Characterization and Determination of Interesterification Markers (Triacylglycerol Regioisomers) in Confectionery Oils by Liquid Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Calibration Curve Preparation
2.4. Instrumentation
2.5. Chromatography
2.6. Silver Modification of SCX Columns
2.7. Statistical Evaluation of Final Data and Validation
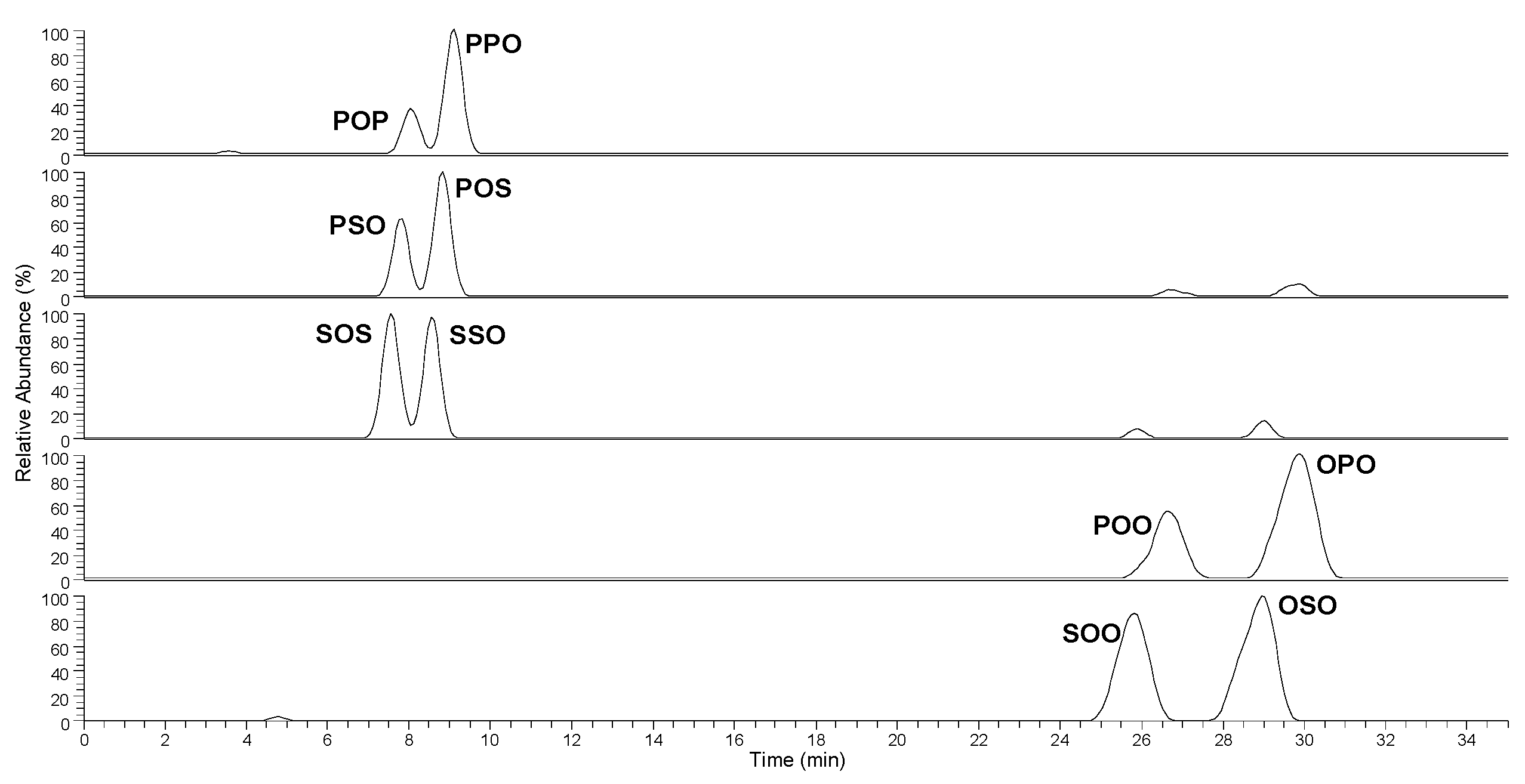
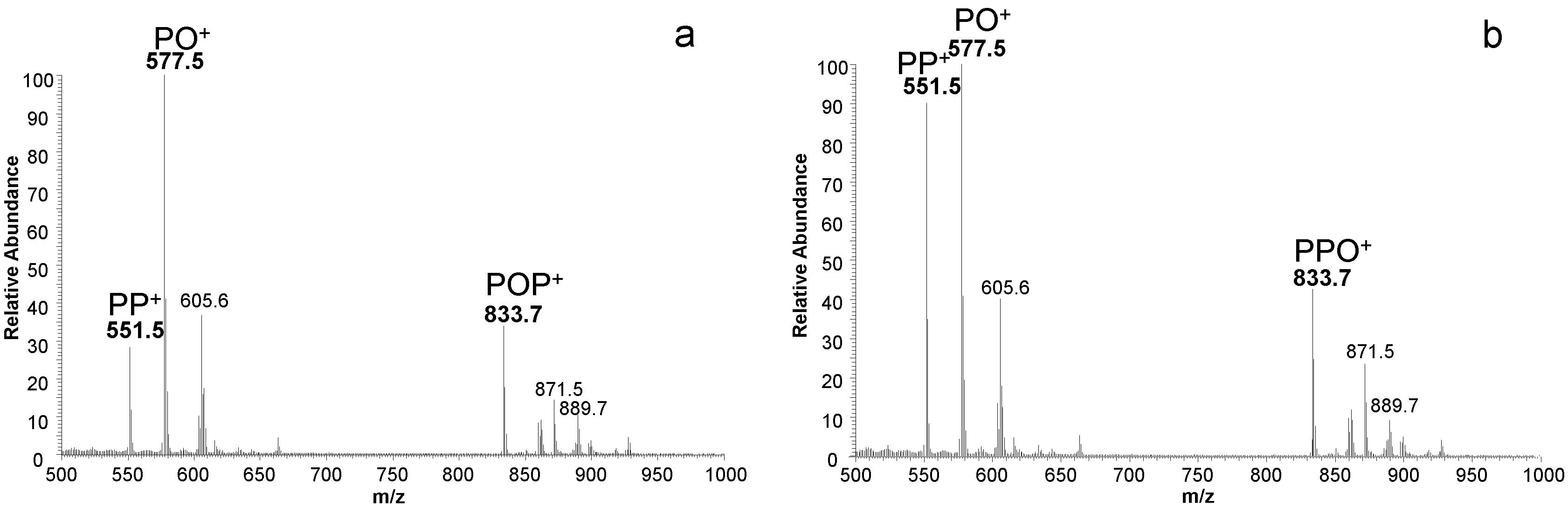
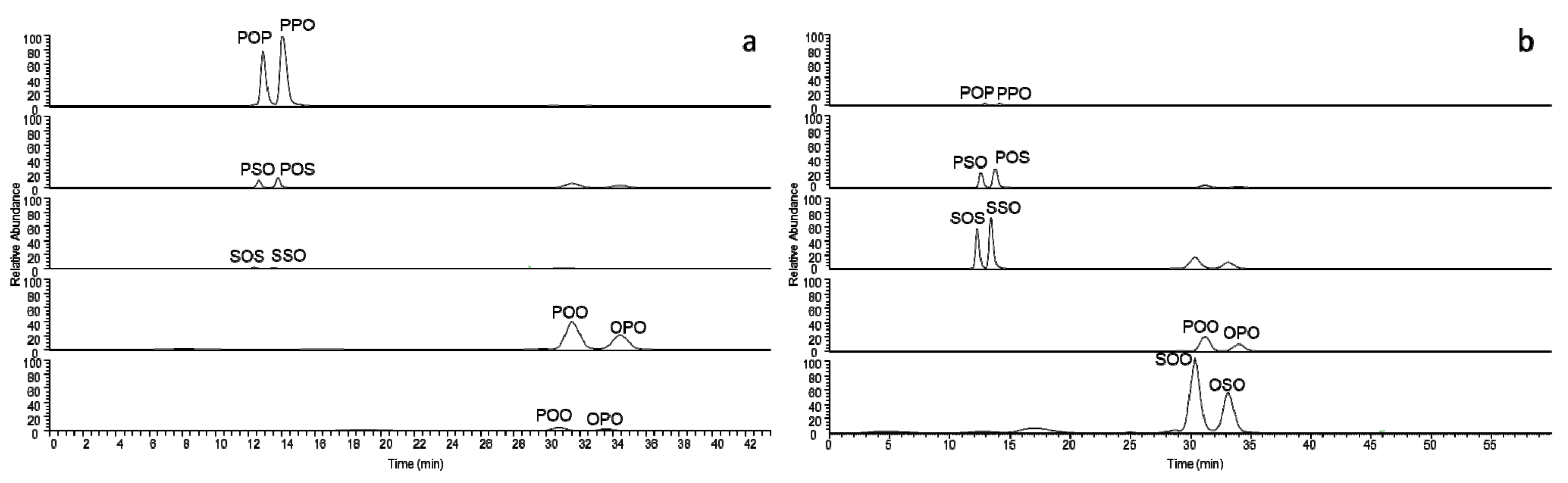
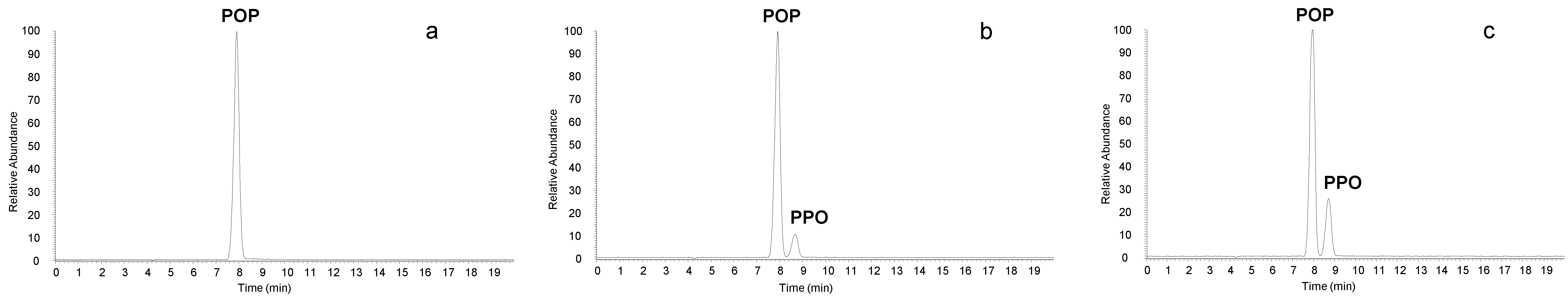
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, M.K. Practical Guide to Vegetable Oil Processing, 1st ed.; AOCS Press: Urbana, IL, USA, 2010; ISBN 978-1-89-399790-5. [Google Scholar]
- De Vries, B. Quantitative separation of lipid materials by column chromatography on silica impregnated with silver nitrate. Chem. Ind. J. 1962, 24, 1049–1050. [Google Scholar]
- Adlof, R.O.; Menzel, A.; Dorovska-Taran, V. Analysis of conjugated linoleic acid-enriched triacylglycerol mixtures by isocratic silver-ion high-performance liquid chromatography. J. Chromatogr. A 2002, 953, 293–297. [Google Scholar] [CrossRef]
- Christie, W.W. Separation of molecular species of triacylglycerols by high-performance liquid chromatography with a silver ion column. J. Chromatogr. A 1988, 454, 273–284. [Google Scholar] [CrossRef]
- Adlof, R.O. Analysis of triacylglycerol positional isomers by silver ion high-performance liquid chromatography. J. High Resolut. Chromatogr. 1995, 18, 105–107. [Google Scholar] [CrossRef]
- Adlof, R.O. Normal-phase separation effects with lipids on a silver ion high-performance liquid chromatography column. J. Chromatogr. A 1997, 764, 337–340. [Google Scholar] [CrossRef]
- Laakso, P.; Voutilainen, P. Analysis of triacylglycerols by silver-ion high-performance liquid chromatography—Atmospheric pressure chemical ionization mass spectrometry. Lipids 1996, 31, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Schuyl, P.J.W.; de Joode, T.; Vasconcellos, M.A.; Duchateau, G.S.M.J.E. Silver-phase high-performance liquid chromatography–electrospray mass spectrometry of triacylglycerols. J. Chromatogr. A 1998, 810, 53–61. [Google Scholar] [CrossRef]
- Mondello, L.; Tranchida, P.Q.; Stanek, V.; Jandera, P.; Dugo, G.; Dugo, P. Silver-ion reversed-phase comprehensive two-dimensional liquid chromatography combined with mass spectrometric detection in lipidic food analysis. J. Chromatogr. A 2005, 1086, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Kumm, T.; Crupi, M.L.; Cotroneo, A.; Mondello, L. Comprehensive two-dimensional liquid chromatography combined with mass spectrometric detection in the analyses of triacylglycerols in natural lipidic matrixes. J. Chromatogr. A 2006, 1112, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Nikolova-Damyanova, B. Retention of lipids in silver ion high-performance liquid chromatography: Facts and assumptions. J. Chromatogr. A 2009, 1216, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Lisa, M.; Velínská, H.; Holčapek, M. Regioisomeric Characterization of Triacylglycerols Using Silver-Ion HPLC/MS and Randomization Synthesis of Standards. Anal. Chem. 2009, 81, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Lisa, M.; Netusilova, K.; Franek, L.; Dvorakova, H.; Vrkoslav, V.; Holcapek, M. Characterization of fatty acid and triacylglycerol composition in animal fats using silver-ion and non-aqueous reversed-phase high-performance liquid chromatography/mass spectrometry and gas chromatography/flame ionization detection. J. Chromatogr. A 2011, 1218, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Lisa, M.; Denev, R.; Holčapek, M. Retention behavior of isomeric triacylglycerols in silver-ion HPLC: Effects of mobile phase composition and temperature. J. Sep. Sci. 2013, 36, 2888–2900. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Velínská, H.; Lísa, M.; Česla, P. Orthogonality of silver-ion and non-aqueous reversed-phase HPLC/MS in the analysis of complex natural mixtures of triacylglycerols. J. Sep. Sci. 2009, 32, 3672–3680. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Dvorakova, H.; Lisa, M.; Giron, A.J.; Sandra, P.; Cvacka, J. Regioisomeric analysis of triacylglycerols using silver-ion liquid chromatography atmospheric pressure chemical ionization mass spectrometry: Comparison of five different mass analyzers. J. Chromatogr. A 2010, 1217, 8186–8194. [Google Scholar] [CrossRef] [PubMed]
- Byrdwell, W.C.; Holčapek, M. Extreme Chromatography: Faster, Hotter, Smaller, 1st ed.; American Oil Chemists Society: Urbana, IL, USA, 2011; pp. 197–230. ISBN 978-1-89-399766-0. [Google Scholar]
- Ovčačíková, M.; Lisa, M.; Cifkova, E.; Holcapek, M. Retention behavior of lipids in reversed-phase ultrahigh-performance liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2016, 1450, 76–85. [Google Scholar] [CrossRef] [PubMed]
- TambaSompila, A.W.G.; Héron, S.; Hmida, D.; Tchapla, A. Fast non-aqueous reversed-phase liquid chromatography separation of triacylglycerol regioisomers with isocratic mobile phase. Application to different oils and fats. J. Chromatogr. B 2017, 1041–1042, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Šala, M.; Lísa, M.; Campbell, J.L.; Holčapek, M. Determination of triacylglycerol regioisomers using differential mobility spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hubaux, A.; Vos, G. Decision and detection limits for linear calibration curves. Anal. Chem. 1970, 42, 849–855. [Google Scholar] [CrossRef]
- Baiocchi, C.; Medana, C.; Dal Bello, F.; Giancotti, V.; Aigotti, R.; Gastaldi, D. Analysis of regioisomers of polyunsaturated triacylglycerols in marine matrices by HPLC/HRMS. Food Chem. 2015, 166, 551–560. [Google Scholar] [CrossRef] [PubMed]
| Tag | Calibration Points (ppm) | Calibration Curve Parameters 1 | Rsd (%) | Calibration Curve Parameters 2 | Rsd (%) | Measure Uncertainty x0 ± t (0.05, 5)sx0 | LOD, LOQ (ppm) |
|---|---|---|---|---|---|---|---|
| SOS | 1 | b = (5.91 ± 0.06) × 106 R² = 0.9996 | 13.9 | b = (5.82 ± 0.10) × 106 R² = 0.9985 | 10.3 | 1.00 ± 0.38 | 0.31, 1.04 |
| 2 | 6.73 | 8.85 | 1.89 ± 0.34 | ||||
| 3 | 5.99 | 6.81 | 2.87 ± 0.32 | ||||
| 5 | 6.39 | 5.09 | 5.01 ± 0.29 | ||||
| 8 | 2.13 | 7.90 | 7.61 ± 0.34 | ||||
| 10 | 1.22 | 2.25 | 10.37 ± 0.46 | ||||
| SSO | 1 | b = (7.79 ± 0.07) × 106 R² = 0.9996 | 2.42 | b = (7.84 ± 0.10) × 106 R² = 0.9992 | 10.6 | 0.97 ± 0.27 | 0.31, 1.03 |
| 2 | 15.5 | 9.96 | 2.19 ± 0.24 | ||||
| 3 | 6.46 | 5.65 | 2.91 ± 0.22 | ||||
| 5 | 4.01 | 5.71 | 4.69 ± 0.21 | ||||
| 8 | 1.82 | 2.30 | 8.09 ± 0.25 | ||||
| 10 | 1.09 | 9.86 | 10.1 ± 0.31 | ||||
| POS | 1 | b = (5.76 ± 0.07) × 106 R² = 0.9995 | 13.9 | b = (5.61 ± 0.04) × 106 R² = 0.9997 | 9.56 | 1.06 ± 0.16 | 0.30, 1.01 |
| 2 | 6.03 | 5.10 | 2.17 ± 0.14 | ||||
| 3 | 2.12 | 4.28 | 3.13 ± 0.13 | ||||
| 5 | 4.13 | 5.18 | 4.91 ± 0.12 | ||||
| 8 | 1.69 | 4.28 | 7.97 ± 0.15 | ||||
| 10 | 8.64 | 10.6 | 9.99 ± 0.18 | ||||
| PSO | 1 | b = (9.34 ± 0.10) × 106 R² = 0.9985 | 5.22 | b = (9.41 ± 0.09) × 106 R² = 0.9995 | 9.86 | 1.02 ± 0.21 | 0.30, 1.00 |
| 2 | 7.16 | 8.57 | 2.03 ± 0.19 | ||||
| 3 | 3.55 | 4.41 | 2.82 ± 0.17 | ||||
| 5 | 0.45 | 3.52 | 4.96 ± 0.16 | ||||
| 8 | 3.64 | 3.78 | 7.85 ± 0.19 | ||||
| 10 | 4.05 | 6.86 | 10.2 ± 0.25 | ||||
| POP | 1 | b = (3.69 ± 0.06) × 106 R² = 0.9989 | 7.76 | b = (3.61 ± 0.05) × 106 R² = 0.9992 | 9.02 | 0.95 ± 0.27 | 0.26, 0.85 |
| 2 | 4.89 | 7.48 | 2.14 ± 0.24 | ||||
| 3 | 5.11 | 11.1 | 3.16 ± 0.22 | ||||
| 5 | 9.37 | 11.3 | 4.84 ± 0.21 | ||||
| 8 | 4.73 | 5.28 | 7.99 ± 0.25 | ||||
| 10 | 2.81 | 12.5 | 10.1 ± 0.32 | ||||
| PPO | 1 | b = (12.60 ± 0.07) × 106 R² = 0.9998 | 11.1 | b = (12.67 ± 0.06) × 106 R² = 0.9999 | 10.02 | 1.07 ± 0.11 | 0.32, 1.07 |
| 2 | 1.97 | 4.38 | 2.02 ± 0.10 | ||||
| 3 | 4.59 | 4.97 | 3.04 ± 0.09 | ||||
| 5 | 0.75 | 4.59 | 4.87 ± 0.08 | ||||
| 8 | 3.42 | 5.14 | 7.99 ± 0.10 | ||||
| 10 | 5.38 | 9.52 | 10.1 ± 0.12 | ||||
| SOO | 1 | b = (16.19 ± 0.21) × 106 R2 = 0.9992 | 6.10 | b = (16.26 ± 0.27) × 106 R² = 0.9960 | 9.10 | 1.19 ± 0.35 | 0.32, 1.08 |
| 2 | 4.13 | 2.62 | 2.07 ± 0.32 | ||||
| 3 | 2.29 | 2.13 | 2.91 ± 0.30 | ||||
| 5 | 0.71 | 8.05 | 5.38 ± 0.28 | ||||
| 8 | 1.73 | 1.80 | 8.10 ± 0.34 | ||||
| 10 | 1.96 | 4.74 | 9.72 ± 0.40 | ||||
| OSO | 1 | b = (22.37 ± 0.25) × 106 R2 = 0.9994 | 0.99 | b = (22.31 ± 0.23) × 106 R² = 0.9990 | 4.25 | 1.20 ± 0.22 | 0.15, 0.51 |
| 2 | 2.94 | 1.95 | 2.14 ± 0.20 | ||||
| 3 | 1.56 | 2.65 | 3.11 ± 0.18 | ||||
| 5 | 3.89 | 5.58 | 4.90 ± 0.17 | ||||
| 8 | 3.49 | 4.66 | 7.86 ± 0.20 | ||||
| 10 | 0.45 | 3.79 | 10.1 ± 0.26 |
| TAG | Biscuit Filling Cream TAG Composition (%) | Original Raw Material | |
|---|---|---|---|
| Shea Butter (%) | High Oleic Rapeseed Oil (%) | ||
| POP | 84.1 | 91.51 | 100 |
| PPO | 15.9 | 8.49 | 0 |
| POS | 77.3 | 100 | 100 |
| PSO | 22.7 | 0 | 0 |
| SOS | 80.3 | 100 | 100 |
| SSO | 19.7 | 0 | 0 |
| POO | 100 | 100 | 100 |
| OPO | 0 | 0 | 0 |
| SOO | 88.2 | 100 | 100 |
| OSO | 11.8 | 0 | 0 |
| Evidence of interesterification | |||
| Tag | Spreading Cream A TAG Composition (%) | Original Raw Material | |
|---|---|---|---|
| High Linoleic Sunflower Oil (%) | Cocoa Butter (%) | ||
| POP | 95.7 | 100 | 100 |
| PPO | 4.3 1 | 0 | 0 |
| POS | 100 | 100 | 100 |
| PSO | 0 | 0 | 0 |
| SOS | 100 | 100 | 100 |
| SSO | 0 | 0 | 0 |
| POO | 100 | 100 | 100 |
| OPO | 0 | 0 | 0 |
| SOO | 100 | 100 | 100 |
| OSO | 0 | 0 | 0 |
| No interesterification | |||
| TAG | Spreading Cream BTAG Composition (%) | Original Raw Material | |
|---|---|---|---|
| High Linoleic Sunflower Oil (%) | Cocoa Butter (%) | ||
| POP | 83.0 | 100 | 100 |
| PPO | 17.0 | 0 | 0 |
| POS | 84.5 | 100 | 100 |
| PSO | 15.5 | 0 | 0 |
| SOS | 84.1 | 100 | 100 |
| SSO | 15.9 | 0 | 0 |
| POO | 91.7 | 100 | 100 |
| OPO | 8.3 | 0 | 0 |
| SOO | 91.7 | 100 | 100 |
| OSO | 8.3 | 0 | 0 |
| Evidence of interesterification | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, V.; Dal Bello, F.; Aigotti, R.; Gastaldi, D.; Romaniello, F.; Forte, E.; Magni, M.; Baiocchi, C.; Medana, C. Characterization and Determination of Interesterification Markers (Triacylglycerol Regioisomers) in Confectionery Oils by Liquid Chromatography-Mass Spectrometry. Foods 2018, 7, 23. https://doi.org/10.3390/foods7020023
Santoro V, Dal Bello F, Aigotti R, Gastaldi D, Romaniello F, Forte E, Magni M, Baiocchi C, Medana C. Characterization and Determination of Interesterification Markers (Triacylglycerol Regioisomers) in Confectionery Oils by Liquid Chromatography-Mass Spectrometry. Foods. 2018; 7(2):23. https://doi.org/10.3390/foods7020023
Chicago/Turabian StyleSantoro, Valentina, Federica Dal Bello, Riccardo Aigotti, Daniela Gastaldi, Francesco Romaniello, Emanuele Forte, Martina Magni, Claudio Baiocchi, and Claudio Medana. 2018. "Characterization and Determination of Interesterification Markers (Triacylglycerol Regioisomers) in Confectionery Oils by Liquid Chromatography-Mass Spectrometry" Foods 7, no. 2: 23. https://doi.org/10.3390/foods7020023






