Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation
2.3. Chromatographic and Quantification Conditions
2.4. Method Validation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analytical Method Development
3.2. Validation of the Analytical Method
3.3. Analysis of Red Wine Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reiter, R.J. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1980, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Huether, G.; Poeggeler, B.; Reimer, A.; George, A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: Evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992, 51, 945–953. [Google Scholar] [CrossRef]
- Huether, G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J. Pineal melatonin—Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef]
- Chattoraj, A.; Liu, T.; Zhang, L.S.; Huang, Z.; Borjigin, J. Melatonin formation in mammals: In vivo perspectives. Rev. Endocr. Metab. Disord. 2009, 10, 37–243. [Google Scholar] [CrossRef]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Intern. 1995, 35, 627–634. [Google Scholar]
- Paredes, S.D.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Reiter, R.J. Phytomelatonin: A review. J. Exp. Bot. 2009, 60, 57–69. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural sciences. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Sturtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, H.; Calvo, J.R.; Maldonado, M.D. High levels of melatonin generated during the brewing process. J. Pineal Res. 2012, 55, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Saracino, M.A.; Bugamelli, F.; Ferranti, A.; Malaguti, M.; Hrelia, S.; Raggi, M.A. HPLC-F analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J. Sep. Sci. 2008, 31, 1007–1014. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.D. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Fico, G.; Faoro, F.; Simonetti, P.; Iriti, M. From vineyard to glass: Agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicol 2010, 278, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vitalini, S. Health-promoting effects of traditional mediterranean diets—A review. Pol. J. Food Nutr. Sci. 2012, 62, 71–76. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Gil-Izquirdo, A.; Moreno, D.A.; Martí, N.; García-Viguera, C. Melatonin is detected in pomegranate wines. Influence of variety and winemaking stage. LWT-Food Sci. Technol. 2012, 47, 13–18. [Google Scholar] [CrossRef]
- Fernandez-Pachon, M.S.; Medina, S.; Herrero-Martin, G.; Cerrillo, I.; Berna, G.; Escudero-Lopez, B.; Ferreres, F.; Martin, F.; Garcia-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Iriti, M.; Stuknyte, M.; De Noni, I.; Simonetti, P. “Melatonin isomer” in wine is not an isomer of the melatonin but tryptophan-ethylester. J. Pineal Res 2014, 57, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vigentini, I. Tryptophan-ethylester, the false (unveiled) melatonin isomer in red wine. Int. J. Tryptophan Res. 2015, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast contribution to melatonin, melatonin isomers and tryptophan ethyl ester during alcoholic fermentation of grape musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and derived L-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuff: Measurement using MEPS-HPLC-F method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef]
- El Moussaoui, N.; Bendriss, A. Analysis of melatonin by high performance liquid chromatography after Solid-Phase Extraction (SPE/HPLC-FD). Int. J. Eng. Res. Technol. 2015, 4, 988–993. [Google Scholar]
- Muñiz-Calvo, S.; Guillamón, J.M.; Domínguez, I.; Doménech-Carbó, A. Detecting and monitoring the production of melatonin and other related indole compounds in different Saccharomyces strain by solid-state electrochemical techniques. Food Anal. Methods 2017, 10, 1408–1418. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud-Funel, A. The microbiology of wine and vinifications. In Handbook of Enology; Ribéreau-Gayon, P., Ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 14 March 2018).
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antioxidant capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Giridhar, O.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- 2002/657/EC: Commission Decision. Official Journal of the European Communities L221, 17.8.2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2002:221:FULL&from=HR (accessed on 14 March 2019).
- Amerin, M.A.; Ough, C.S. Alcohols, in Methods for Wine and Must Analysis; Amerine, M.A., Ough, C.S., Eds.; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.J.V.; Raba, J.; Cerutti, S.; Silva, M.F. Monitoring melatonin and its isomer in Vitis vinifera cv. Malbec by UHPLC-MS/MS from grape to bottle. J. Pineal Res. 2012, 52, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kocadağli, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Vacondio, F.; Silva, C.; Rivara, M.; Mor, M.; Plazzi, P.V.; Zusso, M.; et al. Synthesis, antioxidant activity and structure-activity relationships for a new series of 2-(acylaminoethyl) indoles with melatonin-like cytoprotective activity. J. Pineal Res. 2006, 40, 259–269. [Google Scholar] [CrossRef]

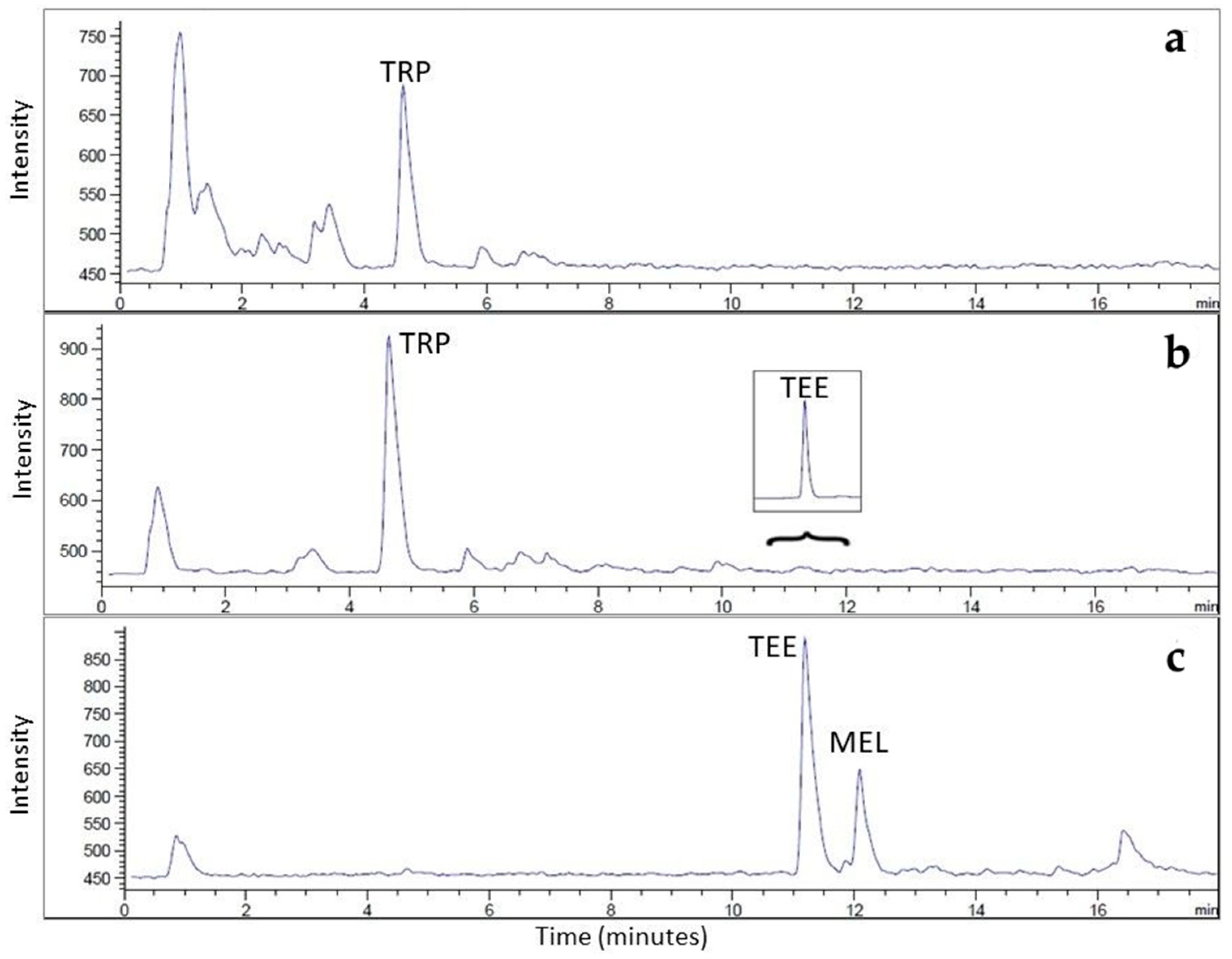
| FLD | MSD | |||||
|---|---|---|---|---|---|---|
| Compound | Concentration Range (μg/L) | Linearity | Concentration Range (μg/L) | Linearity | ||
| Equation | r | Equation | r | |||
| TRP | 110–5500 | 39.03 × x − 0.08 | 0.996 | 110–5500 | 3389.30 × x + 49.99 | 1 |
| TEE | 50–2000 | 0.064 × x + 7.308 | 0.998 | 5–200 | 23.40 × x + 111.85 | 0.999 |
| MEL | 20–500 | 0.15 × x + 0.41 | 1 | 1–200 | 15.04 × x + 109.10 | 0.998 |
| Compound | Concentration Range Added (μg/L) | SWS | Spiked Red Wine | LOD (µg/L) (n = 3) | LOQ (µg/L) (n = 3) | Recovery (%) (n = 6) | Repeatability (%RSD) (n = 9) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Equation | r | Equation | r | SWS | Spiked Red Wine | SWS | Spiked Red Wine | ||||
| TRP | 110–5500 | 3284.2 × x + 328.3 | 0.999 | 3257.4 × x + 3132.1 | 0.995 | 0.75 | 1.25 | 89 | 84 | 9.1 | 10.5 |
| TEE | 5–250 | 659.2 × x − 440.4 | 0.997 | 677.4 × x + 3861.0 | 0.999 | 0.038 | 0.12 | 88 | 76 | 6.5 | 7.9 |
| MEL | 0.05–250 | 242.2 × x + 2748.6 | 0.996 | 269.5 × x + 840.1 | 0.997 | 0.0023 | 0.018 | 86 | 79 | 4.6 | 5.4 |
| Compound | Exact Mass | MS/MS Fragmentation | |
|---|---|---|---|
| [M + H]+ | MS/MS Fragments | Collision Energy (µV) | |
| MEL | 233.1 | 188.1 | 30 |
| 216.1 | 30 | ||
| 174.1 | 30 | ||
| TEE | 233.1 | 174.1 | 20 |
| 159.0 | 36 | ||
| 130.1 | 55 | ||
| 178.1 | 29 | ||
| MIS 1 | 233.1 | 141.0 | 20 |
| 216.0 | 20 | ||
| 174.1 | 20 | ||
| MIS 2 | 233.1 | 141.1 | 20 |
| 196.0 | 20 | ||
| MIS 3 | 233.1 | 130.0 | 50 |
| MIS 4 | 233.1 | 141.0 | 20 |
| 216.0 | 20 | ||
| 174.1 | 20 | ||
| MIS 5 | 233.1 | 141.0 | 35 |
| 159.0 | 35 | ||
| Sample Code | TRP | TEE | MEL | MIS 1 | MIS 2 | MIS 3 | MIS 4 | MIS 5 |
|---|---|---|---|---|---|---|---|---|
| mg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| Red wine 1 | 3.85 ± 0.40 | 172.2 ± 13.6 | 0.057 ± 0.003 | 1.64 ± 0.09 | <LOQ | <LOQ | 0.0043 ± 0.0002 | <LOQ |
| Red wine 2 | 4.39 ± 0.46 | 212.0 ± 16.8 | 0.062 ± 0.003 | 1.97 ± 0.11 | <LOQ | <LOQ | 0.0041 ± 0.0002 | <LOQ |
| Red wine 3 | 1.56 ± 0.16 | 256.2 ± 20.2 | 0.063 ± 0.004 | 0.74 ± 0.04 | <LOQ | <LOQ | <LOQ | <LOQ |
| Red wine 4 | 1.02 ± 0.11 | 223.2 ± 17.6 | 0.038 ± 0.002 | 0.67 ± 0.04 | <LOQ | <LOQ | <LOQ | <LOQ |
| Red wine 5 | 0.98 ± 0.10 | 113.0 ± 8.9 | 0.046 ± 0.003 | 0.58 ± 0.03 | <LOQ | <LOQ | <LOQ | <LOQ |
| Red wine 6 | 0.84 ± 0.09 | 92.9 ± 7.3 | 0.054 ± 0.003 | 0.86 ± 0.05 | <LOQ | <LOQ | <LOQ | <LOQ |
| Red wine 7 | 0.44 ± 0.05 | 71.7 ± 5.7 | 0.063 ± 0.004 | 0.91 ± 0.04 | <LOQ | <LOQ | <LOQ | <LOQ |
| Red wine 8 | 0.57 ± 0.06 | 74.4 ± 5.9 | 0.038 ± 0.001 | 0.80 ± 0.04 | <LOQ | <LOQ | <LOQ | <LOQ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods. Foods 2019, 8, 99. https://doi.org/10.3390/foods8030099
Fracassetti D, Vigentini I, Lo Faro AFF, De Nisi P, Foschino R, Tirelli A, Orioli M, Iriti M. Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods. Foods. 2019; 8(3):99. https://doi.org/10.3390/foods8030099
Chicago/Turabian StyleFracassetti, Daniela, Ileana Vigentini, Alfredo Fabrizio Francesco Lo Faro, Patrizia De Nisi, Roberto Foschino, Antonio Tirelli, Marica Orioli, and Marcello Iriti. 2019. "Assessment of Tryptophan, Tryptophan Ethylester, and Melatonin Derivatives in Red Wine by SPE-HPLC-FL and SPE-HPLC-MS Methods" Foods 8, no. 3: 99. https://doi.org/10.3390/foods8030099







