Effects of Two-Step Transamidation of Wheat Semolina on the Technological Properties of Gluten
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quality Characteristics of Durum Wheat Semolina
2.2. Transamidation Reaction of Durum Wheat Semolina
2.3. Biochemical Analysis of Transamidation of Wheat Semolina
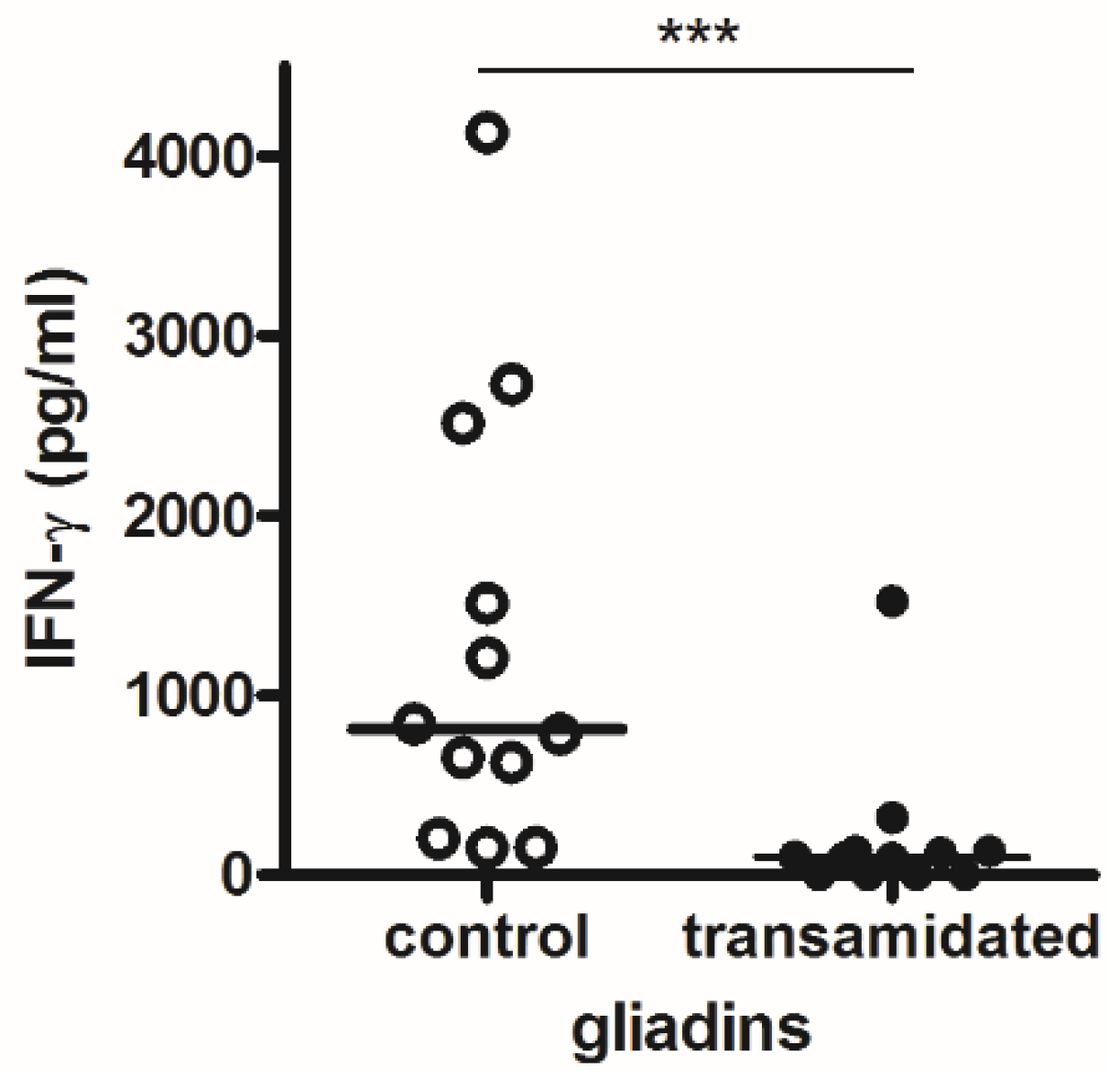
2.4. Immunological Analysis of Transamidation of Wheat Semolina
2.5. Pasta Manufacturing Procedure
2.6. Statistical Evaluation
3. Results
3.1. Qualitative Features of Semolina and Dried Pasta
3.2. Pilot-Scale Production of Transamidated Semolina
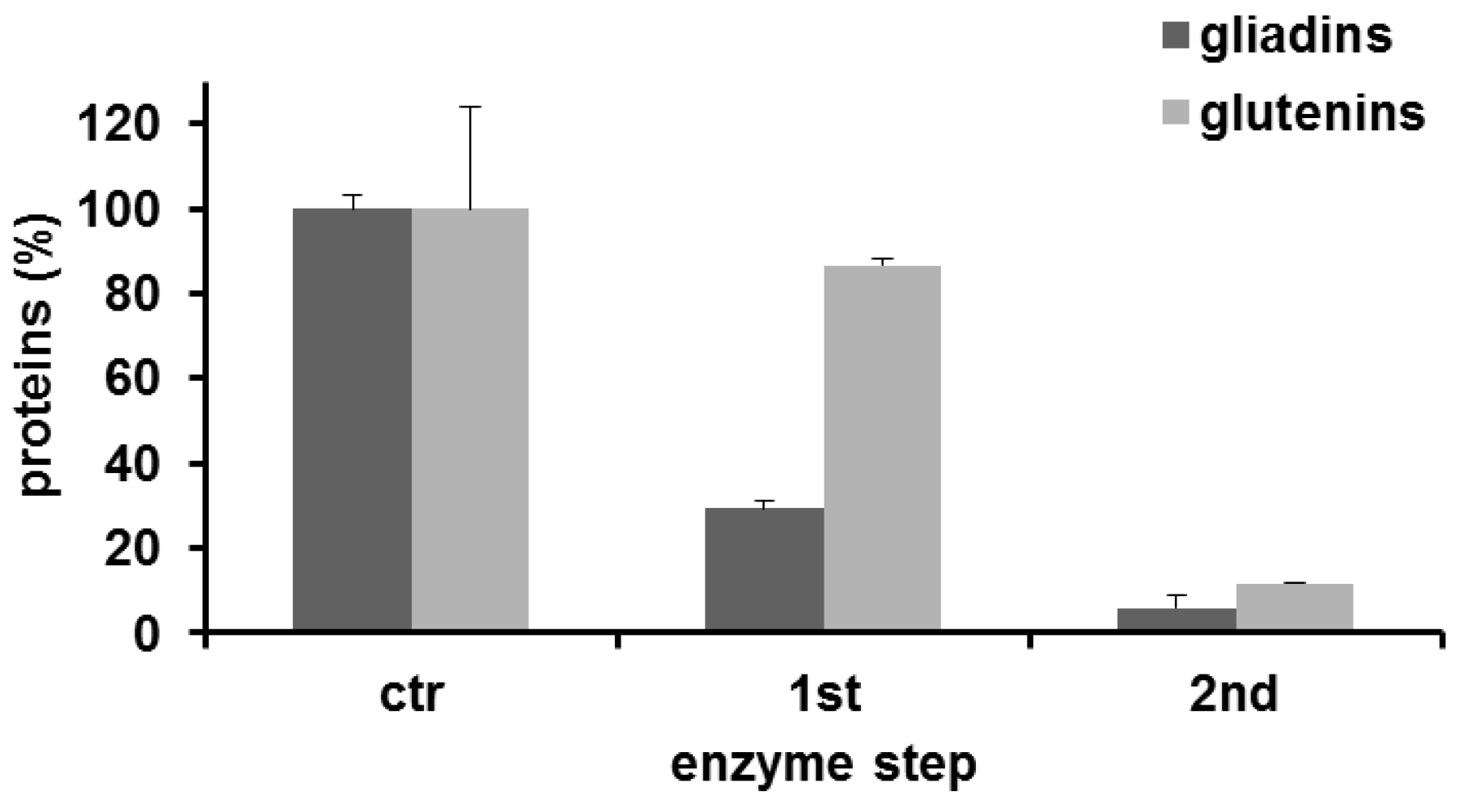
3.3. Analysis of Transamidated Prolamins
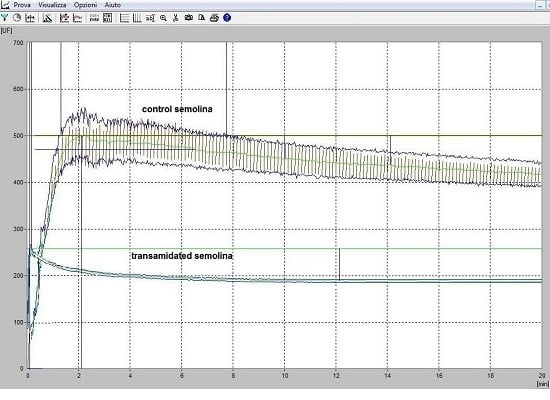
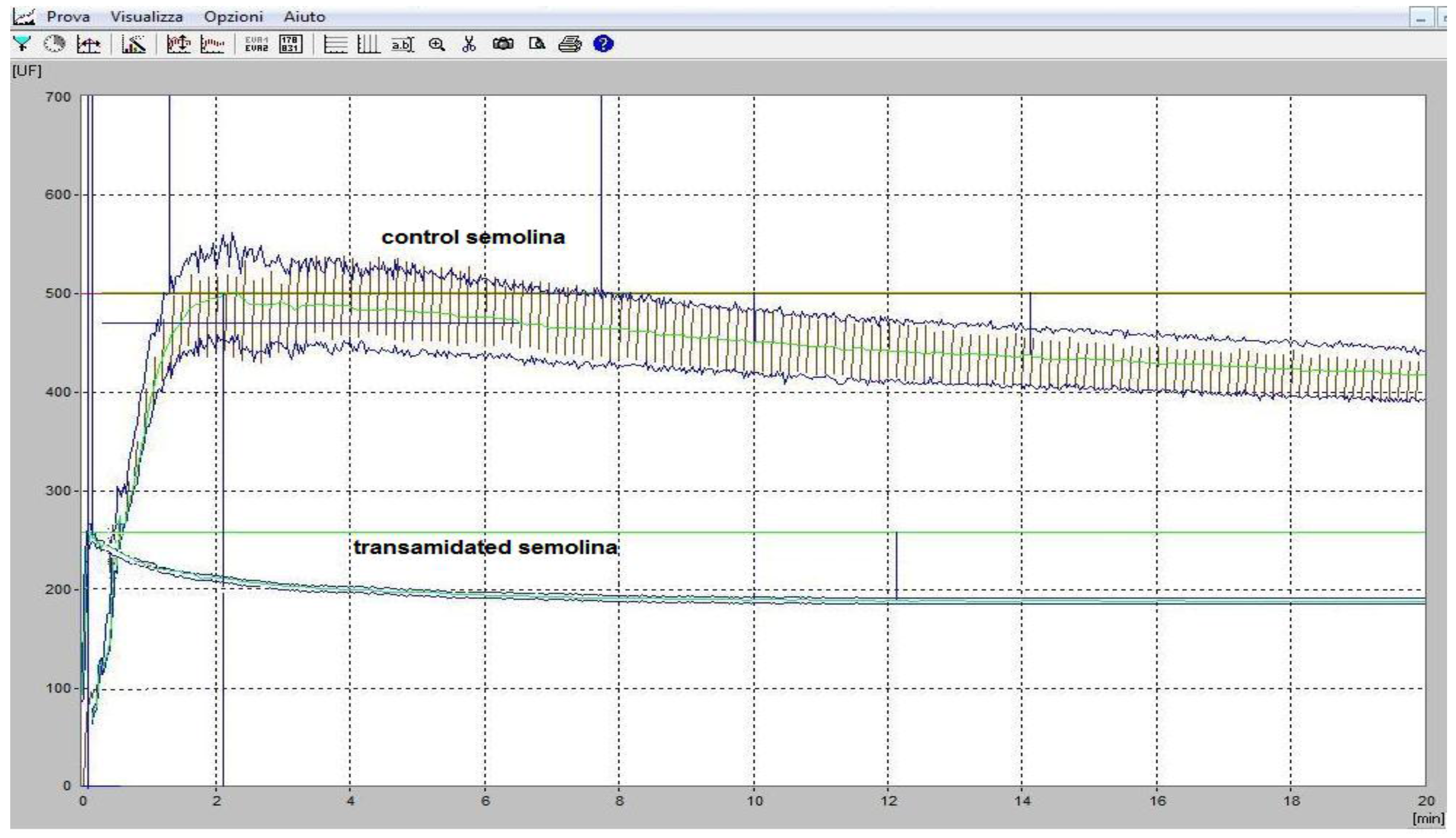
3.4. The Rheological Properties of Transamidated Semolina
3.5. The Technological Properties of Transamidated Semolina
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CD | coeliac disease |
| GF | gluten free |
| K-C2H5 | lysine ethyl ester |
| PEPs | prolyl endopeptidases |
| mTG | microbial transglutaminase |
| tTG | tissue transglutaminase |
References
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Kyle, R.A.; Kaplan, E.L.; Johnson, D.R.; Page, W.; Erdtmann, F.; Brantner, T.L.; Kim, W.R.; Phelps, T.K.; Lahr, B.D.; et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009, 137, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Lohi, S.; Mustalahti, K.; Kaukinen, K.; Laurila, K.; Collin, P.; Rissanen, H.; Lohi, O.; Bravi, E.; Gasparin, M.; Reunanen, A.; et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol. Ther. 2007, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Molberg, O.; Mcadam, S.N.; Körner, R.; Quarsten, H.; Kristiansen, C.; Madsen, L.; Fugger, L.; Scott, H.; Norén, O.; Roepstorff, P.; et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998, 4, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Molberg, O.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 27, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Marti, T.; Sollid, L.M.; Gray, G.M.; Khosla, C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: Implications for coeliac sprue. Biochem. J. 2004, 383, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Gobbetti, M.; Auricchio, R.; Di Mase, R.; Landolfo, F.; Paparo, F.; Di Cagno, R.; De Angelis, M.; Rizzello, C.G.; Cassone, A.; et al. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin. Gastroenterol. Hepatol. 2011, 9, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, T.; Ozaki, H.; Takao, T.; Kawajiri, H.; Ide, H.; Motoki, M.; Shimonishi, Y. Primary structure of microbial transglutaminase from Streptoverticillium sp. strains-8112. J. Biol. Chem. 1993, 268, 11565–11572. [Google Scholar] [PubMed]
- Yokoyama, K.; Nio, N.; Kikuchi, Y. Properties and applications of microbial transglutaminase. Appl. Microbiol. Biotechnol. 2004, 64, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, J.A.; Fayle, S.E.; Wilson, A.J.; Newberry, M.; Ross, M.P.; Kavale, S. Dough properties and crumb strength of white pan bread as affected by microbial transglutaminase. J. Food Sci. 1998, 63, 472–475. [Google Scholar] [CrossRef]
- Iwami, K.; Yasumoto, K. Amine-binding capacities of food proteins in transglutaminase reaction and digestibility of wheat gliadin with ϵ-attached lysine. J. Sci. Food Agric. 1986, 37, 495–503. [Google Scholar] [CrossRef]
- Fink, M.L.; Chung, S.I.; Folk, J.E. gamma-Glutamylamine cyclotransferase: Specificity toward epsilon-(l-gamma-glutamyl)-l-lysine and related compounds. Proc. Natl. Acad. Sci. USA 1980, 77, 4564–4568. [Google Scholar] [CrossRef] [PubMed]
- Gianfrani, C.; Siciliano, R.A.; Facchiano, A.M.; Camarca, A.; Mazzeo, M.F.; Costantini, S.; Salvati, V.M.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; et al. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology 2007, 133, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, E.; Bergamo, P.; Maurano, F.; Bozzella, G.; Luongo, D.; Mazzarella, G.; Rotondi Aufiero, V.; Iaquinto, G.; Rossi, M. Selective inhibition of the gliadin-specific, cell-mediated immune response by transamidation with microbial transglutaminase. J. Leukoc. Biol. 2013, 93, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kick, F.; Belitz, H.D.; Wieser, H.; Kieffer, R. Comparative studies of the Osborne protein fraction of wheat varieties with different dough and baking properties. Z. Lebensm. Unters. Forsch. 1992, 195, 437–442. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cheng, S.; Baisch, J.; Krco, C.; Savarirayan, S.; Hanson, J.; Hodgson, K.; Smart, M.; David, C. Expression and function of HLA-DQ8 (DQA1*0301/DQB1*0302) genes in transgenic mice. Eur. J. Immunogenet. 1996, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- D’Egidio, M.G.; De Stefanis, E.; Fortini, S.; Galterio, G.; Nardi, S.; Sgrulletta, D.; Bozzini, A. Standardization of cooking quality analysis in macaroni and pasta products. Cereal Foods World 1982, 27, 367. [Google Scholar]
- Tye-Din, J.A.; Stewart, J.A.; Dromey, J.A.; Beissbarth, T.; van Heel, D.A.; Tatham, A.; Henderson, K.; Mannering, S.I.; Gianfrani, C.; Jewell, D.P.; et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010, 2, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.; Dorsch, S.; Otterbach, J.T.; Robenek, I.R.; Wolf, S.; Fuchsbauer, H.L. Bacterial pro-transglutaminase from Streptoverticillium mobaraense-purification, characterisation and sequence of the zymogen. Eur. J. Biochem. 1998, 257, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.F.; Bonavita, R.; Maurano, F.; Bergamo, P.; Siciliano, R.A.; Rossi, M. Biochemical modifications of gliadins induced by microbial transglutaminase on wheat flour. Biochim. Biophys. Acta 2013, 1830, 5166–5174. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Salvati, V.M.; Iaquinto, G.; Stefanile, R.; Capobianco, F.; Luongo, D.; Bergamo, P.; Maurano, F.; Giardullo, N.; Malamisura, B.; et al. Reintroduction of gluten following flour transamidation in adult celiac patients: A randomized, controlled clinical study. Clin. Dev. Immunol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.P.; Skerritt, J.H. The glutenin macropolymer of wheat flour doughs: Structure and function perspectives. Trends Food Sci. Technol. 1999, 10, 247–253. [Google Scholar] [CrossRef]
| Parameters | Control Pasta | Experimental Pasta |
|---|---|---|
| Ø (mm) fresh pasta after extrusion | nd | 1.6 (±0.0) |
| Ø (mm) raw dried pasta | 1.3 (±0.0) | 1.3 (±0.0) |
| cooking time until disappearance of the nucleus (min) | 7 | 6 |
| pasta weight after cooking (t 0’) (g) | 280 ± 0.6 | 200 ± 0.5 |
| pasta weight after cooking (t 9’) (g) | 270 ± 0.3 | 193 ± 0.2 |
| springiness | 70 (±5) | 70 (±3) |
| firmness | 75 (±5) | 75 (±5) |
| stickiness (t 9′) | 70 (±5) | 70 (±5) |
| taste (t 9′) | 70 (±5) | 90 (±3) |
| appearance (t 9′) | 70 (±5) | 80 (±3) |
| flavor (t 9′) | 70 (±5) | 75 (±5) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscaritolo, S.; Treppiccione, L.; Ottombrino, A.; Rossi, M. Effects of Two-Step Transamidation of Wheat Semolina on the Technological Properties of Gluten. Foods 2016, 5, 49. https://doi.org/10.3390/foods5030049
Moscaritolo S, Treppiccione L, Ottombrino A, Rossi M. Effects of Two-Step Transamidation of Wheat Semolina on the Technological Properties of Gluten. Foods. 2016; 5(3):49. https://doi.org/10.3390/foods5030049
Chicago/Turabian StyleMoscaritolo, Salvatore, Lucia Treppiccione, Antonio Ottombrino, and Mauro Rossi. 2016. "Effects of Two-Step Transamidation of Wheat Semolina on the Technological Properties of Gluten" Foods 5, no. 3: 49. https://doi.org/10.3390/foods5030049