An Investigation of the Complexity of Maillard Reaction Product Profiles from the Thermal Reaction of Amino Acids with Sucrose Using High Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Methods
2.2.1. ESI-TOF-MS
2.2.2. FT ICR-MS
3. Results and Discussion
3.1. Reactions of Amino Acids with Disaccharides
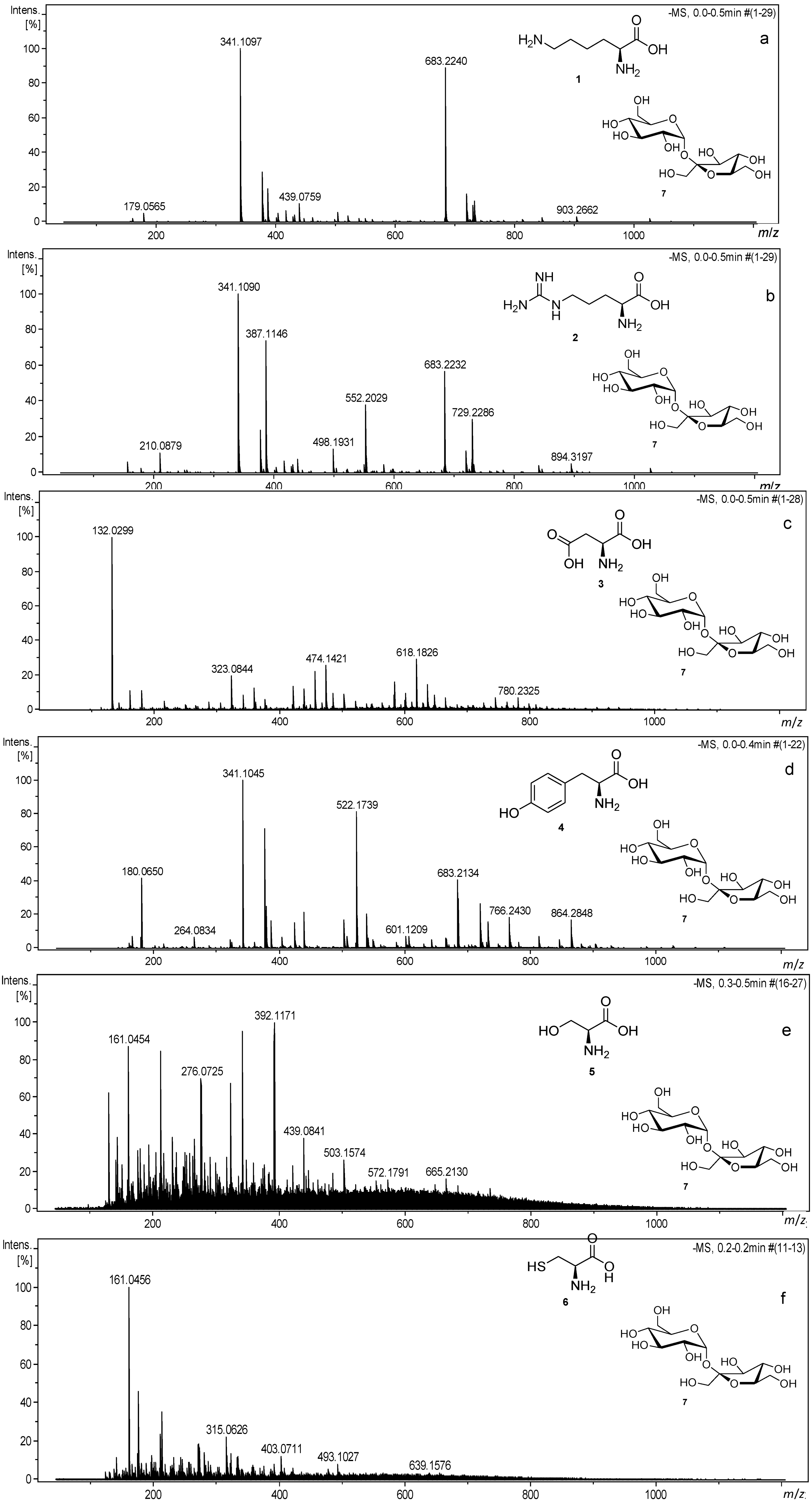
| No. | Sample | Number of Signals with S/N > 10 | Number of Signals with S/N > 3 |
|---|---|---|---|
| 1 | Arginine-sucrose a | 126 | 714 |
| 2 | Lysine-sucrose a | 221 | 670 |
| 3 | Aspartic acid-sucrose a | 297 | 796 |
| 4 | Tyrosine-sucrose a | 420 | 916 |
| 5 | Serine-sucrose a | 691 | 885 |
| 6 | Cysteine-sucrose a | 495 | 791 |
| 7 | Serine-sucrose b | 2682 | 4698 |
| 8 | Cysteine-sucrose b | 5193 | 8527 |
| No. | Assignment | Molecular Formula | Theoretical m/z [M − H]− | Experimental m/z [M − H]− | Error (ppm) |
|---|---|---|---|---|---|
| 1 | Asp-Glu/Fru | C10H17NO9 | 294.0831 | 294.0831 | −0.1 |
| 2 | Asp-Glu/Fru + H2O | C10H19NO10 | 312.0936 | 312.0935 | 0.2 |
| 3 | Asp-Glu/Fru-2 × H2O | C16H23NO12 | 420.1147 | 420.1167 | −4.6 |
| 4 | Asp-Glu/Fru-H2O | C16H25NO13 | 438.1253 | 438.1236 | 3.9 |
| 5 | Asp-Suc | C16H27NO14 | 456.1359 | 456.1357 | 0.3 |
| 6 | Asp-Suc + H2O | C16H29NO15 | 474.1464 | 474.1472 | −1.6 |
| 7 | Asp-3 × Glu/Fru-3 × H2O | C22H31NO16 | 564.1570 | 564.1583 | −2.3 |
| 8 | Asp-3 × Glu/Fru-2 × H2O | C22H33NO17 | 582.1676 | 582.1680 | −0.7 |
| 9 | Asp-3 × Glu/Fru-H2O | C22H35NO18 | 600.1781 | 600.1789 | −1.3 |
| 10 | Asp-3 × Glu/Fru | C22H37NO19 | 618.1887 | 618.1879 | 1.4 |
| 11 | Asp-3 × Glu/Fru + H2O | C22H39NO20 | 636.1993 | 636.1990 | 0.4 |
| 12 | Asp-4 × Glu/Fru-4 × H2O | C28H39NO20 | 708.1993 | 708.2048 | −7.7 |
| 13 | Asp-4 × Glu/Fru-3 × H2O | C28H41NO21 | 726.2098 | 726.2139 | −5.5 |
| 14 | Asp-4 × Glu/Fru-2 × H2O | C28H43NO22 | 744.2204 | 744.2236 | −4.3 |
| 15 | Asp-4 × Glu/Fru-H2O | C28H45NO23 | 762.2310 | 762.2303 | 0.8 |
| 16 | Asp-4 × Glu/Fru | C28H47NO24 | 780.2415 | 780.2385 | 3.9 |
| 17 | Asp-4 × Glu/Fru + H2O | C28H49NO25 | 798.2521 | 798.2507 | 1.7 |
3.2. FT ICR-MS Measurement of Maillard Reactions of Serine and Cysteine with Sucrose
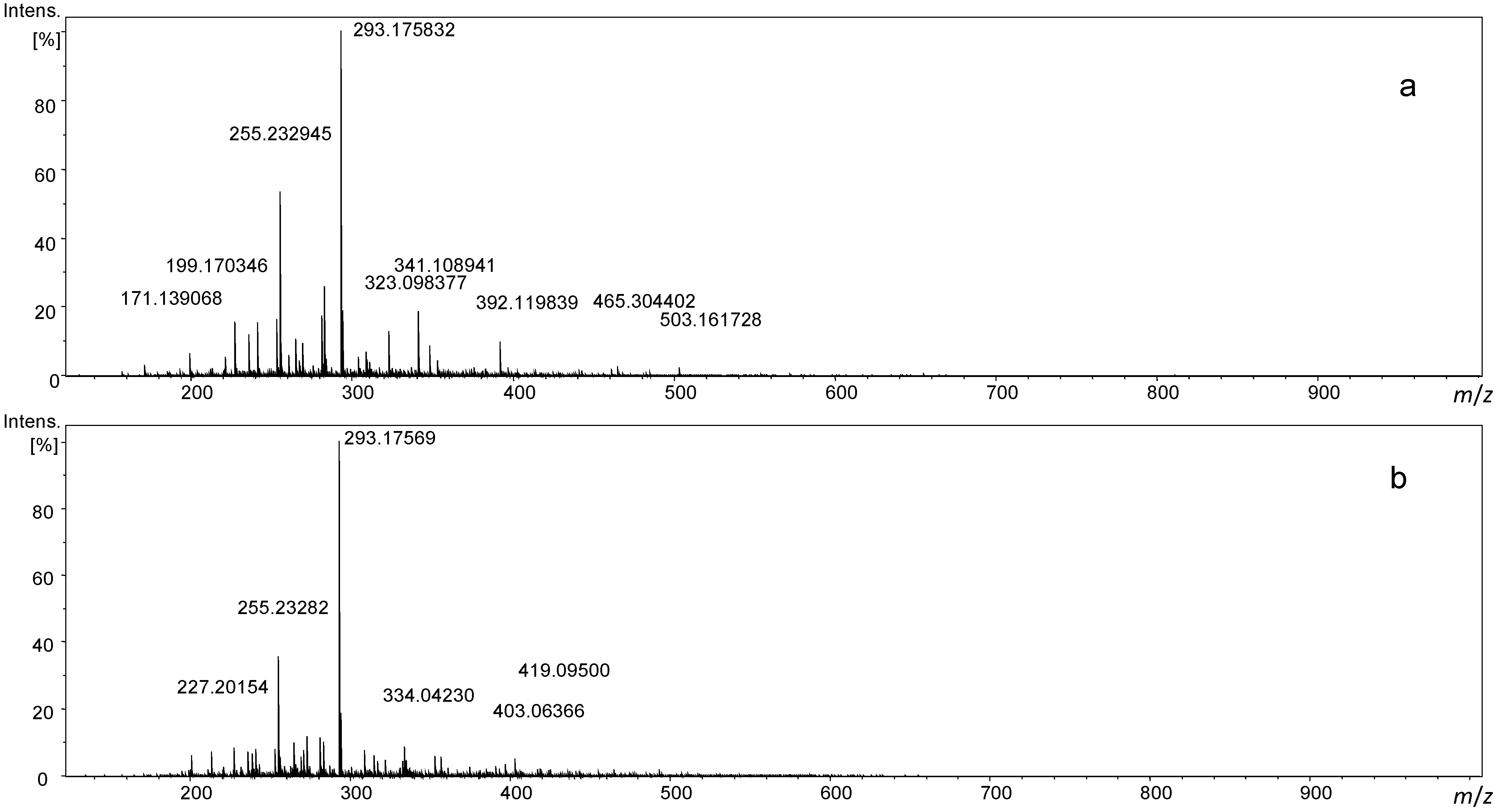
3.3. Graphical Data Interpretation
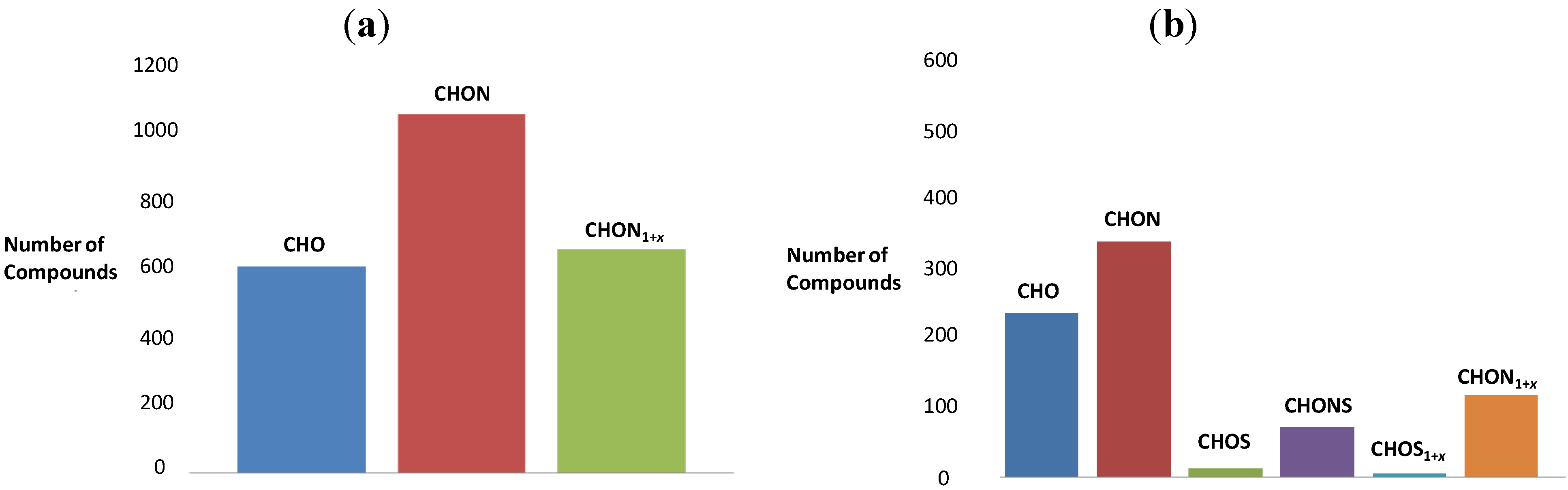
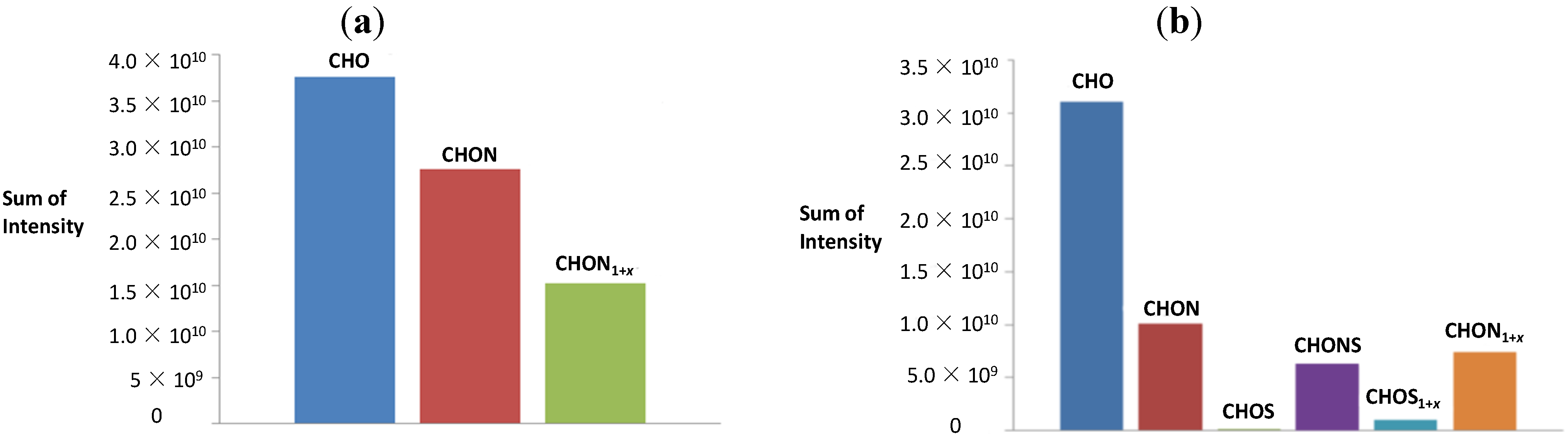
3.4. The van Krevelen Diagrams for the Reaction of Serine with Sucrose
3.5. The van Krevelen Diagrams for the Reaction of Cysteine with Sucrose


4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuhnert, N.; Dairpoosh, F.; Yassin, G.; Golon, A.; Jaiswal, R. What is under the hump? Mass spectrometry based analysis of complex mixtures in processed food—Lessons from the characterisation of black tea thearubigins, coffee melanoidines and caramel. Food Funct. 2013, 4, 1130–1147. [Google Scholar] [CrossRef]
- Fogliano, V.; Morales, F.J. Estimation of dietary intake of melanoidins from coffee and bread. Food Funct. 2011, 2, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Knerr, T.; Pischetsrieder, M.; Severin, T. 5-Hydroxy-2-methyl-4-(alkylamino)-2H-pyran-3(6H)-one: A New Sugar-Derived Aminoreductone. J. Agric. Food Chem. 1994, 42, 1657–1660. [Google Scholar] [CrossRef]
- Du, Q.-Q.; Liu, S.-Y.; Xu, R.-F.; Li, M.; Song, F.-R.; Liu, Z.-Q. Studies on structures and activities of initial Maillard reaction products by electrospray ionisation mass spectrometry combined with liquid chromatography in processing of red ginseng. Food Chem. 2012, 135, 832–838. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Silván, J.M.; van de Lagemaat, J.; Olano, A.; del Castillo, M.D. Analysis and biological properties of amino acid derivates formed by Maillard reaction in foods. J. Pharm. Biomed. Anal. 2006, 41, 1543–1551. [Google Scholar] [CrossRef]
- Cerny, C.; Davidek, T. Formation of aroma compounds from ribose and cysteine during the Maillard reaction. J. Agric. Food Chem. 2003, 51, 2714–2721. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Mennella, C.; Barba, F.; Russo, M.; Russo, G.L.; Krome, K.; Erbersdobler, H.F.; Faist, V.; Fogliano, V. Characterization of coloured compounds obtained by enzymatic extraction of bakery products. Food Chem. Toxicol. 2003, 41, 1367–1374. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Hofmann, T. Influence of baking conditions and precursor supplementation on the amounts of the antioxidant pronyl-l-lysine in bakery products. J. Agric. Food Chem. 2003, 52, 350–354. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Faist, V.; Hofmann, T. Structural and functional characterization of pronyl-lysine, a novel protein modification in bread crust melanoidins showing in vitro antioxidative and phase I/II enzyme modulating activity. J. Agric. Food Chem. 2002, 50, 6997–7006. [Google Scholar] [CrossRef]
- Hofmann, T. AcetylformoinA chemical switch in the formation of colored Maillard reaction products from hexoses and primary and secondary amino acids. J. Agric. Food Chem. 1998, 46, 3918–3928. [Google Scholar] [CrossRef]
- Cerny, C.; Briffod, M. Effect of pH on the Maillard reaction of [13C5]xylose, cysteine, and thiamin. J. Agric. Food Chem. 2007, 55, 1552–1556. [Google Scholar] [CrossRef]
- Mundt, S.; Wedzicha, B.L. Role of glucose in the Maillard browning of maltose and glycine: A radiochemical approach. J. Agric. Food Chem. 2005, 53, 6798–6803. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Lee, S.H.; Woo, K.S.; Ban, J.O.; Hong, J.T.; Yu, K.W.; Lee, J.; Jeong, H.S. Isolation and identification of an antiproliferative substance from fructose-tyrosine Maillard reaction products. Food Chem. 2012, 130, 547–551. [Google Scholar] [CrossRef]
- Kuhnert, N.; Drynan, J.W.; Obuchowicz, J.; Clifford, M.N.; Witt, M. Mass spectrometric characterization of black tea thearubigins leading to an oxidative cascade hypothesis for thearubigin formation. Rapid Commun. Mass Spectrom. 2010, 24, 3387–3404. [Google Scholar] [CrossRef]
- Kuhnert, N.; Clifford, M.N.; Muller, A. Oxidative cascade reactions yielding polyhydroxy-theaflavins and theacitrins in the formation of black tea thearubigins: Evidence by tandem LC-MS. Food Funct. 2010, 1, 180–199. [Google Scholar] [CrossRef]
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984. [Google Scholar] [CrossRef]
- Golon, A.; González, F.J.; Dávalos, J.Z.; Kuhnert, N. Investigating the thermal decomposition of starch and cellulose in model systems and toasted bread using domino tandem mass spectrometry. J. Agric. Food Chem. 2012, 61, 674–684. [Google Scholar]
- Golon, A.; Kuhnert, N. Unraveling the chemical composition of caramel. J. Agric. Food Chem. 2012, 60, 3266–3274. [Google Scholar] [CrossRef]
- Golon, A.; Kuhnert, N. Characterisation of “caramel-type” thermal decomposition products of selected monosaccharides including fructose, mannose, galactose, arabinose and ribose by advanced electrospray ionization mass spectrometry methods. Food Funct. 2013, 4, 1040–1050. [Google Scholar] [CrossRef]
- Pais, P.; Knize, M.G. Chromatographic and related techniques for the determination of aromatic heterocyclic amines in foods. J. Chromatogr. B Biomed. Sci. Appl. 2000, 747, 139–169. [Google Scholar] [CrossRef]
- Wu, Z.; Rodgers, R.P.; Marshall, A.G. Two- and three-dimensional van Krevelen diagrams: A graphical analysis complementary to the Kendrick mass plot for sorting elemental compositions of complex organic mixtures based on ultrahigh-resolution broadband fourier transform ion cyclotron resonance mass measurements. Anal. Chem. 2004, 76, 2511–2516. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Golon, A.; Kropf, C.; Vockenroth, I.; Kuhnert, N. An Investigation of the Complexity of Maillard Reaction Product Profiles from the Thermal Reaction of Amino Acids with Sucrose Using High Resolution Mass Spectrometry. Foods 2014, 3, 461-475. https://doi.org/10.3390/foods3030461
Golon A, Kropf C, Vockenroth I, Kuhnert N. An Investigation of the Complexity of Maillard Reaction Product Profiles from the Thermal Reaction of Amino Acids with Sucrose Using High Resolution Mass Spectrometry. Foods. 2014; 3(3):461-475. https://doi.org/10.3390/foods3030461
Chicago/Turabian StyleGolon, Agnieszka, Christian Kropf, Inga Vockenroth, and Nikolai Kuhnert. 2014. "An Investigation of the Complexity of Maillard Reaction Product Profiles from the Thermal Reaction of Amino Acids with Sucrose Using High Resolution Mass Spectrometry" Foods 3, no. 3: 461-475. https://doi.org/10.3390/foods3030461




