Effects of Drying Methods and Ash Contents on Heat-Induced Gelation of Porcine Plasma Protein Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plasma Powders
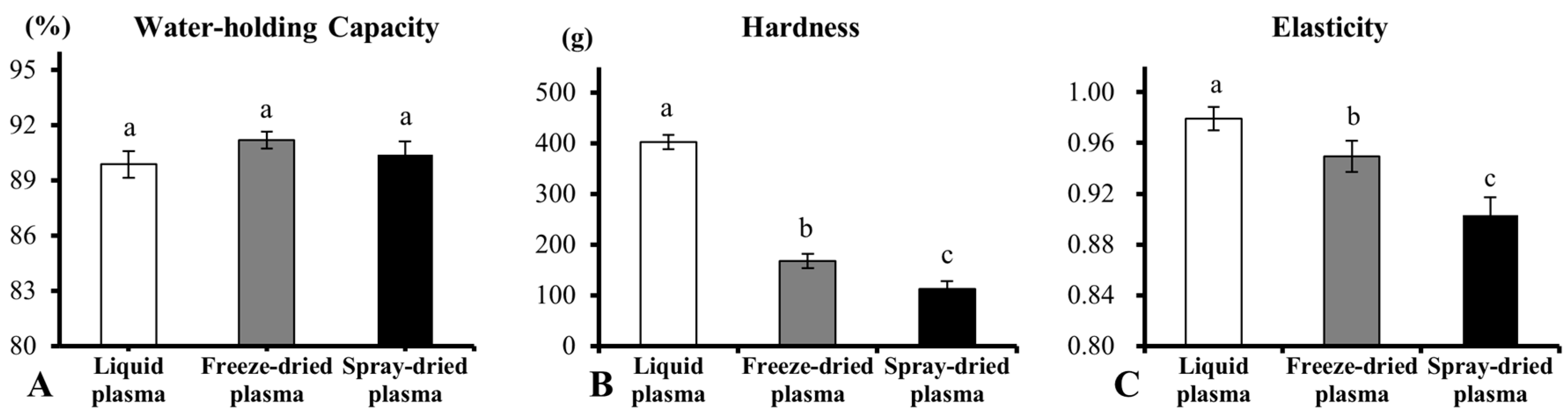
2.3. Water-Holding Capacity and Texture Analyses
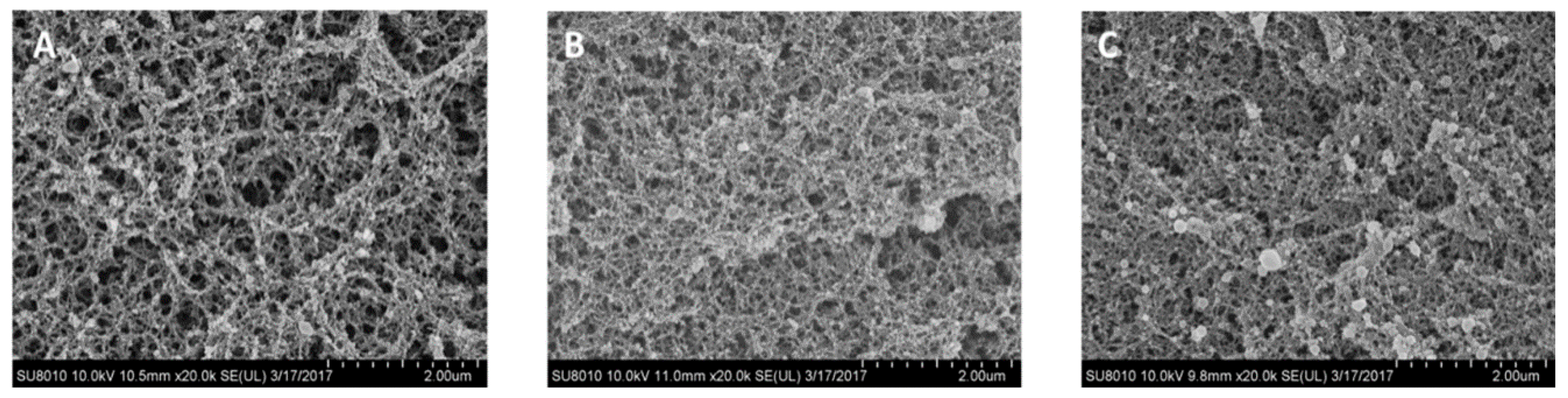
2.4. Microstructure
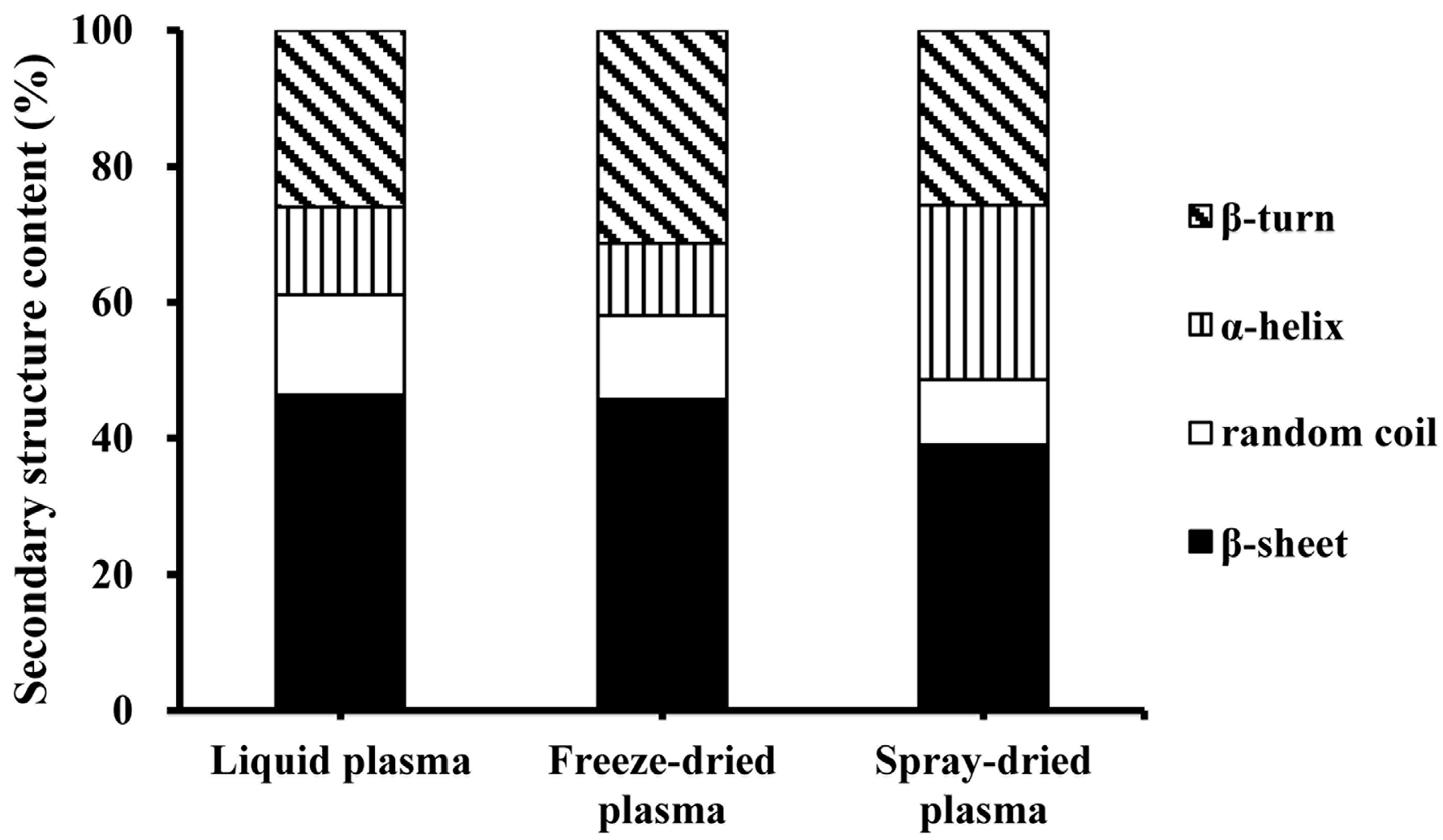
2.5. Fourier Transform Infrared Measurements
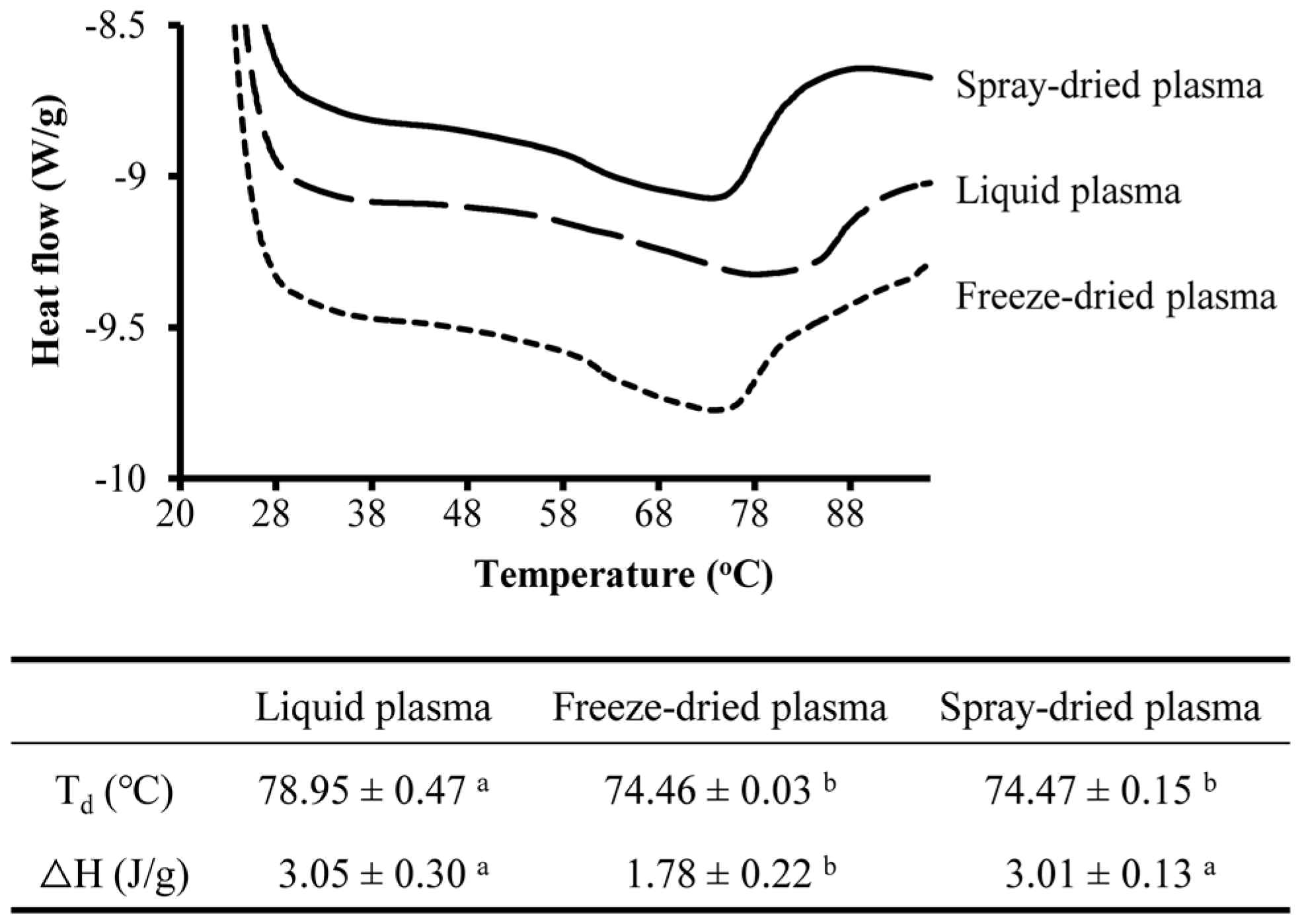
2.6. Differential Scanning Calorimetry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Drying Methods on Heat-Induced Gelation of Plasma Proteins
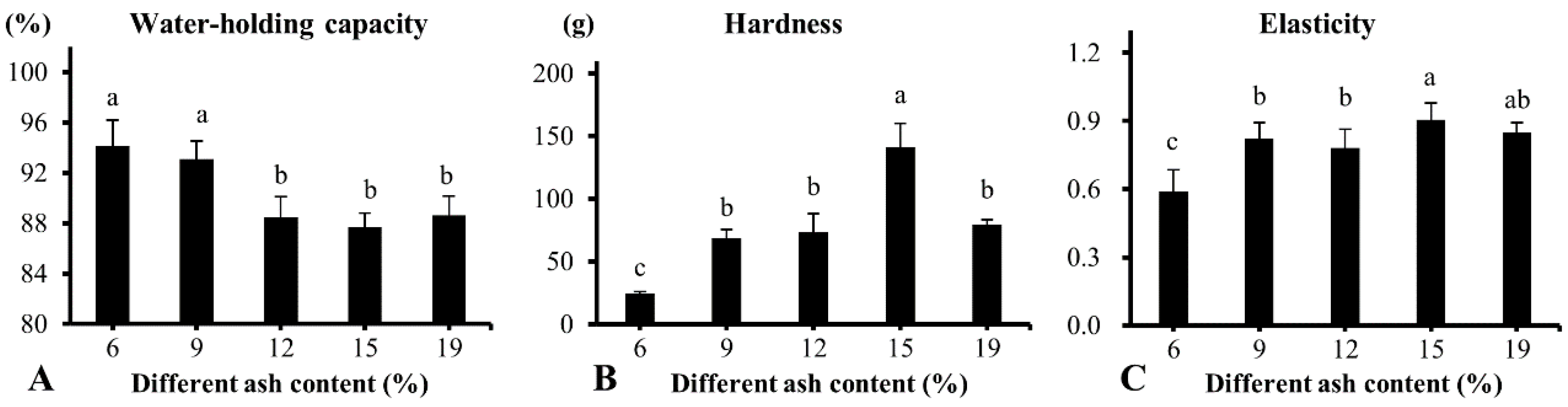
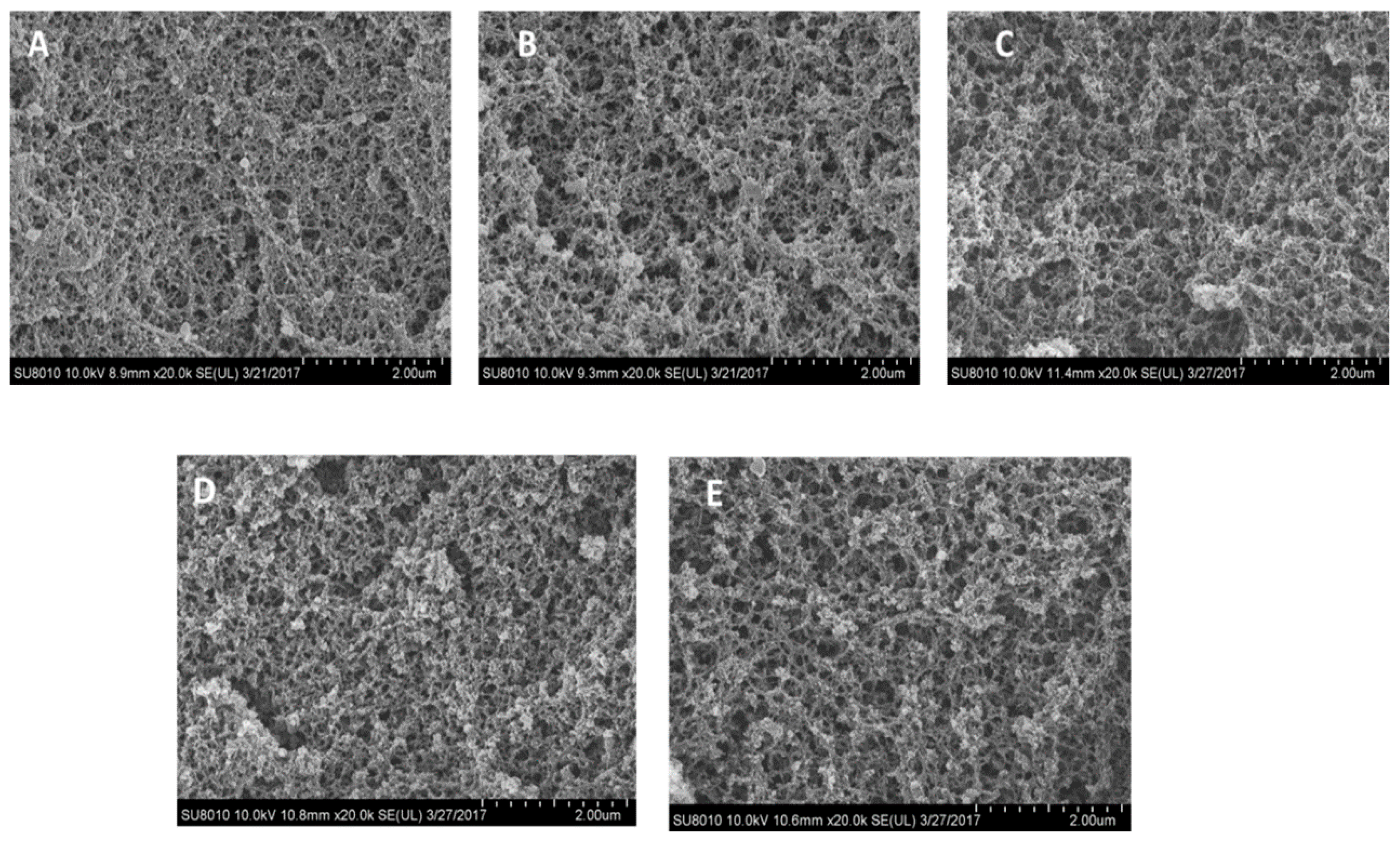
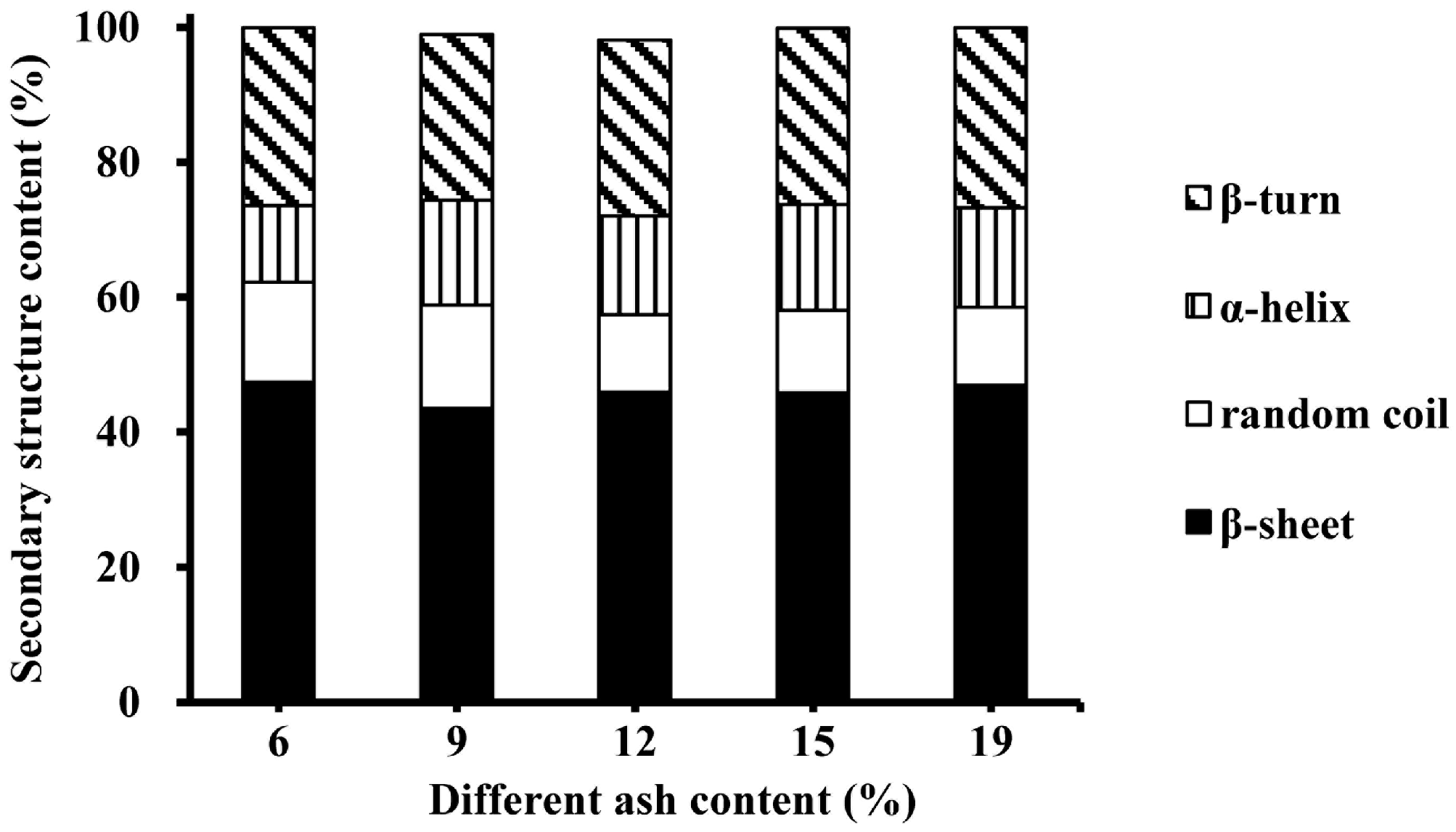
3.2. Effect of Ash Contents on Heat-Induced Gelation of Plasma Protein
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.D.; Wu, H.L.; Zhang, J.; Wan, D.J.; Zhao, J.H. Study of porcine blood decoloration technique. Adv. Mater. Res. 2012, 518–523, 3980–3983. [Google Scholar] [CrossRef]
- Mielink, J.; Slinde, E. Sausage color measured by integrating sphere reflectance spectrophotometry when whole blood or blood cured by nitrite is added to sausages. J. Food Sci. 2010, 48, 1723–1725. [Google Scholar] [CrossRef]
- Ofori, J.A.; Hsieh, Y.H.P. The Use of Blood and Derived Products as Food Additives; El-Samragy, Y., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Fowler, M.R.; Park, J.W. Effect of salmon plasma protein on Pacific whiting surimi gelation under various ohmic heating conditions. LWT Food Sci. Technol. 2015, 61, 309–315. [Google Scholar] [CrossRef]
- Romero de Ávila, M.D.; Ordóñez, J.A.; Escudero, R.; Cambero, M.I. The suitability of plasma powder for cold-set binding of pork and restructured dry ham. Meat Sci. 2014, 98, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Wang, Z.; Wang, L.; He, F.; Liu, J.; Gao, Y.; Zhang, D. Reduction of sodium chloride levels in emulsified lamb sausages: The effect of lamb plasma protein on the gel properties, sensory characteristics, and microstructure. Food Sci. Biotechnol. 2014, 23, 1137–1143. [Google Scholar] [CrossRef]
- Hurtado, S.; Saguer, E.; Toldrà, M.; Parés, D.; Carretero, C. Porcine plasma as polyphosphate and caseinate replacer in frankfurters. Meat Sci. 2012, 90, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Wang, Z.; Chen, L.; Xu, W.; Pan, H.; Gao, Y.; Zhang, D. Effect of pH on the gel properties of lamb plasma protein during heat-induced gelation. Mod. Food Sci. Technol. 2015, 31, 160–166. [Google Scholar]
- Saguer, E.; Alvarez, P.; Fort, N.; Espigulé, E.; Parés, D.; Toldrà, M.; Carretero, C. Heat-induced gelation mechanism of blood plasma modulated by cysteine. J. Food Sci. 2015, 80, 515–521. [Google Scholar] [CrossRef]
- Dàvila, E.; Parés, D.; Cuvelier, G.; Relkin, P. Heat-induced gelation of porcine blood plasma proteins as affected by pH. Meat Sci. 2007, 76, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, X.; Huang, M.; Huang, M.; Zhou, G. Effect of pH on heat-induced gelation of duck blood plasma protein. Food Hydrocoll. 2014, 35, 324–331. [Google Scholar] [CrossRef]
- Eduard, D.; Dolors, P.; Howell, N.K. Fourier transform raman spectroscopy study of heat-induced gelation of plasma proteins as influenced by pH. J. Agric. Food Chem. 2006, 54, 7890–7897. [Google Scholar]
- Huang, Q.; Ma, C.J.; Zhou, J.; Ma, M.H. Effects of drying methods and physico-chemical factors on functional properties of quail egg-white protein powder. Food Sci. 2008, 29, 299–302. [Google Scholar]
- Qiu, C.Y.; Sun, W.Z.; Cui, C.; Zhao, M.M. Effects of drying methods on characteristics of deamidated wheat gluten. J. South China Univ. Technol. 2014, 42, 129–135. [Google Scholar]
- Linarès, E.; Larré, C.; Popineau, Y. Freeze- or spray-dried gluten hydrolysates. 1. Biochemical and emulsifying properties as a function of drying process. J. Food Eng. 2001, 48, 127–135. [Google Scholar] [CrossRef]
- Parés, D.; Saguer, E.; Saurina, J.; Sunol, J.J.; Carretero, A.C. Functional properties of heat induced gels from liquid and spray-dried porcine blood plasma as influenced by pH. J. Food Sci. 1998, 63, 958–961. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Srinivasan, D. Nutritional, chemical, and physical criteria affecting the use and acceptability of proteins in foods. In Criteria of Food Acceptance; Solms, J., Hall, R.L., Eds.; Foster Verlag: Zürich, Switzerland, 1981. [Google Scholar]
- Wang, W.; Hou, C.; Song, X.; Li, Z.; Wu, L.; Boga, L.A.I.; Zhu, J.; Zhang, D. Comparative and analysis of the quality of plasma protein powder. Food Sci. Technol. Int. 2017, 42, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, J.W. Negative roles of salt in gelation properties of fish protein isolate. J. Food Sci. 2008, 73, C585–C588. [Google Scholar] [CrossRef]
- National Health and Family Planning Commission of China. Chinese Standard GB 5009.4-2016. Determination of Ash in Foods; China Standards Press of China: Beijing, China, 2016. [Google Scholar]
- Sun, J.; Wu, Z.; Xu, X.; Li, P. Effect of peanut protein isolate on functional properties of chicken salt-soluble proteins from breast and thigh muscles during heat-induced gelation. Meat Sci. 2012, 91, 88–92. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Xia, X.; Liu, Q.; Diao, X. Structural changes of the myofibrillar proteins in common carp (Cyprinus carpio) muscle exposed to a hydroxyl radical-generating system. Process Biochem. 2013, 48, 863–870. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Y.; Fei, Y.; Xu, X.; Zhou, G. Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2009, 228, 665–670. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J. Rheology and microstructure of myofibrillar protein-plant lipid composite gels: Effect of emulsion droplet size and membrane type. J. Food Eng. 2011, 106, 318–324. [Google Scholar] [CrossRef]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LWT Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Shi, A.; Liu, H.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Samsalee, N.; Sothornvit, R. Effect of natural cross-linkers and drying methods on physicochemical and thermal properties of dried porcine plasma protein. Food Biosci. 2017, 19, 26–33. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Rielly, C.D.; Stapley, A.G.F. Effects of process variables on the denaturation of whey proteins during spray drying. Dry. Technol. 2007, 25, 799–807. [Google Scholar] [CrossRef]
- Samsalee, N.; Sothornvit, R. Effects of drying methods on physicochemical and rheological properties of porcine plasma protein. Kasetsart J. Nat. Sci. 2014, 48, 629–636. [Google Scholar]
- Ismoyo, F.; Wang, Y.; Ismail, A.A. Examination of the effect of heating on the secondary structure of avidin and avidin-biotin complex by resolution-enhanced two-dimensional infrared correlation spectroscopy. Appl. Spectrosc. 2000, 54, 939–947. [Google Scholar] [CrossRef]
- Chantrapornchai, W.; McClements, D.J. Influence of NaCl on optical properties, large-strain rheology and water holding capacity of heat-induced whey protein isolate gels. Food Hydrocoll. 2002, 16, 467–476. [Google Scholar] [CrossRef]
- Shao, J.J.; Zou, Y.F.; Xu, X.L.; Zhou, G.H.; Sun, J.X. Effects of NaCl on water characteristics of heat-induced gels made from chicken breast proteins treated by isoelectric solubilization/precipitation. CyTA J. Food 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Meng, X.; Peng, Z.; Jin, H.; Wu, D.; Feng, Y.; Cui, G. Study on gel properties of bovine plasma proteins. Food Res. Dev. 2012, 33, 157–160. [Google Scholar]
- Croguennec, T.; Nau, F.; Brulé, G. Influence of pH and salts on egg white gelation. J. Food Sci. 2002, 67, 608–614. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef]
- Boye, J.I.; Ismail, A.A.; Alli, I. Effects of physicochemical factors on the secondary structure of beta-lactoglobulin. J. Dairy Res. 1996, 63, 97–109. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Wang, W.; Song, X.; Wu, L.; Zhang, D. Effects of Drying Methods and Ash Contents on Heat-Induced Gelation of Porcine Plasma Protein Powder. Foods 2019, 8, 140. https://doi.org/10.3390/foods8040140
Hou C, Wang W, Song X, Wu L, Zhang D. Effects of Drying Methods and Ash Contents on Heat-Induced Gelation of Porcine Plasma Protein Powder. Foods. 2019; 8(4):140. https://doi.org/10.3390/foods8040140
Chicago/Turabian StyleHou, Chengli, Wenting Wang, Xuan Song, Liguo Wu, and Dequan Zhang. 2019. "Effects of Drying Methods and Ash Contents on Heat-Induced Gelation of Porcine Plasma Protein Powder" Foods 8, no. 4: 140. https://doi.org/10.3390/foods8040140



