Utility of Milk Coagulant Enzyme of Moringa oleifera Seed in Cheese Production from Soy and Skim Milks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vegetal and Dairy Material
2.2. Enzyme Extraction
2.3. Milk-Clotting Activity (MCA)
2.4. Caseinolytic Activity
2.5. Protein Determination
2.6. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Cheese Elaboration
2.8. Statistical Analysis
3. Results and Discussion
3.1. Initial Analysis of Coagulant Activity in Different Parts of the M. oleifera
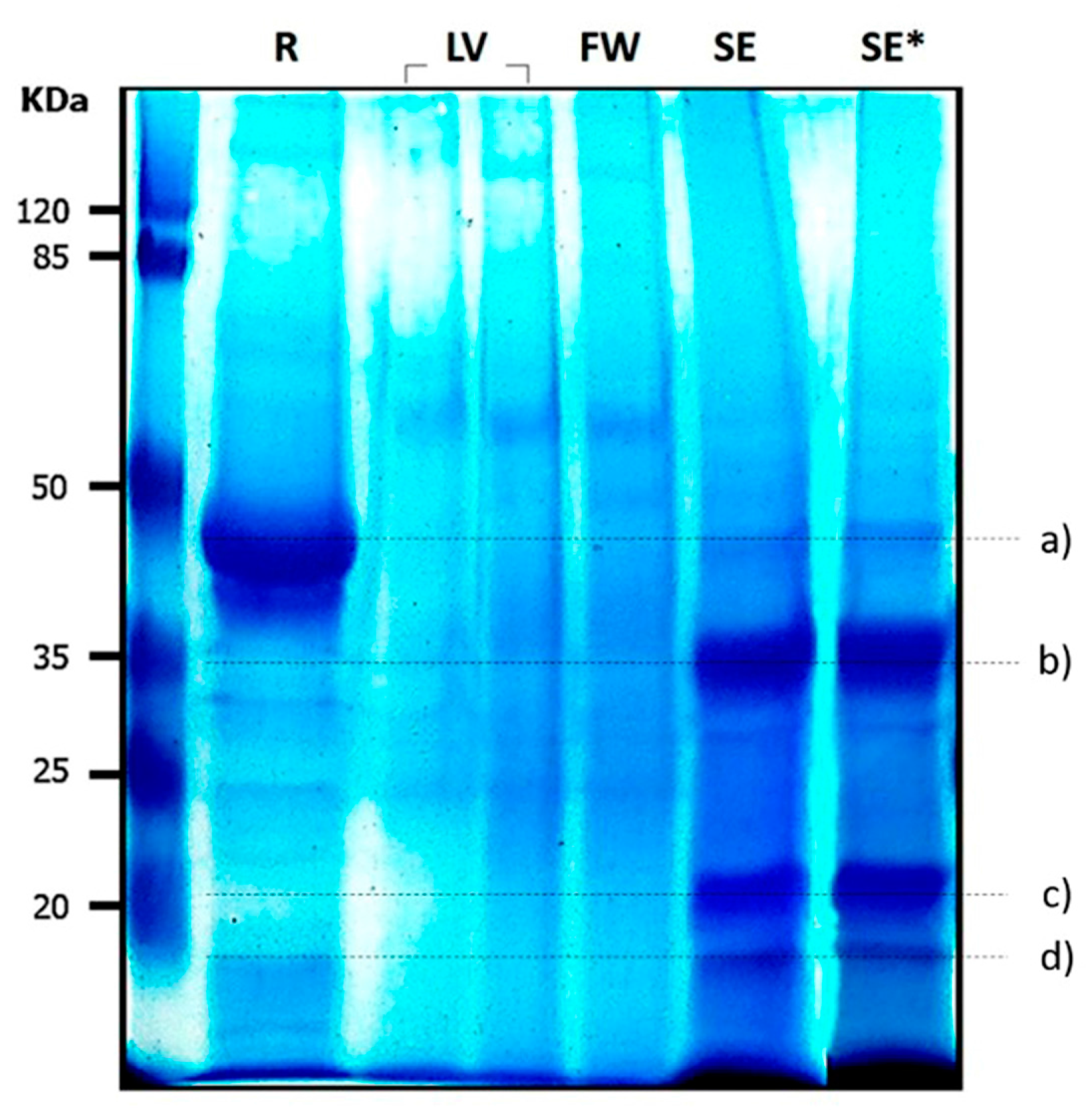
3.2. Electrophoretic Pattern of Moringa oleifera Extracts
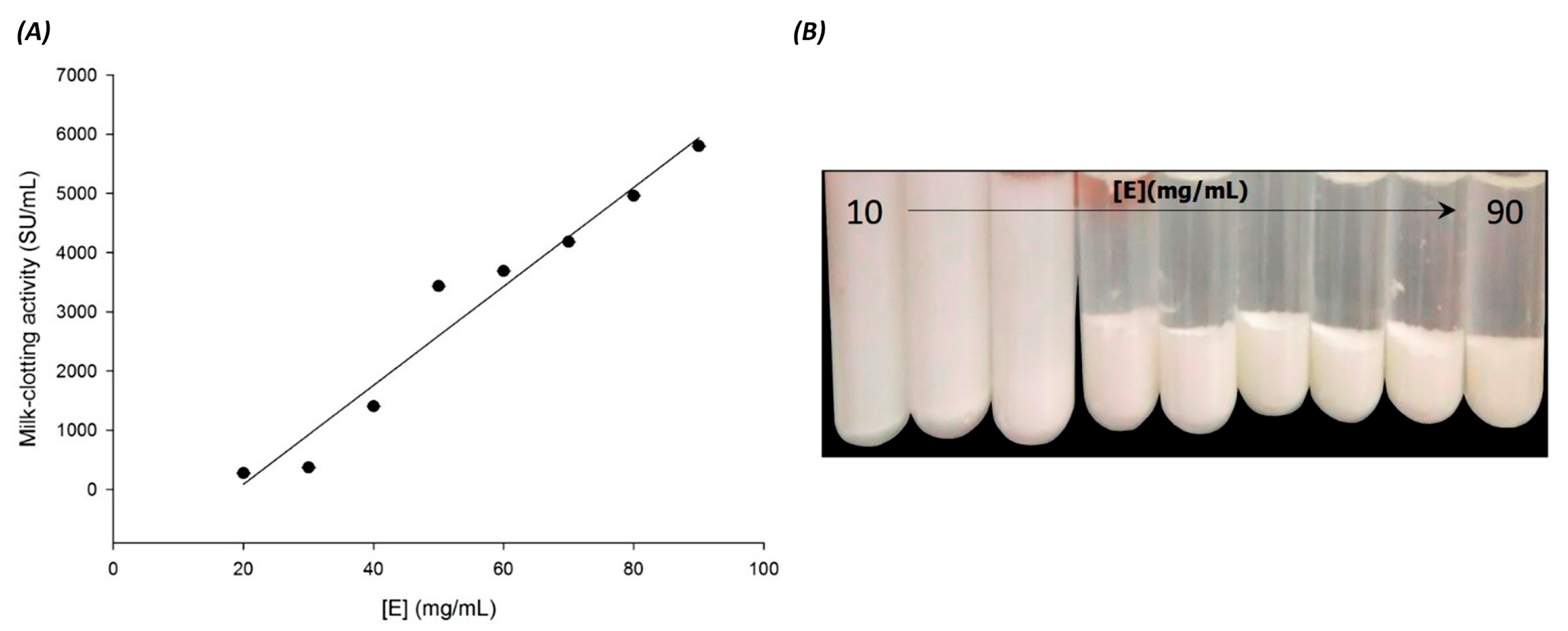
3.3. Effect of Substrate and Enzyme Concentration on Skim Milk Clotting Activity
3.4. Effect of Enzyme Concentration on Caseinolytic Activity
3.5. Composition of Soy Milk
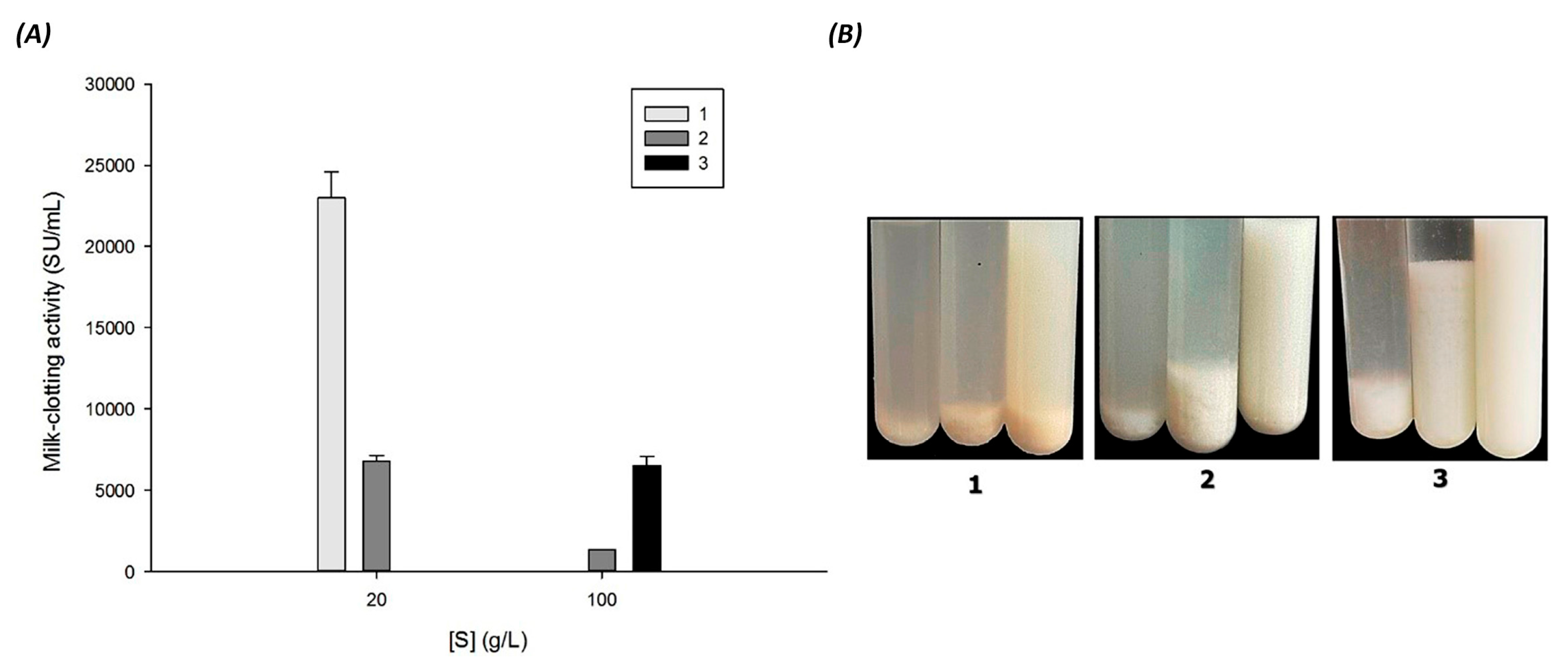
3.6. Effect of Substrate (Soy Milk Types) on Milk Clotting Activity
3.7. Cheese Processing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberfroid, M.B. Concepts and strategy of functional food science: The European perspective. Am. J. Clin. Nutr. 2000, 71, 1660s–1664s. [Google Scholar] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Nutraceuticals and prevention of atherosclerosis: Focus on ω-3 polyunsaturated fatty acids and mediterranean diet polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to mediterranean diet: The medi-lite score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Bonvecchio, A.; Safdie, M.; Monterrubio, E.A.; Gust, T.; Villalpando, S.; Rivera, J.A. Overweight and obesity trends in Mexican children 2 to 18 years of age from 1988 to 2006. Salud Pública México 2009, 51, S586–S594. [Google Scholar] [CrossRef]
- Qin, L.-Q.; Xu, J.-Y.; Han, S.-F.; Zhang, Z.-L.; Zhao, Y.-Y.; Szeto, I.M. Dairy consumption and risk of cardiovascular disease: An updated meta-analysis of prospective cohort studies. Asia Pac. J. Clin. Nutr. 2015, 24, 90–100. [Google Scholar] [PubMed]
- Ahmed, I.A.M.; Morishima, I.; Babiker, E.E.; Mori, N. Characterisation of partially purified milk-clotting enzyme from solanum dubium fresen seeds. Food Chem. 2009, 116, 395–400. [Google Scholar] [CrossRef]
- Ohlsson, L. Dairy products and plasma cholesterol levels. Food Nutr. Res. 2010, 54, 5124. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ning, N.; Wang, C.; Wang, Y.; Li, Q.; Meng, Z.; Liu, Y.; Li, Q. Dairy products consumption and risk of type 2 diabetes: Systematic review and dose-response meta-analysis. PLoS ONE 2013, 8, e73965. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Q.; Giovannucci, E.; Mozaffarian, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014, 12, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, N.A. Review of Egyptian cereal-based fermented product (Kishk). Int. J. Agric. Innov. Res. 2016, 4, 600–609. [Google Scholar]
- O’connell, J.; Fox, P. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.T.; Britten, M. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. LWT-Food Sci. Technol. 2012, 47, 167–174. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Sady, M.; Grega, T.; Walczycka, M. The impact of tea supplementation on microflora, pH and antioxidant capacity of yoghurt. Int. Dairy J. 2011, 21, 568–574. [Google Scholar] [CrossRef]
- Gad, A.S.; El-Salam, M.H.A. The antioxidant properties of skim milk supplemented with rosemary and green tea extracts in response to pasteurisation, homogenisation and the addition of salts. Int. J. Dairy Technol. 2010, 63, 349–355. [Google Scholar] [CrossRef]
- Chen, G.; Kocaoglu-Vurma, N.; Harper, W.J.; Rodriguez-Saona, L.E. Application of infrared microspectroscopy and multivariate analysis for monitoring the effect of adjunct cultures during Swiss cheese ripening. J. Dairy Sci. 2009, 92, 3575–3584. [Google Scholar] [CrossRef] [PubMed]
- Frazier, R.A.; Deaville, E.R.; Green, R.J.; Stringano, E.; Willoughby, I.; Plant, J.; Mueller-Harvey, I. Interactions of tea tannins and condensed tannins with proteins. J. Pharm. Biomed. Anal. 2010, 51, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.-A. Interaction of milk α-and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Kanakis, C.; Hasni, I.; Bourassa, P.; Tarantilis, P.; Polissiou, M.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and their impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Giroux, H.J.; De Grandpré, G.; Fustier, P.; Champagne, C.P.; St-Gelais, D.; Lacroix, M.; Britten, M. Production and characterization of cheddar-type cheese enriched with green tea extract. Dairy Sci. Technol. 2013, 93, 241–254. [Google Scholar] [CrossRef]
- Gad, A.S.; Sayd, A.F. Antioxidant properties of rosemary and its potential uses as natural antioxidant in dairy products—A review. Food Nutr. Sci. 2015, 6, 179. [Google Scholar] [CrossRef]
- Yadav, D.; Vij, S.; Hati, S.; Singh, B.P.; Dhanday, M.; Dahiya, M.; Vandna, V. Evaluation of total antioxidant activity of soy yoghurt. Indian J. Dairy Sci. 2012, 65, 220–224. [Google Scholar]
- Murata, K.; Kusakabe, I.; Kobayashi, H.; Akaike, M.; Park, Y.W.; Murakami, K. Studies on the coagulation of soymilk-protein by commercial proteinases. Agric. Biol. Chem. 1987, 51, 385–389. [Google Scholar]
- Galán, E.; Prados, F.; Pino, A.; Tejada, L.; Fernández-Salguero, J. Influence of different amounts of vegetable coagulant from cardoon cynara cardunculus and calf rennet on the proteolysis and sensory characteristics of cheeses made with sheep milk. Int. Dairy J. 2008, 18, 93–98. [Google Scholar] [CrossRef]
- Pino, A.; Prados, F.; Galán, E.; McSweeney, P.L.; Fernández-Salguero, J. Proteolysis during the ripening of goats’ milk cheese made with plant coagulant or calf rennet. Food Res. Int. 2009, 42, 324–330. [Google Scholar] [CrossRef]
- Öner, M.; Akar, B. Separation of the proteolytic enzymes from fig tree latex and its utilization in gaziantep cheese production. LWT-Food Sci. Technol. 1993, 26, 318–321. [Google Scholar] [CrossRef]
- Ilany, J.; Netzer, A. Milk-clotting activity of proteolytic enzymes. J. Dairy Sci. 1969, 52, 43–46. [Google Scholar] [CrossRef]
- Xiu-ling, Z. Study on physical, chemical and biochemical characteristics of different milk-clotting enzymes during cheesemaking. Resour. Dev. Mark. 2009, 2, 002. [Google Scholar] [CrossRef]
- Chye, S.J.; Ahmad, R.; Noor Aziah, A.A. Studies on the physicochemical and sensory characteristics of goat’s milk dadih incorporated with tropical- fruit purees. Int. Food Res. J. 2012, 19, 1387–1392. [Google Scholar]
- Hatanaka, S.; Maegawa, M.; Kanauchi, M.; Kasahara, S.; Shimoyamada, M.; Ishida, M. Characteristics and purification of soybean milk curdling enzyme-producing yeast Saccharomyces bayanus SCY003. Food Sci. Technol. Res. 2014, 20, 927–938. [Google Scholar] [CrossRef]
- Idris, M.A.; Jami, M.S.; Hammed, A.M.; Jamal, P. Moringa oleifera seed extract: A review on its environmental applications. Int. J. Appl. Environ. Sci. 2016, 11, 1469–1486. [Google Scholar]
- Pontual, E.V.; Carvalho, B.E.; Bezerra, R.S.; Coelho, L.C.; Napoleão, T.H.; Paiva, P.M. Caseinolytic and milk-clotting activities from Moringa oleifera flowers. Food Chem. 2012, 135, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Tajalsir, A.E.; Ebraheem, A.S.; Abdallah, A.M.; Khider, F.J.; Elsamani, M.O.; Ahmed, I.A.M. Partial purification of milk-clotting enzyme from the seeds of Moringa oleifera. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 58. [Google Scholar] [CrossRef]
- Arima, K.; Yu, J.; Iwasaki, S. Milk-clotting enzyme from Mucor pusillus var. Lindt. Methods Enzymol. 1970, 19, 446–459. [Google Scholar]
- Sarath, G.; Motte, R.S.; Wagner, F.M. Protease assay methods. In Proteolytic Enzyme—A Practical Approach; Beynon, R.J., Bond, J.S., Eds.; IRL Press: Oxford, UK, 1989; pp. 25–55. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Laemmli, V. Determination of protein molecular weight in polyacrylamide gels. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Chazarra, S.; Sidrach, L.; Lopez-Molina, D.; Rodríguez-López, J.N. Characterization of the milk-clotting properties of extracts from artichoke (Cynara scolymus, L.) flowers. Int. Dairy J. 2007, 17, 1393–1400. [Google Scholar] [CrossRef]
- Egito, A.; Girardet, J.-M.; Laguna, L.; Poirson, C.; Mollé, D.; Miclo, L.; Humbert, G.; Gaillard, J.-L. Milk-clotting activity of enzyme extracts from sunflower and albizia seeds and specific hydrolysis of bovine κ-casein. Int. Dairy J. 2007, 17, 816–825. [Google Scholar] [CrossRef]
- Raposo, S.; Domingos, A. Purification and characterization milk-clotting aspartic proteinases from Centaurea calcitrapa cell suspension cultures. Process Biochem. 2008, 43, 139–144. [Google Scholar] [CrossRef]
- De Silva, A.C.; da Silva Nascimento, T.C.E.; da Silva, S.A.; Herculano, P.N.; Moreira, K.A. Potential of quixaba (Sideroxylon obtusifolium) latex as a milk-clotting agent. Food Sci. Technol. (Camp.) 2013, 33, 494–499. [Google Scholar] [CrossRef]
- Cavalli, S.V.; Silva, S.V.; Cimino, C.; Malcata, F.X.; Priolo, N. Hydrolysis of caprine and ovine milk proteins, brought about by aspartic peptidases from Silybum marianum flowers. Food Chem. 2008, 106, 997–1003. [Google Scholar] [CrossRef]
- Llorente, B.E.; Obregón, W.D.; Avilés, F.X.; Caffini, N.O.; Vairo-Cavalli, S. Use of artichoke (Cynara scolymus) flower extract as a substitute for bovine rennet in the manufacture of Gouda-type cheese: Characterization of aspartic proteases. Food Chem. 2014, 159, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.; Duarte, D.M.R.; Moreira, K.A.; Cavalcanti, M.T.H.; de Lima-Filho, J.L.; Porto, A.L.F. Jacaratia corumbensis O. Kuntze a new vegetable source for milk-clotting enzymes. Braz. Arch. Biol. Technol. 2009, 52, 1–9. [Google Scholar] [CrossRef]
- Hashim, M.M.; Dong, M.; Iqbal, M.F.; Chen, X. Ginger rhizome as a potential source of milk coagulating cysteine protease. Phytochemistry 2011, 72, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Néstor, G.M.; Dely Rubí, C.G.; Héctor, J.C. Exploring the milk-clotting properties of a plant coagulant from the berries of S. elaeagnifolium var. Cavanilles. J. Food Sci. 2012, 77, C89–C94. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Luz, L.A.; Argolo, A.C.; Teixeira, J.A.; Paiva, P.M.; Coelho, L.C. Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem. 2009, 44, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.; Napoleão, T.H.; Santos, A.F.; Sá, R.; Carneiro-da-Cunha, M.; Morais, M.; Silva-Lucca, R.A.; Oliva, M.L.V.; Coelho, L.; Paiva, P.M. Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera. Lett. Appl. Microbiol. 2011, 53, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.I.; Mohamed Ahmed, I.A.; Hamid, O.I. Characterization of partially purified milk-clotting enzyme from sunflower (Helianthus annuus) seeds. Food Sci. Nutr. 2016, 4, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Helmy, W. Comparative evaluation of Bacillus licheniformis 5A5 and Aloe variegata milk-clotting enzymes. Braz. J. Chem. Eng. 2012, 29, 69–76. [Google Scholar] [CrossRef]
- Wahba, A.; El-Abbassy, F.; El-Shafei, H.; Awad, S. Effect of some factors on the activity of milk clotting enzymes. Egypt. J. Food Sci. (Egypt) 1995, 23, 27–35. [Google Scholar]
- Zhang, J.; Sun, Y.; Li, Z.; Luo, Q.; Li, T.; Wang, T. Structure-based design of mucor pusillus pepsin for the improved ratio of clotting activity/proteolytic activity in cheese manufacture. Protein Pept. Lett. 2015, 22, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, A.; Mabrouk, S.S.; El-Hawwary, N.M. Production and some properties of rennin-like milk-clotting enzyme from Penicillium citrinum. Microbiology 1972, 70, 151–155. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Brinkhuis, J.; Payens, T.A. The coagulation of differently sized casein micelles by rennet. Eur. J. Biochem. 1981, 119, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Low, Y.H.; Agboola, S.; Zhao, J.; Lim, M.Y. Clotting and proteolytic properties of plant coagulants in regular and ultrafiltered bovine skim milk. Int. Dairy J. 2006, 16, 335–343. [Google Scholar] [CrossRef]
- Hashem, A.M. Optimization of milk-clotting enzyme productivity by Penicillium oxalicum. Bioresour. Technol. 1999, 70, 203–207. [Google Scholar] [CrossRef]
- Nájera, A.; De Renobales, M.; Barron, L. Effects of pH, temperature, CaCl2 and enzyme concentrations on the rennet-clotting properties of milk: A multifactorial study. Food Chem. 2003, 80, 345–352. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Olano, A. Effects of high pressures combined with moderate temperatures on the rennet coagulation properties of milk. Int. Dairy J. 1998, 8, 623–627. [Google Scholar] [CrossRef]
- Van Hooydonk, A.; Walstra, P. Interpretation of the kinetics of the renneting reaction in milk. Neth. Milk Dairy J. 1987, 41, 19–48. [Google Scholar]
- Kappeler, S.R.; Rahbek-Nielsen, H.; Farah, Z.; Puhan, Z.; Hansen, E.B.; Johansen, E. Characterization of recombinant camel chymosin reveals superior properties for the coagulation of bovine and camel milk. Biochem. Biophys. Res. Commun. 2006, 342, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Zheng, Z.; Zhao, A.; Yang, Z. Production of a milk-clotting enzyme by glutinous rice fermentation and partial characterization of the enzyme. J. Food Biochem. 2015, 39, 70–79. [Google Scholar] [CrossRef]
- Hajirostamloo, B. Comparison of nutritional and chemical parameters of soymilk and cow milk. World Acad. Sci. Eng. Technol. 2009, 57, 436–438. [Google Scholar]
- Kanauchi, M.; Kanauchi, K. Diet quality and adherence to a healthy diet in Japanese male workers with untreated hypertension. BMJ Open 2015, 5, e008404. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Ano, T.; Shoda, M. Use of soybean curd residue, okara, for the solid state substrate in the production of a lipopeptide antibiotic, iturin A, by Bacillus subtilis NB22. Process Biochem. 1996, 31, 801–806. [Google Scholar] [CrossRef]
- Ono, T.; Katho, S.; Mothizuki, K. Influences of calcium and pH on protein solubility in soybean milk. Biosci. Biotechnol. Biochem. 1993, 57, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Guinee, T.P.; O’Callaghan, D.J.; Pudja, P.D.; O’Brien, N. Rennet coagulation properties of retentates obtained by ultrafiltration of skim milks heated to different temperatures. Int. Dairy J. 1996, 6, 581–596. [Google Scholar] [CrossRef]
- Mehaia, M.A. Studies on rennet coagulation of skim camel milk concentrated by ultrafiltration. J. King Saud Univ. 1997, 9, 11–23. [Google Scholar]
- Bruno, M.A.; Trejo, S.A.; Avilés, F.X.; Caffini, N.O.; López, L.M.I. Cloning, sequencing, and identification using proteomic tools of a protease from Bromelia hieronymi mez. Appl. Biochem. Biotechnol. 2011, 165, 583. [Google Scholar] [CrossRef] [PubMed]
- Guiama, V.; Libouga, D.; Ngah, E.; Beka, R.; Ndi, K.; Maloga, B.; Bindzi, J.; Donn, P.; Mbofung, C. Milk-clotting potential of fruit extracts from Solanum esculentum, Solanum macrocarpon L. And Solanum melongena. Afr. J. Biotechnol. 2010, 9. [Google Scholar] [CrossRef]
- Hailu, Y.; Seifu, E.; Yilma, Z. Physicochemical properties and consumer acceptability of soft unripened cheese made from camel milk using crude extract of ginger (Zingiber officinale) as coagulant. Afr. J. Food Sci. 2014, 8, 87–91. [Google Scholar]
- Liu, K. Chemistry and nutritional value of soybean components. In Soybeans; Springer US, International Thomson Publishing Asia: Henderson Road, Singapore, 1997; pp. 25–113. [Google Scholar]
- Liong, M.T.; Easa, A.M.; Lim, P.T.; Kang, J.Y. Survival, growth characteristics and bioactive potential of Lactobacillus acidophilus in a soy-based cream cheese. J. Sci. Food Agric. 2009, 89, 1382–1391. [Google Scholar] [CrossRef]
- Rinaldoni, A.N.; Palatnik, D.R.; Zaritzky, N.; Campderrós, M.E. Soft cheese-like product development enriched with soy protein concentrates. LWT-Food Sci. Technol. 2014, 55, 139–147. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.; Shah, N.E. Stability of β-glucosidase activity produced by Bifidobacterium and Lactobacillus spp. In fermented soymilk during processing and storage. J. Food Sci. 2005, 70, 236–241. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Chang, S.K.-C. Effect of soy milk characteristics and cooking conditions on coagulant requirements for making filled tofu. J. Agric. Food Chem. 2004, 52, 3405–3411. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, M.; Taira, H.; Igarashi, Y.; Yagasaki, K.; Ono, T. Properties of tofus and soy milks prepared from soybeans having different subunits of glycinin. J. Agric. Food Chem. 2000, 48, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, K.; Kousaka, F.; Kitamura, K. Potential improvement of soymilk gelation properties by using soybeans with modified protein subunit compositions. Breed. Sci. 2000, 50, 101–107. [Google Scholar] [CrossRef]




| Crude Extract | Protein Concentration (mg/mL) | Total MCA (SU/mL) | Specific Activity (SU/mg Protein) |
|---|---|---|---|
| Seeds | 10.30 ± 0.45 | 3419.26 ± 186.80 | 331.96 |
| Flowers | 10.62 ± 0.77 | 13.66 ± 1.12 ** | 0.77 |
| Leaves | 28.43 ± 3.04 * | ND | ND |
| Calf rennet | 14.10 ± 0.95 | 6060.6 ± 0.71 * | 429.82 |
| Commercial Milk Type | Fat (g) | Fiber (g) | Protein (g) | CHO * (g) | Ratio + | Ca | Fe | P | Zn | Calories (Kcal) |
|---|---|---|---|---|---|---|---|---|---|---|
| [50] | 4.67 | 3.18 | 6.73 | 4.43 | 0.69 | 9.8 mg | 1.4 mg | 120.05 mg | NR | 79 |
| AdeS | 4.5 | 3.0 | 6.2 | 10.8 | 0.72 | 27% | 9% | NR | 22% | 109 |
| Soyalac | 1.0 | 2.0 | 2.0 | 10.0 | 0.50 | 19% | 21% | NR | NR | 60 |
| Soyapac | 6.0 | 0 | 6.6 | 9.1 | 0.90 | 25% | 13% | 28% | 25% | 117 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Muñoz, M.A.; Valdez-Solana, M.A.; Avitia-Domínguez, C.; Ramírez-Baca, P.; Candelas-Cadillo, M.G.; Aguilera-Ortíz, M.; Meza-Velázquez, J.A.; Téllez-Valencia, A.; Sierra-Campos, E. Utility of Milk Coagulant Enzyme of Moringa oleifera Seed in Cheese Production from Soy and Skim Milks. Foods 2017, 6, 62. https://doi.org/10.3390/foods6080062
Sánchez-Muñoz MA, Valdez-Solana MA, Avitia-Domínguez C, Ramírez-Baca P, Candelas-Cadillo MG, Aguilera-Ortíz M, Meza-Velázquez JA, Téllez-Valencia A, Sierra-Campos E. Utility of Milk Coagulant Enzyme of Moringa oleifera Seed in Cheese Production from Soy and Skim Milks. Foods. 2017; 6(8):62. https://doi.org/10.3390/foods6080062
Chicago/Turabian StyleSánchez-Muñoz, María Alejandra, Mónica Andrea Valdez-Solana, Claudia Avitia-Domínguez, Patricia Ramírez-Baca, María Guadalupe Candelas-Cadillo, Miguel Aguilera-Ortíz, Jorge Armando Meza-Velázquez, Alfredo Téllez-Valencia, and Erick Sierra-Campos. 2017. "Utility of Milk Coagulant Enzyme of Moringa oleifera Seed in Cheese Production from Soy and Skim Milks" Foods 6, no. 8: 62. https://doi.org/10.3390/foods6080062
APA StyleSánchez-Muñoz, M. A., Valdez-Solana, M. A., Avitia-Domínguez, C., Ramírez-Baca, P., Candelas-Cadillo, M. G., Aguilera-Ortíz, M., Meza-Velázquez, J. A., Téllez-Valencia, A., & Sierra-Campos, E. (2017). Utility of Milk Coagulant Enzyme of Moringa oleifera Seed in Cheese Production from Soy and Skim Milks. Foods, 6(8), 62. https://doi.org/10.3390/foods6080062





