Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157:H7 in Laboratory Medium
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacterial Strains and Inoculm Prpearation
2.2. Experimental Design
2.3. Measuring Bacterial Growth
2.4. Bacterial Enumeration
2.5. Statistical Analysis
3. Results and Discussion
3.1. Antibacterial Activity of Armoise and Clove Bud EOs against E. coli O157:H7 in BHI Medium
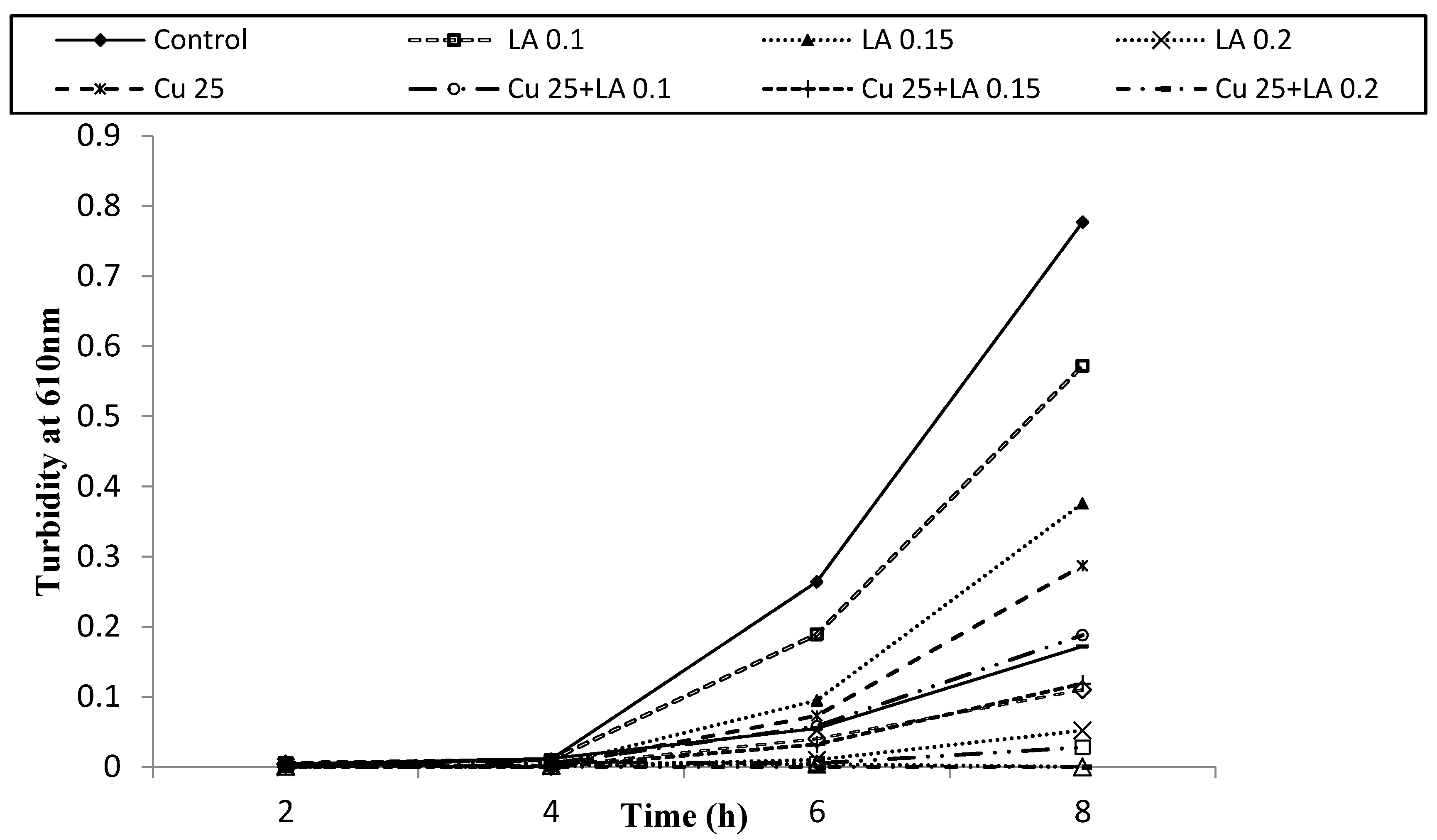
3.2. Antibacterial Activity of Copper (Cu) and Lactic Acid (LA) against E. coli O157:H7 in BHI Medium
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). CDC Estimates of Foodborne Illness in the United States, CDC 2011 Estimates: Factsheet Findings Updated on April 2013. Available online: http://www.cdc.gov/foodborneburden/pdfs/factsheet_a_findings_updated4-13.pdf (accessed on 22 February 2016).
- Abdulmumeen, H.A.; Risikat, A.N.; Sururah, A.R. Food: Its preservatives, additives and applications. Int. J. Chem. Biol. Sci. 2012, 1, 36–47. [Google Scholar]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A.; Abu Hasfa, S.H.; Smqadri, S.Q.; Haik, Y. Antimicrobial activity of copper alone and in combination with lactic acid against Escherichia coli O157: H7 in laboratory medium and on the surface of lettuce and tomatoes. J. Pathog. 2011, 2011. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Yang, H.; Seo, C.W. Antimicrobial activity of lactic acid and copper on growth of salmonella and Escherichia coli O157: H7 in laboratory medium and carrot juice. Food Chem. 2008, 109, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Davidson, P.M.; Zhong, Q. Antimicrobial activity of nanodispersed thymol in tryptic soy broth. J. Food Prot. 2013, 76, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Imelouane, B.; El Bachiri, A.; Ankit, M.; Khedid, K.; Wathelet, J.-P.; Amhamdi, H. Essential oil composition and antimicrobial activity of Artemisia herba-alba Asso grown in Morocco. Banat. J. Biotechnol. 2010, 1, 48–55. [Google Scholar]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [PubMed]
- Al-Holy, M.A.; Castro, L.F.; Al-Qadiri, H. Inactivation of Cronobacter spp. (Enterobacter sakazakii) in infant formula using lactic acid, copper sulfate and monolaurin. Lett. Appl. Microbial. 2010, 50, 246–251. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Hazard. Analysis and Critical Control Point (HACCP); Procedures for the Safe and Sanitary Processing and Importing of Juice; Final Rule (21 CFR Part 120); Federal Register US Food and Drug Administration: Washington, DC, USA, 2001; Volume 66, pp. 6138–6202.
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
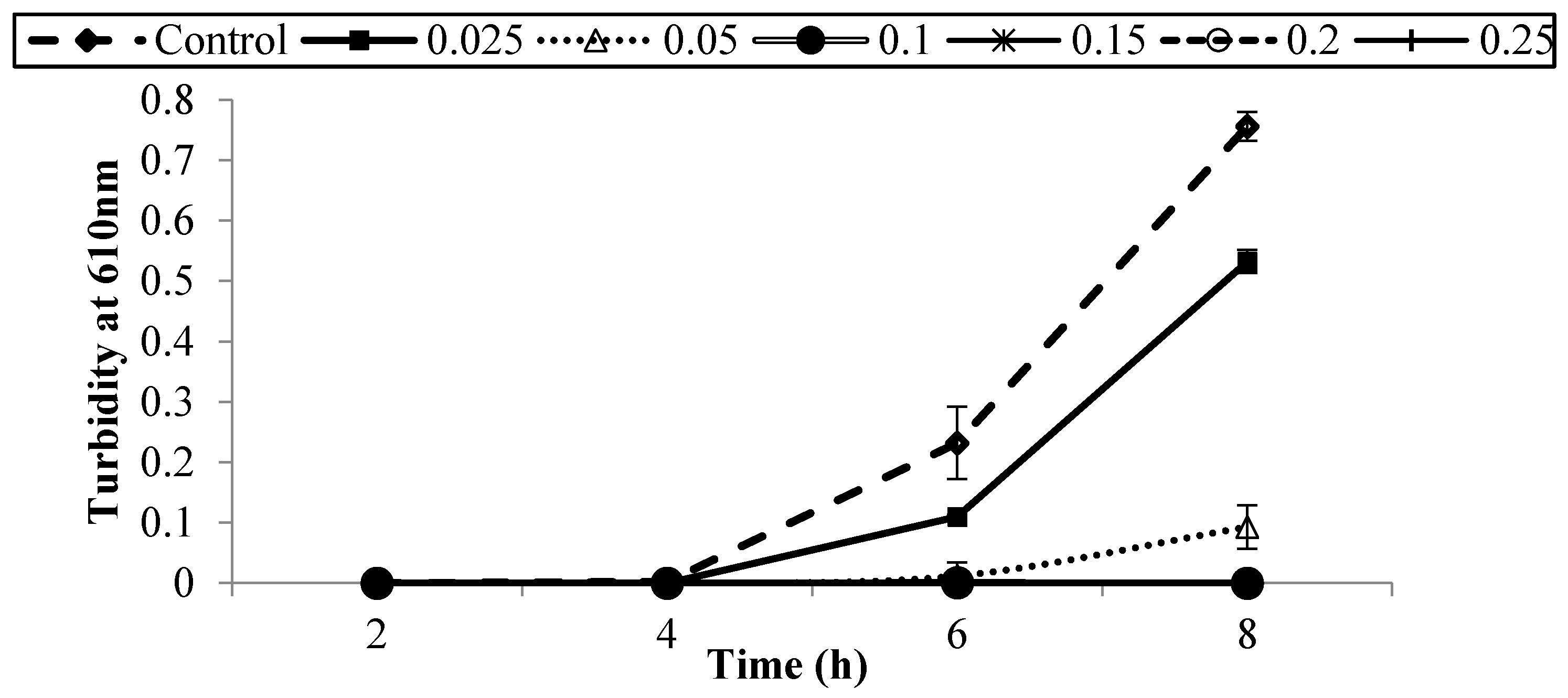



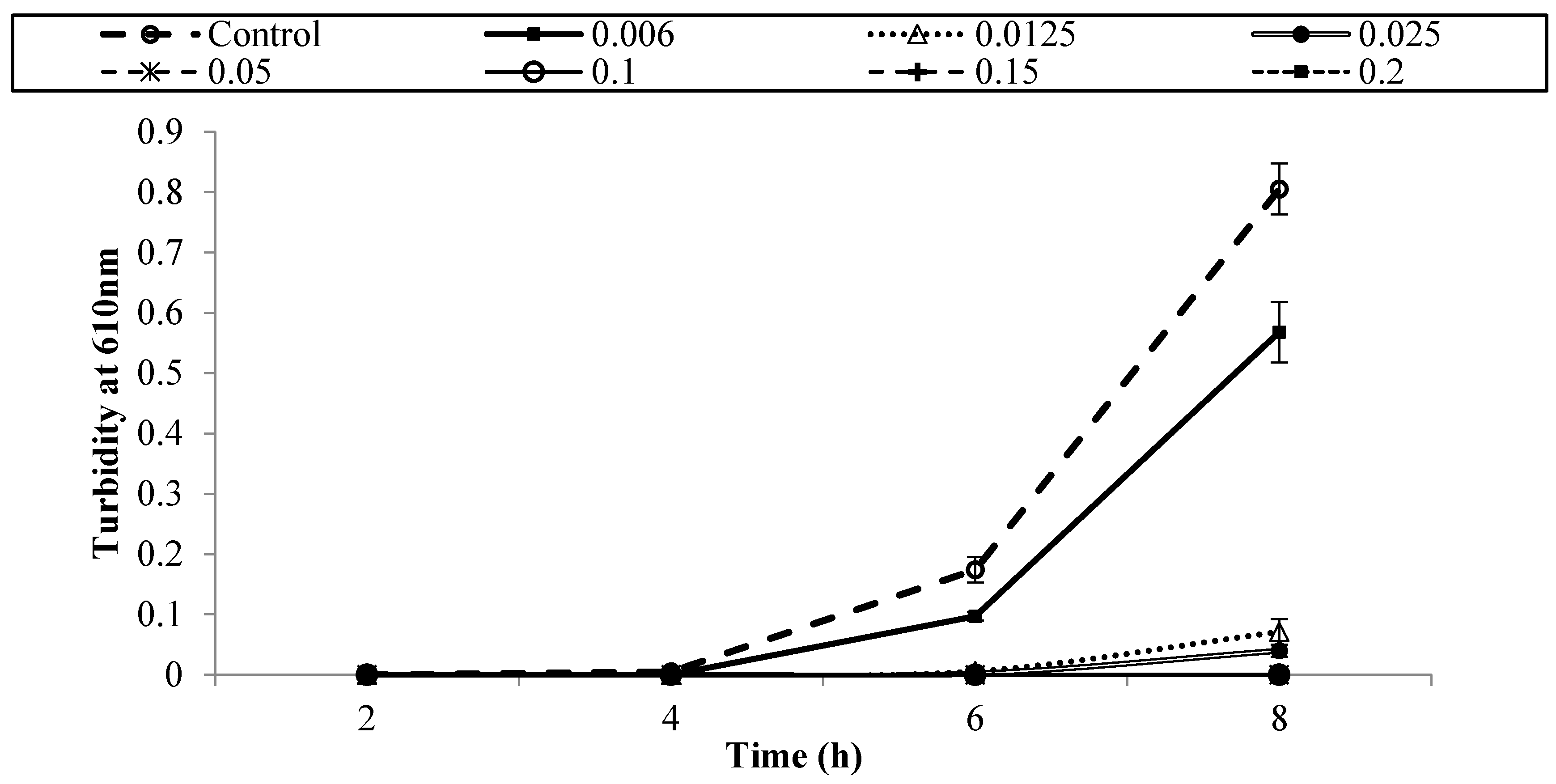
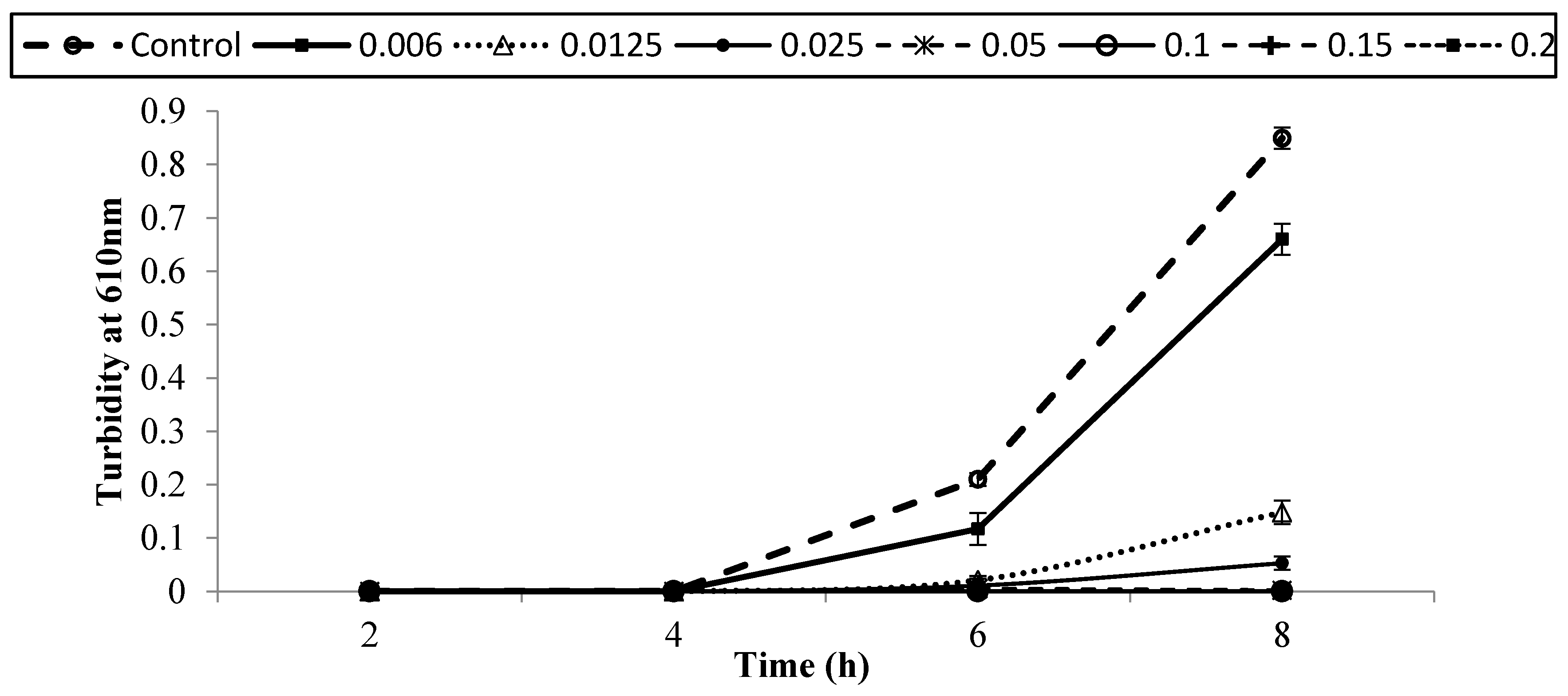
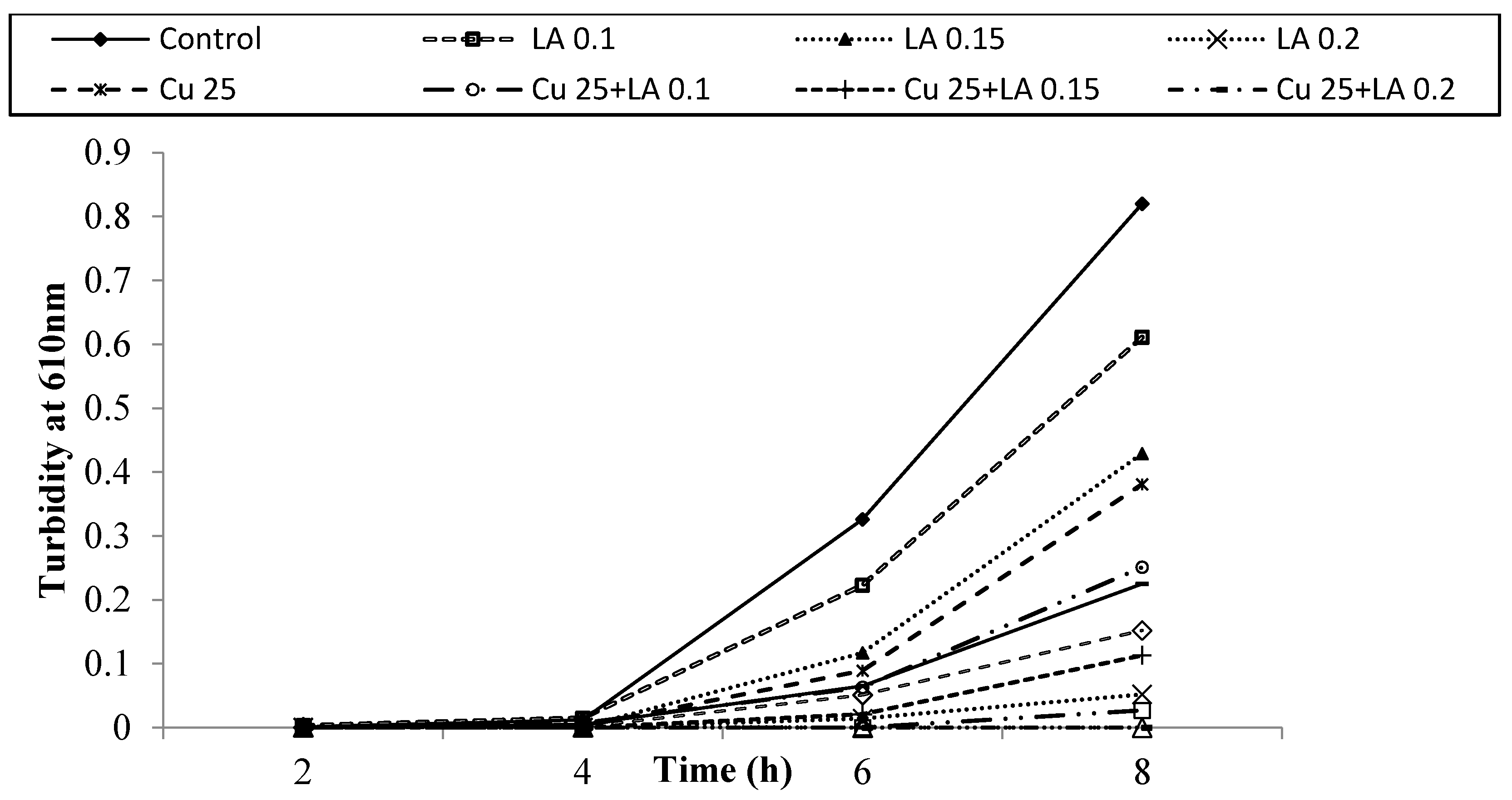

| Treatment (% v/v) | Population of E. coli O157:H7 Strains (log CFU/mL) | ||
|---|---|---|---|
| ATCC 700599 | ATCC 51659 | ATCC 43895 | |
| Control | 9.01 ± 0.11 a | 8.82 ± 0.11 a | 8.41 ± 0.53 a |
| EO 0.025 | 8.26 ± 0 b | 8.68 ± 0.26 a | 8.22 ± 0.29 b |
| EO 0.05 | 8.1 ± .005 b | 7.96 ± 0.18 b | 6.93 ± 0.28 c |
| EO 0.1 | <1 c | 2.34 ± 0.19 c | <1 d |
| EO 0.15 | <1 c | <1 d | <1 d |
| EO 0.2 | <1 c | <1 d | <1 d |
| EO 0.25 | <1 c | <1 d | <1 d |
| Treatment (% v/v) | Population of E. coli O157:H7 Strains (log CFU/mL) | ||
|---|---|---|---|
| ATCC 700599 | ATCC 51659 | ATCC 43895 | |
| Control | 8.48 ± 0.05 a | 8.52 ± 0.47 a | 7.23 ± 0.26 a |
| EO 0.006 | 7.71 ± 0.04 b | 7.22 ± 0.35 b | 6.74 ± 0.13 a |
| EO 0.0125 | 7.06 ± 0.02 c | 5.2 ± 0.14 c | 4.66 ± 0.19 b |
| EO 0.025 | 3.06 ± 0.41 d | 4.6 ± 0.11 d | 4.23 ± 0.59 b |
| EO 0.05 | 2.27 ± 0.04 e | 1.94 ± 0.02 e | 1.89 ± 0.01 c |
| EO 0.1 | <1 f | <1 f | <1 d |
| EO 0.15 | <1 f | <1 f | <1 d |
| EO 0.2 | <1 f | <1 f | <1 d |
| Treatment | Population of E. coli O157:H7 Strains (log CFU/mL) | ||
|---|---|---|---|
| ATCC 700599 | ATCC 51659 | ATCC 43895 | |
| CONTROL | 8.79 ± 0.21 a | 8.70 ± 0.25 a | 8.74 ± 0.23 a |
| LA 0.1 | 8.55 ± 0.23 a | 8.18 ± 0.56 ab | 8.35 ± 0.19 ab |
| LA 0.15 | 8.1 ± 0.30 ab | 7.46 ± 0.27 bc | 6.55 ± 0.16 d |
| LA 0.2 | 5.69 ± 0.08 e | 5.49 ± 0.38 ef | 6.10 ± 0.41 de |
| Cu 25 ppm | 7.62 ± 0.14 bc | 6.98 ± 0.63 cd | 7.77 ± 0.32 bc |
| Cu 25 + LA 0.1 | 6.98 ± 0.18 c | 6.40 ± 0.20 cde | 7.28 ± 0.41 c |
| Cu 25 + LA 0.15 | 6.01 ± 0.48 de | 6.18 ± 0.27 de | 5.46 ± 0.23 ef |
| Cu 25 + LA 0.2 | 4.58 ± 0.26 f | 4.92 ± 0.21 f | 4.58 ± 0.26 g |
| Cu 50 | 7.16 ± 0.61 c | 6.98 ± 0.81 bd | 6.47 ± 0.15 d |
| Cu 50 + LA 0.1 | 6.79 ± 0.40 cd | 5.31 ± 1.19 ef | 5.01 ± 0.47 fg |
| Cu 50 + LA 0.15 | 5.28 ± 0.49 ef | 4.80 ± 0.10 f | 3.24 ± 0.15 h |
| Cu 50 + LA 0.2 | 1.61 ± 0.67 g | 1.50 ± 0.92 g | 0.84 ± 0.20 i |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bor, T.; Gyawali, R.; Ibrahim, S.A. Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157:H7 in Laboratory Medium. Foods 2016, 5, 14. https://doi.org/10.3390/foods5010014
Bor T, Gyawali R, Ibrahim SA. Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157:H7 in Laboratory Medium. Foods. 2016; 5(1):14. https://doi.org/10.3390/foods5010014
Chicago/Turabian StyleBor, Tarik, Rabin Gyawali, and Salam A. Ibrahim. 2016. "Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157:H7 in Laboratory Medium" Foods 5, no. 1: 14. https://doi.org/10.3390/foods5010014
APA StyleBor, T., Gyawali, R., & Ibrahim, S. A. (2016). Evaluating the Effectiveness of Essential Oils and Combination of Copper and Lactic Acid on the Growth of E. coli O157:H7 in Laboratory Medium. Foods, 5(1), 14. https://doi.org/10.3390/foods5010014






