Physico-Chemical Characteristics of pH-Driven Active Film Loading with Curcumin Based on the Egg White Protein and Sodium Alginate Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Formation
2.3. Characterization of the Film-Forming Solution
2.4. Characterization of the Physicochemical Properties of the Films
2.4.1. Mechanical Performance
2.4.2. Optical Properties
2.4.3. Water Vapor Permeability (WVP) of the Films
2.4.4. Water Vapor Sorption (WVA)
2.4.5. Water Contact Angle
2.4.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.7. X-ray Diffraction (XRD)
2.5. Functional Characterization of the Films
2.5.1. Antioxidant Activity
2.5.2. Volatile Amine Detection
2.6. Curcumin Release
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization of the Film-Forming Solution
3.2. Physical Performance of the Films
3.2.1. Mechanical Performance
3.2.2. Optical Properties
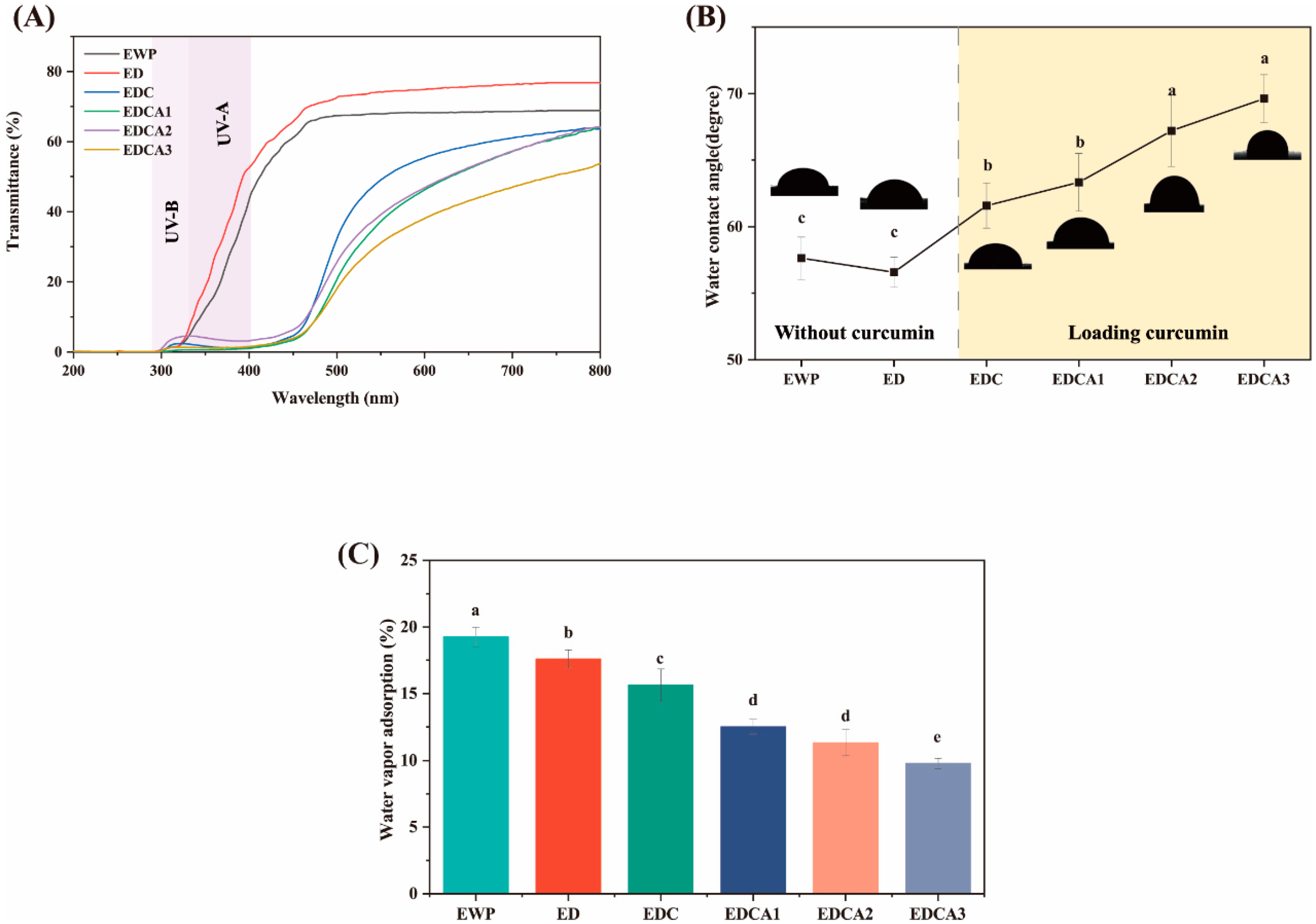
3.3. Water Resistance and Sensitivity
3.4. FTIR Spectra
3.5. XRD Patterns
3.6. Antioxidant Properties
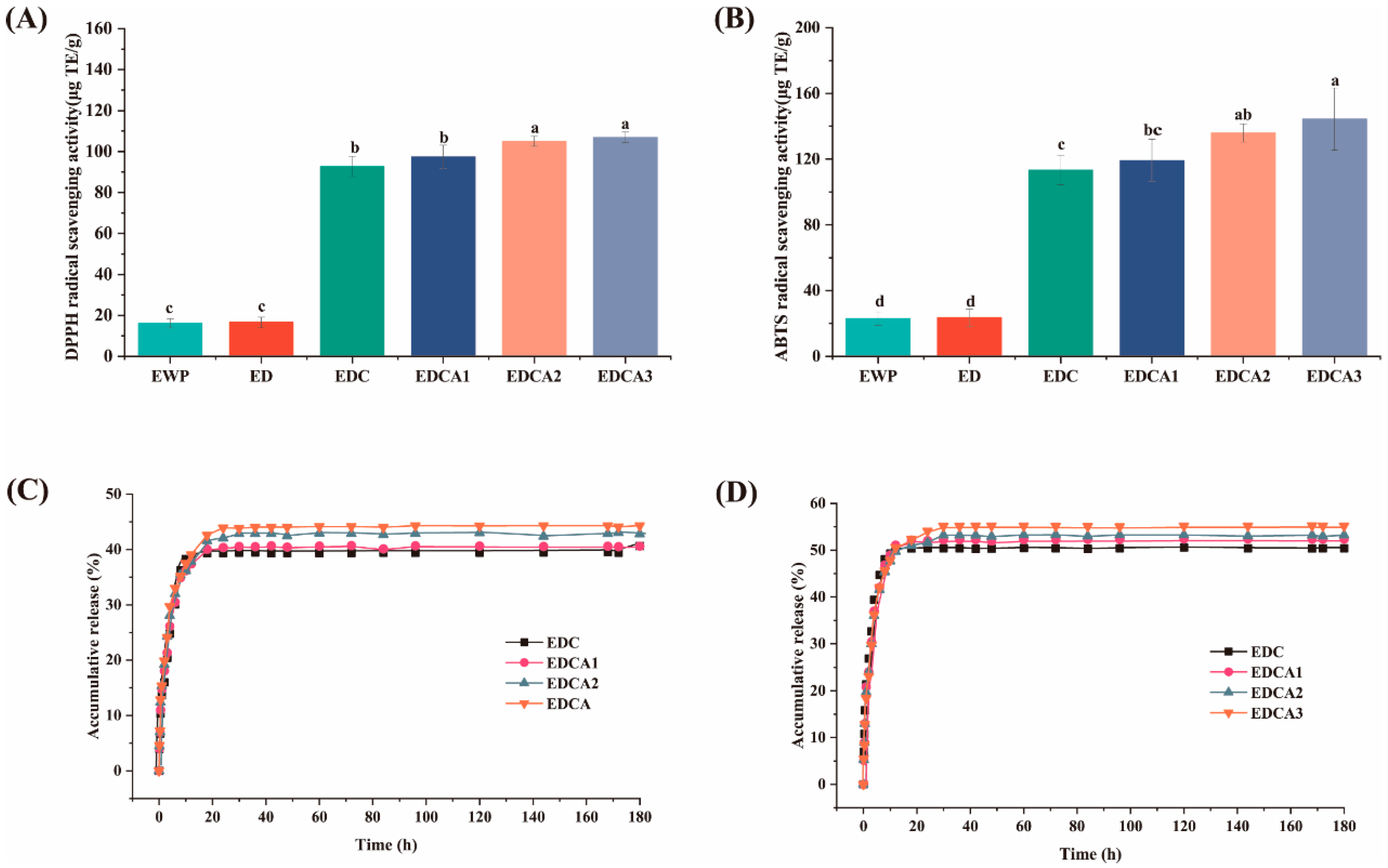
3.6.1. DPPH and ABTS Radical Scavenging
3.6.2. Curcumin Release
3.7. Color Responsiveness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akhila, V.; Badwaik, L.S. Recent advancement in improvement of properties of polysaccharides and proteins based packaging film with added nanoparticles: A review. Int. J. Biol. Macromol. 2022, 203, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Pedersen, G.A. Perspectives on sustainable food packaging: Is bio-based plastics a solution? Trends Food Sci. Technol. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de de Carvalho, A.P.A.; Conte, C.A., Jr. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef] [PubMed]
- Koshy, R.R.; Reghunadhan, A.; Mary, S.K.; Sadanandan, S.; Jose, S.; Thomas, S.; Pothen, L.A. AgNP anchored carbon dots and chitin nanowhisker embedded soy protein isolate films with freshness preservation for active packaging. Food Packag. Shelf Life 2022, 33, 100876. [Google Scholar] [CrossRef]
- Gagliarini, N.; Figoli, C.B.; Piermaria, J.; Bosch, A.; Abraham, A.G. Unraveling molecular interactions in whey protein-kefiran composite films to understand their physicochemical and mechanical properties. Food Biosci. 2022, 50, 102012. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Chen, M.; Jiang, S.; Jiang, S.; Li, X.; Wang, Q. Butylated hydroxyanisole encapsulated in gelatin fiber mats: Volatile release kinetics, functional effectiveness and application to strawberry preservation. Food Chem. 2018, 269, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zarandona, I.; Correia, D.M.; Moreira, J.; Costa, C.M.; Lanceros-Mendez, S.; Guerrero, P.; de la Caba, K. Magnetically responsive chitosan-pectin films incorporating Fe3O4 nanoparticles with enhanced antimicrobial activity. Int. J. Biol. Macromol. 2023, 227, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Rhim, J.-W. Recent progress in konjac glucomannan-based active food packaging films and property enhancement strategies. Food Hydrocoll. 2022, 128, 107572. [Google Scholar] [CrossRef]
- Huang, X.; Luo, X.; Liu, L.; Dong, K.; Yang, R.; Lin, C.; Song, H.; Li, S.; Huang, Q. Formation mechanism of egg white protein/κ-Carrageenan composite film and its application to oil packaging. Food Hydrocoll. 2020, 105, 105780. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Liu, X.; Ma, J.; Ban, H.; Bian, H.; Huang, G. High strength, controlled release of curcumin-loaded ZIF-8/chitosan/zein film with excellence gas barrier and antibacterial activity for litchi preservation. Carbohydr. Polym. 2023, 306, 120612. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Cabaj, A.; Tkaczewska, J.; Kawecka, A.; Krzyściak, P.; Szuwarzyński, M.; Mazur, T.; Juszczak, L. Incorporation of curcumin extract with lemongrass essential oil into the middle layer of triple-layered films based on furcellaran/chitosan/gelatin hydrolysates—In vitro and in vivo studies on active and intelligent properties. Food Chem. 2023, 402, 134476. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Tan, S.; Tan, G.; Zhang, H.; Xia, N.; Jiang, L.; Ren, H.; Rayan, A.M. Intelligent colorimetric soy protein isolate-based films incorporated with curcumin through an organic solvent-free pH-driven method: Properties, molecular interactions, and application. Food Hydrocoll. 2022, 133, 107904. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Qian, L.-H.; Zhang, Y.-H.; Gong, P.-X.; Liu, W.; Li, H.-J. Fabrication of foxtail millet prolamin/caseinate/chitosan hydrochloride composite nanoparticles using antisolvent and pH-driven methods for curcumin delivery. Food Chem. 2023, 404, 134604. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xiang, C.; Li, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery. Food Hydrocoll. 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Kong, L.; Hou, H. Effects of preparation conditions on the properties of agar/maltodextrin-beeswax pseudo-bilayer films. Carbohydr. Polym. 2020, 236, 116029. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; You, F.; Zhou, M.; Shi, S. Fabrication, Characterization, and Antimicrobial Activity of Carvacrol-Loaded Zein Nanoparticles Using the pH-Driven Method. Int. J. Mol. Sci. 2022, 23, 9227. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, M.; Zhou, S.; Zhang, H.; Wang, J.; Xia, N.; Liu, Y.; Hua, S.; Tan, G. Enhancing encapsulation of curcumin by pH-driven and sodium alginate blending with ovalbumin as a carrier. Food Hydrocoll. 2024, 149, 109623. [Google Scholar] [CrossRef]
- Kolotova, D.S.; Borovinskaya, E.V.; Bordiyan, V.V.; Zuev, Y.F.; Salnikov, V.V.; Zueva, O.S.; Derkach, S.R. Phase Behavior of Aqueous Mixtures of Sodium Alginate with Fish Gelatin: Effects of pH and Ionic Strength. Polymers 2023, 15, 2253. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, G.; Zhang, Y.; Yu, J.; Wang, Y.; Ma, X. Active edible films with plant extracts: A updated review of their types, preparations, reinforcing properties, and applications in muscle foods packaging and preservation. Crit. Rev. Food Sci. Nutr. 2022, 63, 11425–11447. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant protein-based food packaging films; recent advances in fabrication, characterization, and applications. Trends Food Sci. Technol. 2022, 120, 154–173. [Google Scholar] [CrossRef]
- Wiącek, A.E. Effect of surface modification on starch biopolymer wettability. Food Hydrocoll. 2015, 48, 228–237. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Dul, K. Effect of surface modification on starch/phospholipid wettability. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 351–359. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Wu, Y.; Huang, X.; Wang, G.; Song, H.; Geng, F.; Luo, P. Mechanism of differences in characteristics of thick/thin egg whites during storage: Physicochemical, functional and molecular structure characteristics analysis. Food Chem. 2022, 369, 130828. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Kordowska-Wiater, M.; Karaś, M.; Zięba, E.; Mężyńska, M.; Wiącek, A.E. Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocoll. 2020, 101, 105539. [Google Scholar] [CrossRef]
- Bishnoi, S.; Trifol, J.; Moriana, R.; Mendes, A.C. Adjustable polysaccharides-proteins films made of aqueous wheat proteins and alginate solutions. Food Chem. 2022, 391, 133196. [Google Scholar] [CrossRef] [PubMed]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Feng, J.; Xu, H.; Zhang, L.; Wang, H.; Liu, S.; Liu, Y.; Hou, W.; Li, C. Development of Nanocomplexes for Curcumin Vehiculization Using Ovalbumin and Sodium Alginate as Building Blocks: Improved Stability, Bioaccessibility, and Antioxidant Activity. J. Agric. Food Chem. 2018, 67, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Xu, H. Insight into the formation mechanism of soy protein isolate films improved by cellulose nanocrystals. Food Chem. 2021, 359, 129971. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Rhim, J.-W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhang, M.; Wang, G.; Meng, W.; Zhang, X.; Wang, D.; Zhou, Y.; Wang, Z. Characterization of polyvinyl alcohol/starch composite films incorporated with p-coumaric acid modified chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2021, 262, 117930. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lim, L.-T. Curcumin-loaded electrospun nonwoven as a colorimetric indicator for volatile amines. LWT 2020, 128, 109493. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, T.; Jiang, H.; Liu, J.; Wang, E.; Zhang, M.; Liu, X. pH-induced egg white protein foaming properties enhancement: Insight into protein structure and quantitative proteomic analysis at protein adsorption layer. Food Hydrocoll. 2023, 144, 109060. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Wang, Y.; Li, K.; Du, J.; Bai, Y. Effect of pH-shifting treatment on structural and heat induced gel properties of peanut protein isolate. Food Chem. 2020, 325, 126921. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yan, J.; Li, W.; Wang, Y.; McClements, D.J.; Liu, X.; Liu, F. Enhanced printability of food-grade edible inks: Emulsions formulated with modified pea protein and sodium alginate. Food Hydrocoll. 2024, 152, 109946. [Google Scholar] [CrossRef]
- Hou, J.; Tan, G.; Hua, S.; Zhang, H.; Wang, J.; Xia, N.; Zhou, S.; An, D. Development of high internal phase Pickering emulsions stabilized by egg yolk and carboxymethylcellulose complexes to improve β-carotene bioaccessibility for the elderly. Food Res. Int. 2024, 177, 113835. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Li, H.; Zhao, M.; Li, J.; Wang, J.; Zhang, H.; Wang, J.; Xia, N.; Wang, Z.; Rayan, A.M. Advancing the pH-driven encapsulation technique of curcumin: Molecular interaction shifts due to structural and charge variations. Food Hydrocoll. 2024, 109952. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, N.; Yang, J.; Hu, J.; Zhang, K.; Nishinari, K.; Phillips, G.O.; Fang, Y. Protein/polysaccharide intramolecular electrostatic complex as superior food-grade foaming agent. Food Hydrocoll. 2020, 101, 105474. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, T.; Xu, X.; Zhou, G. Influence of extreme alkaline pH induced unfolding and aggregation on PSE-like chicken protein edible film formation. Food Chem. 2020, 319, 126574. [Google Scholar] [CrossRef] [PubMed]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Rosa, M.D.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Guan, Y.; Wen, H.; Zhang, Y.; Liu, J.; Zhang, T. Mild heating assisted alkaline pH shifting modify the egg white protein: The mechanism and the enhancement of emulsifying properties. LWT 2021, 151, 112094. [Google Scholar] [CrossRef]
- Xie, H.; Ouyang, K.; Zhang, L.; Hu, J.; Huang, S.; Sun, W.; Xiong, H.; Zhao, Q. Chitosan/rice hydrolysate/curcumin composite film: Effect of chitosan molecular weight. Int. J. Biol. Macromol. 2022, 210, 53–62. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Filho, J.G.d.O.; Bertolo, M.R.V.; Rodrigues, M.Á.V.; Marangon, C.A.; Silva, G.d.C.; Odoni, F.C.A.; Egea, M.B. Curcumin: A multifunctional molecule for the development of smart and active biodegradable polymer-based films. Trends Food Sci. Technol. 2021, 118, 840–849. [Google Scholar] [CrossRef]
- Dangaran, K.L.; Cooke, P.; Tomasula, P.M. The Effect of Protein Particle Size Reduction on the Physical Properties of CO2-Precipitated Casein Films. J. Food Sci. 2006, 71, E196–E201. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Kevij, H.T.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the pH-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Dammak, I.; De Carvalho, R.A.; Favaro-Trindade, C.S.; Lourenço, R.V.; do Amaral Sobral, P.J. Properties of active gelatin films incorporated with rutin-loaded nanoemulsions. Int. J. Biol. Macromol. 2017, 98, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zioga, M.; Papantonopoulou, G.; Evageliou, V. High internal phase emulsions and edible films with high methoxyl pectin and pea protein isolate or sodium caseinate. Food Hydrocoll. 2023, 140, 108605. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, C.; Dai, L.; Zhang, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. The construction of resveratrol-loaded protein–polysaccharide–tea saponin complex nanoparticles for controlling physicochemical stability and in vitro digestion. Food Funct. 2020, 11, 9973–9983. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, B.; McClements, D.J.; Xu, X.; Cui, S.; Gao, L.; Zhou, L.; Xiong, L.; Sun, Q.; Dai, L. Properties of curcumin-loaded zein-tea saponin nanoparticles prepared by antisolvent co-precipitation and precipitation. Food Chem. 2022, 391, 133224. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Chen, W.; Zhong, Q.; Chen, W.; Xu, Y.; Wu, J.; Chen, H. Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin. Foods 2023, 12, 1577. [Google Scholar] [CrossRef]
- Chen, H.; Wu, C.; Feng, X.; He, M.; Zhu, X.; Li, Y.; Teng, F. Effects of two fatty acids on soy protein isolate/sodium alginate edible films: Structures and properties. LWT 2022, 159, 113221. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Exploration of the antioxidant and antimicrobial capacity of two sunflower protein concentrate films with naturally present phenolic compounds. Food Hydrocoll. 2012, 29, 374–381. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Kasilingam, R.; Gopal, D. Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Mater. Sci. Eng. C 2021, 120, 111737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Xiang, C.; Gao, J.; Ye, H.; Ren, G.; Ma, X.; Xie, H.; Fang, S.; Lei, Q.; Fang, W. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Li, J.; Zhang, X.; Zhang, H.; Zheng, L.; Xia, N.; We, A.; Hua, S. 3D Printing of smart labels with curcumin-loaded soy protein isolate. Int. J. Biol. Macromol. 2024, 255, 128211. [Google Scholar] [CrossRef]



| Sample Nomenclature | Film-Forming Liquid Formulations | ||
|---|---|---|---|
| EWP:Alg | Curcumin (%, w/v) | Method | |
| EWP | 0 | 0 | Blending |
| ED | 0 | 0 | pH-driven |
| EDC | 0 | 0.03 | pH-driven |
| EDCA1 | 8:1 | 0.03 | pH-driven |
| EDCA2 | 4:1 | 0.03 | pH-driven |
| EDCA3 | 2:1 | 0.03 | pH-driven |
| Films | Thickness (μm) | TS (MPa) | EB (%) | WVP (1010 g·m/m2⋅Pa·s) |
|---|---|---|---|---|
| EWP | 126 ± 5 a | 2.72 ± 0.54 d | 62.68 ± 6.09 c | 1.41 ± 0.09 a |
| ED | 108 ± 5 b | 2.96 ± 0.43 cd | 96.44 ± 4.22 b | 1.35 ± 0.05 ab |
| EDC | 100 ± 4 c | 3.80 ± 0.18 bc | 108.86 ± 2.13 a | 1.33 ± 0.06 bc |
| EDCA1 | 98 ± 2 c | 4.31 ± 0.32 b | 102.41 ± 2.10 ab | 1.31 ± 0.05 bc |
| EDCA2 | 94 ± 4 c | 5.24 ± 0.63 a | 104.82 ± 0.97 a | 1.30 ± 0.04 bc |
| EDCA3 | 94 ± 2 c | 5.68 ± 0.57 a | 103.56 ± 3.13 a | 1.25 ± 0.02 c |
| Films | UV-A Blocking (%) | UV-B Blocking (%) | Opacity (mm−1) | L* | a* | b* | ΔE* | Yellowness Index (YI) |
|---|---|---|---|---|---|---|---|---|
| EWP | 77.2 ± 50.48 b | 99.56 ± 0.64 a | 1.36 ± 0.11 d | 89.74 ± 0.71 a | −2.97 ± 0.34 c | 9.70 ± 0.81 e | 11.74 ± 1.81 b | 18.43 ± 1.51 e |
| ED | 78.12 ± 0.60 b | 99.60 ± 0.90 a | 1.25 ± 0.12 d | 85.85 ± 2.86 b | −0.61 ± 0.22 c | 5.66 ± 1.65 d | 14.31 ± 2.13 b | 18.27 ± 1.34 e |
| EDC | 99.03 ± 0.48 a | 99.58 ± 0.81 a | 2.34 ± 0.29 c | 76.04 ± 1.36 d | 32.37 ± 2.37 b | 73.93 ± 6.38 a | 69.19 ± 14.81 a | 106.81 ± 2.26 d |
| EDCA1 | 99.21 ± 0.54 a | 99.61 ± 0.16 a | 3.17 ± 0.35 b | 81.68 ± 1.16 c | 33.03 ± 4.89 b | 63.92 ± 4.25 c | 62.44 ± 8.18 a | 118.41 ± 2.74 c |
| EDCA2 | 99.38 ± 0.67 a | 99.70 ± 0.10 a | 3.35 ± 0.27 b | 82.04 ± 2.41 c | 36.70 ± 5.05 ab | 66.34 ± 3.825 bc | 70.12 ± 3.52 a | 123.97 ± 2.91 b |
| EDCA3 | 99.35 ± 0.91 a | 99.66 ± 0.71 a | 3.68 ± 0.17 a | 82.30 ± 0.75 c | 41.16 ± 5.21 a | 70.40 ± 1.36 ab | 70.88 ± 4.34 a | 130.31 ± 4.08 a |
| Models | 50% Ethanol (Semifat Food Simulant) | 95% Ethanol (Fatty Food Simulant) | ||||||
|---|---|---|---|---|---|---|---|---|
| EDC | EDCA1 | EDCA2 | EDCA3 | EDC | EDCA1 | EDCA2 | EDCA3 | |
| Ritger-peppas | 0.7959 | 0.8205 | 0.8366 | 0.8395 | 0.7818 | 0.7974 | 0.8182 | 0.8242 |
| n | 0.16 | 0.16 | 0.16 | 0.16 | 0.13 | 0.15 | 0.16 | 0.17 |
| First-order | 0.9832 | 0.9796 | 0.9737 | 0.9730 | 0.9839 | 0.9860 | 0.9834 | 0.9860 |
| Higuchi | 0.2869 | 0.3017 | 0.3166 | 0.3224 | 0.2335 | 0.2715 | 0.2967 | 0.3131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, M.; Ju, X.; Zhang, H.; Xia, N.; Wang, J.; Wang, Z.; Rayan, A.M. Physico-Chemical Characteristics of pH-Driven Active Film Loading with Curcumin Based on the Egg White Protein and Sodium Alginate Matrices. Foods 2024, 13, 1340. https://doi.org/10.3390/foods13091340
Li H, Liu M, Ju X, Zhang H, Xia N, Wang J, Wang Z, Rayan AM. Physico-Chemical Characteristics of pH-Driven Active Film Loading with Curcumin Based on the Egg White Protein and Sodium Alginate Matrices. Foods. 2024; 13(9):1340. https://doi.org/10.3390/foods13091340
Chicago/Turabian StyleLi, Hanyu, Mengzhuo Liu, Xinyi Ju, Huajiang Zhang, Ning Xia, Jing Wang, Zhongjiang Wang, and Ahmed M. Rayan. 2024. "Physico-Chemical Characteristics of pH-Driven Active Film Loading with Curcumin Based on the Egg White Protein and Sodium Alginate Matrices" Foods 13, no. 9: 1340. https://doi.org/10.3390/foods13091340






