CrossRef]
- Escuredo, O.; Fernández-González, M.; Rodríguez-Flores, M.S.; Seijo-Rodríguez, A.; Seijo, M.C. Influence of the botanical origin of honey from North western Spain in some antioxidant components. J. Apic. Sci. 2013, 57, 5–14. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, A.P.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Kolayl, S.; Karaoglu, S.; Ulusoy, E.; Baltac, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Vela, L.; De Lorenzo, C.; Pérez, R.A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007, 87, 1069–1075. [Google Scholar] [CrossRef]
- Ciappini, M.C.; Gatti, M.B.; Di Vito, M.V. El color como indicador del contenido de flavonoides en miel. Rev. Cienc. Tecnol. 2013, 19, 53–63. [Google Scholar]
- Combarros-Fuertes, P.; Valencia-Barrera, R.M.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tornadijo, M.E.; Fresno, J.M. Spanish honeys with quality brand: A multivariate approach to physicochemical parameters, microbiological quality, and floral origin. J. Apic. Res. 2019, 58, 92–103. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Sagdic, O.; Ekici, L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Revised Codex Standard for Honey Codex Stan 12–1981. Available online: http://teca.fao.org/sites/default/files/resources/Annex%20A%20Codex%20Alimentarius%20Honey%20Standard.pdf (accessed on 8 June 2019).
- Piana, M.L.; Persano-Oddo, L.; Bentabol, A.; Bruneau, E.; Bogdanov, S.; Guyot-Declerck, C. Sensory analysis applied to honey: State of the art. Apidologie 2004, 35, S26–S37. [Google Scholar] [CrossRef]
- Dominguez, M.A.; Centurión, M.E. Application of digital images to determine color in honey samples from Argentina. Microchem. J. 2015, 118, 110–114. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Dezmirean, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; et al. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35 (Suppl. 1), S38–S81. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C. Characterization of Eucalyptus globulus honeys produced in the Eurosiberian area of the Iberian Peninsula. Int. J. Food Prop. 2014, 17, 2177–2191. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C. Assessment of physicochemical and antioxidant characteristics of Quercus pyrenaica honeydew honeys. Food Chem. 2015, 166, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Gurak, P.D.; Ferreira-Marczak, L.D.; Tessaro, I.C. Tracking bioactive compounds with colour changes in foods—A review. Dyes Pigments 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Wilczyńska, A. Effect of filtration on colour, antioxidant activity and total phenolics of honey. LWT-Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Pereir, V.; Pereira, A.C.; Pinto, J.L.; Marques, J.C. Evaluation of Wine colour under accelerated and Oak-Cask ageing using CIELab and chemometric approaches. Food Bioprocess Technol. 2015, 8, 2309–2318. [Google Scholar] [CrossRef]
- Mateo-Castro, R.; Jimenez-Escamilla, M.; Bosch-Reig, F. Evaluation of the color of some unifloral honey types as a characterization parameter. J. AOAC Int. 1992, 75, 537–542. [Google Scholar]
- Terrab, A.; González-Miret, L.; Heredia, F.J. Colour characterisation of thyme and avocado honeys by diffuse reflectance spectrophotometry and spectroradiometry. Eur. Food Res. Technol. 2004, 218, 488–492. [Google Scholar] [CrossRef]
- Bettar, I.; González-Miret, M.L.; Hernanz, D.; Marconi, A.; Heredia, F.J.; Terrab, A. Characterisation of Moroccan Spurge (Euphorbia) honeys by their physicochemical characteristics, mineral contents and colour. Arab. J. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Negueruela, A.I.; Perez-Arquillue, C. Color measurement of rosemary honey in the solid state by reflectance spectroscopy with black background. J. AOAC Int. 2000, 83, 669–674. [Google Scholar]
- Oroian, M.; Leahu, A.; Damian, C.; Buculei, A. Honey classification using colour measurement. Food Environ. Saf. 2017, 11, 29–32. [Google Scholar]
- González-Miret, M.L.; Ayala, F.; Terrab, A.; Echávarri, J.F.; Negueruela, A.I.; Heredia, F.J. Simplified method for calculating colour of honey by application of the characteristic vector method. Food Res. Int. 2007, 40, 1080–1086. [Google Scholar] [CrossRef]
- Nikolova, K.; Tsankova, D.; Evtimov, T. Fluorescence spectroscopy, colorimetry and neural networks in distinguishing different types of honey. Ann. Fac. Eng. Hunedoara Int. J. Eng. 2015, 14, 317–322. [Google Scholar]
- Lekova, S.; Tsankova, D. Determination of botanical origin of honey by mid infrared spectroscopy (mid-FTIR), colorimetry and chemometric analysis. J. Chem. Technol. Metall. 2017, 52, 52–57. [Google Scholar]
- Commission Regulation (EC) No 868/2007 of 23 July 2007 Entering a Designation in the Register of Protected Designations of Origin and Protected Geographical Indications (Miel de Galicia or Mel de Galicia (PGI)). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:192:0011:0018:EN:PDF (accessed on 6 June 2019).
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardisation d’un extrait de propolis et identification des principaux constituants. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Brainard, D. Color Appearance and Color Difference Specification. Available online: https://app.dimensions.ai/details/publication/pub.1038183049 (accessed on 6 June 2019).
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Physicochemical Properties and Pollen Profile of Oak Honeydew and Evergreen Oak Honeydew Honeys from Spain: A Comparative Study. Foods 2019, 8, 126. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo-Rodríguez, A.; Seijo, M.C. Characterization of the honey produced in heather communities (NW Spain). J. Apic. Res. 2019, 58, 84–91. [Google Scholar] [CrossRef]
- Özkök, A.; D’arcy, B.; Sorkun, K. Total phenolic acid and total flavonoid content of Turkish pine honeydew honey. J. ApiProduct ApiMedical Sci. 2010, 2, 65–71. [Google Scholar] [CrossRef]
- Estevinho, L.M.; Feás, X.; Seijas, J.A.; Vázquez-Tato, M.P. Organic honey from Trás-Os-Montes region (Portugal): Chemical, palynological, microbiological and bioactive compounds characterization. Food Chem. Toxicol. 2012, 50, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Vavoura, M.V.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Differentiation of Greek thyme honeys according to geographical origin based on the combination of phenolic compounds and conventional quality parameters using chemometrics. Food Anal. Methods 2014, 7, 2113–2121. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Flores, S.; Escuredo, O.; Seijo, M.C. Characterization and antioxidant capacity of sweet chestnut honey produced in North-West Spain. J. Apic. Res. 2016, 60, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.; Karabournioti, S. Discrimination of clover and citrus honeys from Egypt according to floral type using easily assessable physicochemical parameters and discriminant analysis: An external validation of the chemometric approach. Foods 2018, 7, 70. [Google Scholar] [CrossRef]
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.Á.; Heredia, F.J. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Anupama, D.; Bhat, K.K.; Sapna, V.K. Sensory and physico-chemical properties of commercial samples of honey. Food Res. Int. 2003, 36, 183–191. [Google Scholar] [CrossRef]

| Main Pollen Type (%) | Secondary Pollen (%) | Minor Pollen (%) | Pollen Combination (%) | ||
|---|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | ||||
| Blackberry (n = 15) | Rubus | Mean ± SD (53.3 ± 8.9) Max–Min (75.1–45.3) | Castanea (33.9 ± 9.3) Erica (3.3 ± 5.4) | Cytisus type (2.2 ± 1.7) Echium (1.1 ± 1.4) | Rubus, Castanea, Erica, Cytisus (89%) |
| Chestnut (n = 17) | Castanea | Mean ± SD (77.6 ± 5.7) Max − Min (90.7–70.4) | Rubus (14.5 ± 5.2) | Erica (2.5 ± 1.9) Cytisus type (1.8 ± 1.0) | Castanea, Rubus, Erica, Cytisus (95%) |
| Eucalyptus (n = 11) | Eucalyptus | Mean ± SD (79.9 ± 10.2) Max–Min (95.7–61.3) | Castanea (7.1 ± 6.7) | Cytisus type (2.6 ± 2.1) Erica (1.7 ± 2.2) Quercus (1.4 ± 1.0) Salix (1.3 ± 2.2) | Eucalyptus, Castanea, Cytisus, Erica, Quercus (90%) |
| Heather (n = 22) | Erica | Mean ± SD (38.2 ± 12.7) Max–Min (68.6–22.7) | Castanea (27.1 ± 12.2) Rubus (13.1 ± 8.5) Cytisus type (9.6 ± 6.0) | Eucalyptus (2.9 ± 3.9) Crataegus type (1.5 ± 3.7) Quercus (1.4 ± 1.4) | Erica, Castanea, Rubus, Cytisus, Quercus (95%) |
| Honeydew (n = 28) | Castanea | Mean ± SD (44.5 ± 15.1) Max–Min (83.6–17.7) | Erica (4.3 ± 4.3) | Eucalyptus (1.2 ± 2.2) Echium (1.1 ± 2.7) Frangula alnus (1.1 ± 3.1) | Castanea, Rubus, Cytisus, Erica (100%) |
| Rubus | Mean± SD (37.2 ± 13.5) Max–Min (39.0–7.8) | ||||
| Honey Type | Blackberry | Chestnut | Eucalyptus | Heather | Honeydew | |
|---|---|---|---|---|---|---|
| (n = 15) | (n = 17) | (n = 11) | (n = 22) | (n = 28) | ||
| EC (mS/cm) | Mean ± SD | 0.624 ± 0.25 b | 1.050 ± 0.19 a | 0.568 ± 0.08 b | 0.777 ± 0.11 | 1.016 ± 0.14 a |
| Range | 0.320–1.065 | 0.737–1.235 | 0.479–0.769 | 0.501–0.922 | 0.712–1.267 | |
| pH | Mean ± SD | 4.26 ± 0.19 cde | 4.35 ± 0.20 abc | 4.22 ± 0.20 ad | 3.97 ± 0.19 | 4.46 ± 0.25 be |
| Range | 4.00–4.63 | 3.93–4.65 | 3.94–4.62 | 3.43–4.20 | 4.12–5.10 | |
| Polyphenols (mg/100 g) | Mean ± SD | 93.30 ± 39.78 cef | 114.36 ± 26.08 ade | 75.04 ± 29.33 f | 114.44 ± 18.58 abc | 129.09 ± 28.50 bd |
| Range | 39.82–140.91 | 88.43–166.45 | 48.68–146.02 | 95.75–167.16 | 83.20–186.97 | |
| Flavonoids (mg/100 g) | Mean ± SD | 4.32 ± 1.61 d | 8.55 ± 2.27 ac | 4.04 ± 0.75 d | 8.13 ± 1.27 ab | 9.48 ± 1.74 bc |
| Range | 2.00–7.23 | 6.60–11.78 | 2.73–5.20 | 5.54–10.66 | 6.62–13.12 | |
| Pfund (mm) | Mean ± SD | 72 ± 23 b | 142 ± 14 a | 73 ± 13 b | 116 ± 15 | 136 ± 16 a |
| Range | 28–99 | 123–150 | 52–92 | 89–140 | 108–150 | |
| L | Mean ± SD | 73.46 ± 6.10 c | 53.03 ± 2.28 ab | 75.41 ± 5.37 c | 56.11 ± 2.88 a | 51.62 ± 3.59 b |
| Range | 66.98–83.77 | 50.17–58.61 | 66.88–83.05 | 50.72–59.76 | 45.63–56.85 | |
| a* | Mean ± SD | 4.04 ± 5.01 c | 9.89 ± 1.43 ab | 2.55 ± 4.42 c | 10.89 ± 2.10 a | 8.75 ± 2.79 b |
| Range | −4.55–9.84 | 7.87–13.13 | −3.43–9.94 | 7.97–14.78 | 4.87–15.58 | |
| b* | Mean ± SD | 27.54 ± 3.45 b | 11.10 ± 2.15 a | 28.68 ± 4.22 b | 11.01 ± 2.21 a | 7.4 ± 2.98 |
| Range | 23.79–34.60 | 8.00–14.00 | 21.75–34.08 | 8.48–16.71 | 3.63–4.15 |
| Parameter | Pfund | L | a* | b* | Chroma | Hue |
|---|---|---|---|---|---|---|
| Pfund | −0.784 ** | 0.183 | −0.930 ** | −0.923 ** | −0.898 ** | |
| EC | 0.815 ** | −0.672 ** | 0.170 ** | −0.756 ** | −0.741 ** | −0.793 ** |
| pH | 0.334 * | −0.217 * | −0.400 ** | −0.333 ** | −0.332 ** | −0.332 ** |
| Polyphenols | 0.592 ** | −0.521 ** | 0.226 * | −0.520 ** | −0.514 ** | −0.544 ** |
| Flavonoids | 0.889 ** | −0.789 ** | 0.273 * | −0.853 ** | −0.834 ** | −0.864 ** |
| Erica | 0.247 * | −0.244 * | 0.588 ** | −0.194 | −0.204 | 0.213 * |
| Castanea | 0.478 ** | −0528 ** | 0.203 | −0.483 ** | −0.449 ** | −0.545 ** |
| Cytisus | 0.268 * | −0.256 * | 0.270 * | −0.282 ** | −0.313 ** | −0.222 * |
| Eucalyptus | −0.466 ** | 0.258 * | −0.041 | 0.458 ** | 0.435 ** | 0.460 ** |
| Rubus | 0.020 | −0.0463 | −0.098 | −0.084 | −0.080 | −0.067 |
| Model Summary (Dependent Variable = Pfund) | ||||
| R2 | R2 adjusted | Est. error | F | p |
| 0.86 | 0.86 | 12.3 | 182.9 | <0.001 |
| Coefficients | B | Est. Err. B | t | p |
| Constant | 17.03 | 4.59 | 3.71 | 0.001 |
| EC | 51.78 | 7.71 | 6.72 | <0.001 |
| Flavonoids | 6.61 | 0.74 | 8.92 | <0.001 |
| Erica | 0.22 | 0.08 | 2.58 | 0.011 |
| Pfund = 17.03 + 51.78 EC + 6.61 Flavonoids + 0.22 Erica | ||||
| Model Summary (Dependent Variable = Chroma) | ||||
| R2 | R2 adjusted | Est. error | F | p |
| 0.75 | 0.74 | 4.26 | 89.22 | <0.001 |
| Coefficients | B | Est. Err. B | t | p |
| Constant | 36.97 | 1.84 | 20.1 | <0.001 |
| EC | −6.72 | 2.49 | −2.69 | 0.008 |
| Flavonoids | −1.90 | 0.25 | −7.63 | <0.001 |
| Eucalyptus | 0.04 | 0.02 | 2.11 | 0.037 |
| Chroma = 36.97 − 6.72EC − 1.90 Flavonoids + 0.04 Eucalyptus | ||||
| Model Summary (Dependent Variable = Hue) | ||||
| R2 | R2 adjusted | Est. error | F | p |
| 0.87 | 0.86 | 8.2 | 116.8 | <0.001 |
| Coefficients | B | Est. Err. B | t | p |
| Constant | 119.20 | 3.5 | 33.55 | <0.001 |
| EC | −20.19 | 5.51 | −3.67 | <0.001 |
| Polyphenols | −0.070 | 0.03 | −2.11 | <0.001 |
| Flavonoids | −4.32 | 0.56 | −7.65 | <0.001 |
| Erica | −0.25 | 0.06 | −4.17 | <0.001 |
| Castanea | −0.17 | 0.04 | −3.64 | <0.001 |
| Hue = 119.20 − 20.19 EC − 0.07 Polyphenols − 4.32 Flavonoids − 0.25 Erica − 0.17 Castanea | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escuredo, O.; Rodríguez-Flores, M.S.; Rojo-Martínez, S.; Seijo, M.C. Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain). Foods 2019, 8, 233. https://doi.org/10.3390/foods8070233
Escuredo O, Rodríguez-Flores MS, Rojo-Martínez S, Seijo MC. Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain). Foods. 2019; 8(7):233. https://doi.org/10.3390/foods8070233
Chicago/Turabian StyleEscuredo, Olga, María Shantal Rodríguez-Flores, Sergio Rojo-Martínez, and María Carmen Seijo. 2019. "Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain)" Foods 8, no. 7: 233. https://doi.org/10.3390/foods8070233





