Iron Bioavailability and Provitamin A from Sweet Potato- and Cereal-Based Complementary Foods
Abstract
:1. Introduction
2. Experimental Section
2.1. Complementary Food Samples
2.2. In Vitro Digestion/Caco-2 Model to Measure Iron Bioavailability
2.3. Compositional Analysis: β-Carotene, Vitamin C, Fructose, Iron, Total Polyphenols and Phytate
2.4. Statistical Analysis
3. Results
3.1. Iron Bioavailability of Tested Complementary Foods as Provided

3.2. Iron Bioavailability of OFSP ComFa Compared with CFSP ComFa Fortified with Fructose and β-Carotene
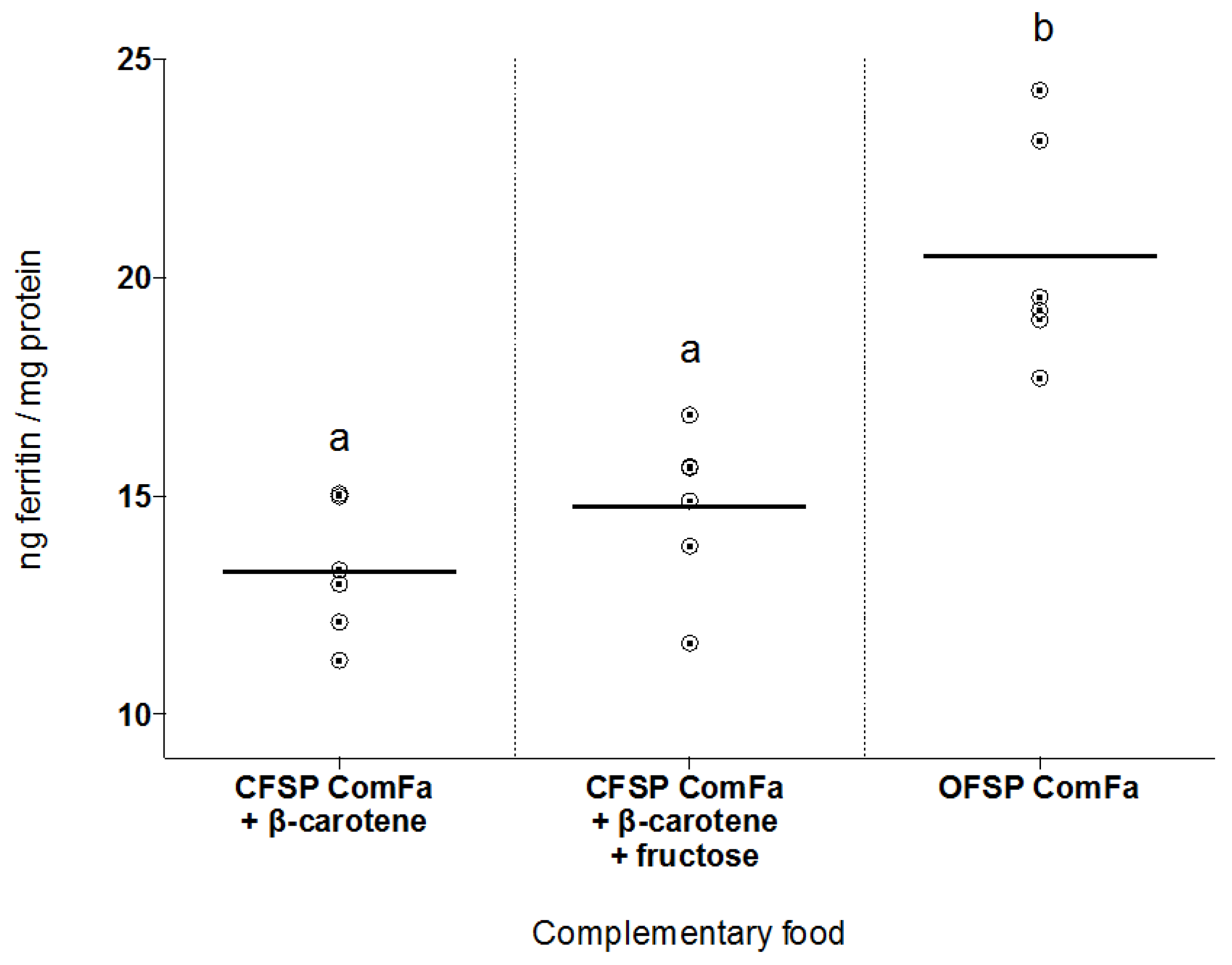
3.3. Concentrations of Total Iron and Iron Absorption Inhibitors in Tested Foods
| Complementary Food | Inhibitor (/100 g) | Enhancer (/100 g) | Iron (mg/100 g) | ||||
|---|---|---|---|---|---|---|---|
| Phytate (mg) | Total Polyphenols (mg GA equ) | Total Dietary Fibre (g) | Ascorbic Acid (mg) | β-Carotene (µg) | Fructose (g) | ||
| OFSP ComFa | 229.85 ± 19.36 b | 466.28 ± 9.68 a | 12.28 ± 1.04 a | 32.48 ± 0.48 b | 13353 ± 1792.60 a | 7.15 ± 0.17 a | 7.76 ± 1.22 a |
| CFSP ComFa | 78.62 ± 3.50 c | 466.42 ± 34.98 a | 10.05 ± 0.18 b | 37.40 ± 0.61 a,b | 1263 ± 22.30 b | 3.09 ± 0.07 b | 7.26 ± 0.08 a |
| Weanimix | 438.10 ± 8.58 a | 263.68 ± 17.82 b | 6.90 ± 0.64 c | BDL | 34 ± 11.77 b | BDL | 6.53 ± 1.55 a |
| Cerelac | 66.92 ± 3.96 c | 213.45 ± 29.92 b | 1.48 ± 0.50 d | 53.11± 12.07 a | 66 ± 15.83 b | BDL | 8.85 ± 0.17 a |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.10 |
3.4. Concentrations of Iron Absorption Enhancers in Tested Foods
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Nutrition Health Topics: Iron deficiency anaemia; Vitamin A deficiency. Available online: http://www.who.int/nutrition/topics/en/ (accessed on 6 June 2015).
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B. Child development in developing countries 1: Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef]
- Khan, Y.; Bhutta, Z.A. Nutritional Deficiencies in the Developing World: Current Status and Opportunities for intervention. Pediatr. Clin. N. Am. 2010, 57, 1409–1441. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.K.; Hardacre, A.; Mutukumira, A.N.; Weber, J.L.; Brough, L.; Coad, J. A household-level sweet potato-based infant food to complement vitamin A supplementation initiatives. Matern. Child Nutr. 2012, 8, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.K.; Coad, J. Orange-fleshed sweet potato-based infant food is a better source of dietary vitamin A than a maize-legume blend as complementary food. Food Nutr. Bull. 2014, 35, 51–59. [Google Scholar] [PubMed]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010, 31, S134–S146. [Google Scholar] [PubMed]
- Roos, N.; Sorensen, J.C.; Sorensen, H.; Rasmussen, S.K.; Briend, A.; Yang, Z.; Huffman, S.L. Screening for anti-nutritional compounds in complementary foods and food aid products for infants and young children. Matern. Child Nutr. 2013, 9 (Suppl. 1), 47–71. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.K.; Weber, J.L.; Brough, L.; Hardacre, A.; Mutukumira, A.N.; Coad, J. Complementary food blends and malnutrition among infants in Ghana—A review and a proposed solution. Sci. Res. Essays 2012, 7, 972–988. [Google Scholar]
- Mahmoud, A.H.; Anany, A.M.E. Nutritional and sensory evaluation of a complementary food formulated from rice, faba beans, sweet potato flour, and peanut oil. Food Nutr. Bull. 2014, 35, 403–413. [Google Scholar] [PubMed]
- Amagloh, F.K.; Mutukumira, A.N.; Brough, L.; Weber, J.L.; Hardacre, A.; Coad, J. Carbohydrate composition, viscosity, solubility, and sensory acceptance of sweet potato- and maize-based complementary foods. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Phillippy, B.Q.; Bland, J.M.; Evens, T.J. Ion chromatography of phytate in roots and tubers. J. Agric. Food Chem. 2003, 51, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Phillippy, B.Q. Inositol phosphates in foods. Adv. Food Nutr. Res. 2003, 45, 1–60. [Google Scholar] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- García-Casal, M.N. Carotenoids increase iron absorption from cereal-based food in the human. Nutr. Res. 2006, 26, 340–344. [Google Scholar] [CrossRef]
- Christides, T.; Sharp, P. Sugars increase non-heme iron bioavailability in human epithelial intestinal and liver cells. PLoS ONE 2013, 8, e83031. [Google Scholar] [CrossRef] [PubMed]
- Lung’aho, M.G.; Glahn, R.P. In vitro estimates of iron bioavailability in some Kenyan complementary foods. Food Nutr. Bull. 2009, 30, 145–152. [Google Scholar] [PubMed]
- Glahn, R. The use of Caco-2 cells in defining nutrient bioavailability: Application to iron bioavailability of foods. In Designing Functional Foods: Measuring and Controlling Food Structure Breakdown and Nutrient Absorption; McClements, D.J., Decker, E.D., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 340–361. [Google Scholar]
- Ojong, P.B.; Njiti, V.; Guo, Z.B.; Gao, M.; Besong, S.; Barnes, S.L. Variation of flavonoid content among sweet potato accessions. J. Am. Soc. Hortic. Sci. 2008, 133, 819–824. [Google Scholar]
- Teow, C.C.; Truong, V.D.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and beta-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Tako, E.; Beebe, S.E.; Reed, S.; Hart, J.J.; Glahn, R.P. Polyphenolic compounds appear to limit the nutritional benefit of biofortified higher iron black bean (Phaseolus vulgaris L.). Nutr. J. 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Tako, E.; Reed, S.M.; Budiman, J.; Hart, J.J.; Glahn, R.P. Higher iron pearl millet (Pennisetum glaucum L.) provides more absorbable iron that is limited by increased polyphenolic content. Nutr. J. 2015, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Kim, E.Y.; Han, O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. Nutr. J. 2010, 140, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.P.; Wortley, G.M.; South, P.K.; Miller, D.D. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: Studies using an in vitro digestion/Caco-2 cell model. J. Agric. Food Chem. 2002, 50, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Rossander, L.; Hallberg, L. Iron absorption and phenolic compounds: Importance of different phenolic structures. Eur. J. Clin Nutr. 1989, 43, 547–557. [Google Scholar] [PubMed]
- Reddy, M.B.; Hurrell, R.F.; Cook, J.D. Estimation of nonheme-iron bioavailability from meal composition. Am. J. Clin. Nutr. 2000, 71, 937–943. [Google Scholar] [PubMed]
- Tuntipopipat, S.; Judprasong, K.; Zeder, C.; Wasantwisut, E.; Winichagoon, P.; Charoenkiatkul, S.; Hurrell, R.; Walczyk, T. Chili, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal. J. Nutr. 2006, 136, 2970–2974. [Google Scholar] [PubMed]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion Caco-2 cell culture model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [PubMed]
- Lung’aho, M.G.; Glahn, R.P. Use of white beans instead of red beans may improve iron bioavailability from a Tanzanian complementary food mixture. Int. J. Vitam. Nutr. Res. 2010, 80, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.K.; Coad, J. Weaning food [audio clip of an interview (online)]. In Our Changing World; Beran, R., Ed.; Radio New Zealand National: Wellington, New Zealand, 2012; Available online: http://www.radionz.co.nz/national/programmes/afternoons/audio/2535256/our-changing-world-weaning-food (accessed on 11 October 2012).
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron bioavailability from commercially available iron supplements. Eur. J. Nutr. 2014. [Google Scholar] [CrossRef]
- Amagloh, F.K.; Brough, L.; Weber, J.L.; Mutukumira, A.N.; Hardacre, A.; Coad, J. Sweetpotato-based complementary food would be less inhibitory on mineral absorption than a maize-based infant food assessed by compositional analysis. Int. J. Food Sci. Nutr. 2012, 63, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Use of ferrous fumarate to fortify foods for infants and young children. Nutr. Rev. 2010, 68, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Miret, S.; Simpson, R.J.; McKie, A.T. Physiology and molecular biology of dietary iron absorption. Annu. Rev. Nutr. 2003, 23, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. The role of diet- and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Interaction of iron with other nutrients. Food Nutr. Bull. Suppl. 2007, 28, s77–s100. [Google Scholar]
- Lestienne, I.; Besancon, P.; Caporiccio, B.; Lullien-Pellerin, V.; Treche, S. Iron and zinc in vitro availability in pearl millet flours (Pennisetum glaucum) with varying phytate, tannin, and fiber contents. J. Agric. Food Chem. 2005, 53, 3240–3247. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [PubMed]
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; MacPhail, P.; Schmidt, U.; Tal, A.; Mayet, F. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [PubMed]
- Petry, N.; Egli, I.; Campion, B.; Nielsen, E.; Hurrell, R. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J. Nutr. 2013, 143, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Abizari, A.R.; Moretti, D.; Schuth, S.; Zimmermann, M.B.; Armar-Klemesu, M.; Brouwer, I.D. Phytic acid-to-iron molar ratio rather than polyphenol concentration determines iron bioavailability in whole-cowpea meal among young women. J. Nutr. 2012, 142, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Rossander, L.; Skanberg, A.B. Phytates and the inhibitory effect of bran on iron absorption in man. Am. J. Clin. Nutr. 1987, 45, 988–996. [Google Scholar] [PubMed]
- Hallberg, L.; Brune, M.; Rossander, L. Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [PubMed]
- Hallberg, L.; Brune, M.; Rossander, L. Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum. Nutr. Appl. Nutr. 1986, 40, 97–113. [Google Scholar] [PubMed]
- Ma, Q.; Kim, E.Y.; Lindsay, E.A.; Han, O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011, 76, H143–H150. [Google Scholar] [CrossRef] [PubMed]
- Lartey, A.; Manu, A.; Brown, K.H.; Peerson, J.M.; Dewey, K.G. A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. Am. J. Clin. Nutr. 1999, 70, 391–404. [Google Scholar] [PubMed]
- Fairweather-Tait, S.; Lynch, S.; Hotz, C.; Hurrell, R.; Abrahamse, L.; Beebe, S.; Bering, S.; Bukhave, K.; Glahn, R.; Hambidge, M.; et al. The usefulness of in vitro models to predict the bioavailability of iron and zinc: A consensus statement from the Harvestplus expert consultation. Int. J. Vitam. Nutr. Res. 2005, 75, 371–374. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christides, T.; Amagloh, F.K.; Coad, J. Iron Bioavailability and Provitamin A from Sweet Potato- and Cereal-Based Complementary Foods. Foods 2015, 4, 463-476. https://doi.org/10.3390/foods4030463
Christides T, Amagloh FK, Coad J. Iron Bioavailability and Provitamin A from Sweet Potato- and Cereal-Based Complementary Foods. Foods. 2015; 4(3):463-476. https://doi.org/10.3390/foods4030463
Chicago/Turabian StyleChristides, Tatiana, Francis Kweku Amagloh, and Jane Coad. 2015. "Iron Bioavailability and Provitamin A from Sweet Potato- and Cereal-Based Complementary Foods" Foods 4, no. 3: 463-476. https://doi.org/10.3390/foods4030463






