Mackerel and Seaweed Burger as a Functional Product for Brain and Cognitive Aging Prevention
Abstract
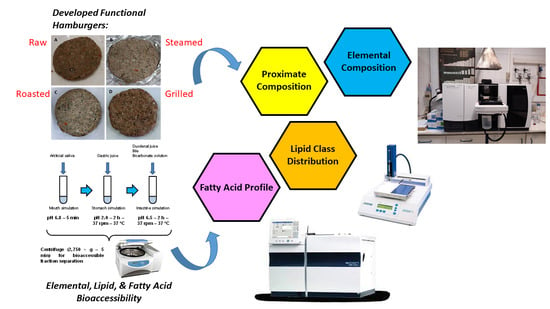
:1. Introduction
2. Materials and Methods
2.1. Chub Mackerel and Seaweed
2.2. Preparation of the Functional Hamburger
2.3. Moisture, Protein, Ash, Carbohydrate, and Energy Values
2.4. In Vitro Digestion Model
Calculation of Bioaccessibility
- [FA] = Concentration of FA;
- [S] = [FA] before digestion.
- [E] = Concentration of the element;
- [T] = [E] in the bioaccessible fraction + [E] in the non-digested fraction.
2.5. Lipid Determination
2.6. Lipid Class Determination
2.7. Fatty Acid Profile
2.8. Selenium and Iodine Determination
2.9. Polyphenolic Compound Determination
2.10. Antioxidant Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Lipid Classes
3.3. Fatty Acid Profile
3.4. Selenium and Iodine Contents
3.5. Polyphenol Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology diagnostic criteria risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Moradi, S.; Moradinazar, M.; Mostafai, R.; Pasdar, Y. Dietary pattern in relation to the risk of Alzheimer’s disease:A systematic review. Neurol. Sci. 2019, 40, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- McGurran, H.; Glenn, J.; Madero, E.; Bott, N. Risk reduction and prevention of Alzheimer’s Disease: Biological mechanisms of diet. Curr. Alzheimer Res. 2020, 17, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Tangalakis, K.; Bosevski, M.; Apostolopoulos, V. Cognitive decline: A vitamin B perspective. Maturitas 2016, 93, 108–113. [Google Scholar] [CrossRef]
- Aaseth, J.; Alexander, J.; Børklund, G.; Hestad, K.; Dusek, P.; Roos, P.M.; Alehagen, U. Treatment strategies in Alzheimer’s disease: A review with focus on selenium supplementation. BioMetals 2016, 29, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-L.; Chen, C.; Wu, Q.-Y.; Zhang, Z.-H.; Zheng, R.; Chen, Y.; Jia, S.-Z.; Ni, J.-Z. Selenium-enriched yeast inhibited β-amyloid production and modulated autophagy in a triple transgenic mouse model of Alzheimer’s disease. Metallomics 2018, 10, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Redman, K.; Ruffman, T.; Fitzgerald, P.; Skeaff, S. Iodine deficiency and the brain: Effects and mechanisms. Cr. Rev. Food Sci. Nutr. 2016, 56, 2695–2713. [Google Scholar] [CrossRef] [PubMed]
- Kurtys, E.; Eisel, U.L.M.; Verkuyl, J.M.; Broersen, L.M.; Dierckx, R.A.J.O.; de Vries, E.F.J. The combination of vitamins and omega-3 fatty acids has an enhanced anti-inflammatory effect on microglia. Neurochem. Int. 2016, 99, 206–214. [Google Scholar] [CrossRef]
- Ferreira, I.; Gomes-Bispo, A.; Lourenço, H.; Matos, J.; Afonso, C.; Cardoso, C.; Castanheira, I.; Motta, C.; Prates, J.A.M.; Bandarra, N.M. The chemical composition and lipid profile of the chub mackerel (Scomber colias) show a strong seasonal dependence: Contribution to a nutritional evaluation. Biochimie 2020, 178, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Almeida, J.; Coelho, I.; Delgado, I.; Gomes, R.; Quintã, R.; Bandarra, N.M.; Afonso, C. Farming a wild seaweed and changes to its composition bioactivity and bioaccessibility: The Saccorhiza polyschides case study. Aquaculture 2023, 766, 739217. [Google Scholar] [CrossRef]
- Afonso, C.; Cardoso, C.; Gomes-Bispo, A.; Ferreira, I.; Rego, A.; Coelho, I.; Motta, C.; Prates, J.A.M.; Castanheira, I.; Bandarra, N.M. Fatty acids, selenium and vitamin B12 in chub mackerel (Scomber colias) as nourishment considering seasonality and bioaccessibility as factors. Food Chem. 2023, 403, 134455. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.-D.A.; Kumar, S.; Bhat, H.F. Thermal processing implications on the digestibility of meat, fish and seafood proteins. Comp. Rev. Food Sci. Food Technol. 2021, 20, 4511–4548. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Afonso, C.; Cardoso, C.; Batista, I.; Chaveiro, N.; Nunes, M.L.; Bandarra, N.M. Fatty acids mercury and methylmercury bioaccessibility in salmon (Salmo salar) using an in vitro model: Effect of culinary treatment. Food Chem. 2015, 185, 268–276. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Weihrauch, J.L.; Posati, L.P.; Anderson, B.A.; Exler, J. Lipid conversion factors for calculating fatty acids contents in foods. JAOCS 1977, 54, 36–40. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pellegrini, N.; Fogliano, V. Cooking industrial processing and caloric density of foods. Curr. Opt. Food Sci. 2017, 14, 98–102. [Google Scholar] [CrossRef]
- Aursand, M.; Bleivik, B.; Rainuzzo, J.R.; Leif, J.; Mohr, V. Lipid distribution and composition of commercially farmed Atlantic salmon (Salmo salar). J. Sci. Food Agric. 1994, 64, 239–248. [Google Scholar] [CrossRef]
- Ampong, I.; Ikwuobe, O.J.; Brown, J.E.P.; Bailey, C.J.; Gao, D.; Gutierrez-Merino, J.; Griffiths, H.R. Odd chain fatty acid metabolism in mice after a high fat diet. Int. J. Biochem. Cell Biol. 2022, 143, 106135. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd) an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Brühl, L. Fatty acid alterations in oils and fats during heating and frying. Eur. J. Lipid Sci. Technol. 2014, 116, 707–715. [Google Scholar] [CrossRef]
- Choo, P.Y.; Azlan, A.; Khoo, H.E. Cooking methods affect total fatty acid composition and retention of DHA and EPA in selected fish fillets. ScienceAsia 2018, 44, 92–101. [Google Scholar] [CrossRef]
- Schneedorferová, I.; Tomčala, A.; Valterová, I. Effect of heat treatment on the n-3/n-6 ratio and content of polyunsaturated fatty acids in fish tissues. Food Chem. 2015, 176, 205–211. [Google Scholar] [CrossRef]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victório, A.M.; Emanuelli, T. Effect of different cooking methods on the oxidation proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Fu, S.-G.; Wang, S.-Y.; Yang, D.-J.; Wu, Y.-H.S.; Chen, Y.C. Effects of a natural antioxidant polyphenol-rich rosemary (Rosmarinus officinalis L.) extract on lipid stability of plant-derived omega-3 fatty-acid rich oil. LWT Food Sci. Technol. 2018, 89, 210–216. [Google Scholar] [CrossRef]
- Serdaroğlu, M.; Felekoğlu, E. Effects of using rosemary extract and onion juice on oxidative stability of sardine (Sardina pilchardus) mince. J. Food Qual. 2005, 28, 109–120. [Google Scholar] [CrossRef]
- Wang, J.; Ossemond, J.; Jardin, J.; Briard-Bion, V.; Henry, G.; Le Gouar, Y.; Ménard, O.; Lê, S.; Madadlou, A.; Dupont, D.; et al. Encapsulation of DHA oil with heat-denatured whey protein in Pickering emulsion improves its bioaccessibility. Food Res. Int. 2022, 162 Pt B, 112112. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Q.; Li, W.; Wright, A.J. Emulsification of algal oil with soy lecithin improved DHA bioaccessibility but did not change overall in vitro digestibility. Food Funct. 2014, 5, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Dietary reference values for nutrients: Summary report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Fernandez-Tejedor, M.; Tediosi, A.; Kotterman, M.; van den Heuvel, F.H.M.; Robbens, J.; Fernandes, J.O.; Rasmussen, R.R.; et al. Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets. Food Chem. 2018, 267, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, F.; Iacoponi, F.; Raggi, A.; Baldi, F.; Fretigny, M.; Mantovani, A.; Cubadda, F. Essential and toxic elements in sustainable and underutilized seafood species and derived semi-industrial ready-to-eat products. Food Chem. Toxicol. 2021, 154, 112331. [Google Scholar] [CrossRef] [PubMed]
- Jahreis, G.; Hausmann, W.; Kiessling, G.; Leiterer, M. Bioavailability of iodine from normal diets rich in dairy products—Results of balance studies in women. Exp. Clin. Endocrinol. Diabetes 2001, 109, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols:A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; Del Nobile, M.A.; Conte, A. Fish burger enriched by olive oil industrial by-product. Food Sci. Nutr. 2017, 5, 837–844. [Google Scholar] [CrossRef]
- Hentati, F.; Barkallah, M.; Atitallah, A.B.; Dammak, M.; Louati, I.; Pierre, G.; Fendri, I.; Attia, H.; Michaud, P.; Abdelkafi, S. Quality characteristics and functional and antioxidant capacities of algae-fortified fish burgers prepared from common barbel (Barbus barbus). BioMed Res. Int. 2019, 2019, 2907542. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Mercatante, D.; Balzan, S.; Esposto, S.; Cardenia, V.; Servili, M.; Novelli, E.; Taticchi, A.; Rodriguez-Estrada, M.T. Improved oxidative stability and sensory quality of beef hamburgers enriched with a phenolic extract from olive vegetation water. Antioxidants 2021, 10, 1969. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | (%, ww) |
|---|---|
| Chub mackerel | 72.43 |
| Boiled quinoa | 15.73 |
| Onion | 4.73 |
| Red pepper | 3.73 |
| Garlic | 1.60 |
| Seaweed | 0.53 |
| Salt | 0.47 |
| Thyme | 0.33 |
| Oregano | 0.16 |
| Parsley | 0.16 |
| Coriander | 0.13 |
| Moisture (%) | Lipid (%) | Protein (%) | Ash (%) | Carbohydrates (%) * | Energy Value (kcal/100 g) | |
|---|---|---|---|---|---|---|
| Chub mackerel (raw) | 68.5 ± 0.1 c | 7.22 ± 1.32 b | 22.7 ± 0.1 a | 1.31 ± 0.03 b | 0.20 | 157 |
| Quinoa (boiled) | 80.2 ± 0.8 a | 0.27 ± 0.05 a | 2.70 ± 0.21 d | 0.20 ± 0.01 a | 16.63 | 80 |
| Raw hamburger | 71.5 ± 0.3 b | 5.13 ± 0.60 b | 18.0 ± 0.6 c | 1.74 ± 0.05 c | 3.58 | 133 |
| Steamed hamburger | 70.3 ± 1.0 bc | 5.65 ± 0.23 b | 18.3 ± 0.8 c | 1.84 ± 0.02 c | 3.91 | 140 |
| Roasted hamburger | 67.8 ± 0.4 c | 6.49 ± 0.30 b | 20.0 ± 0.3 b | 2.02 ± 0.02 d | 3.73 | 153 |
| Grilled hamburger | 64.0 ± 0.2 d | 6.71 ± 0.32 b | 22.0 ± 0.2 a | 2.21 ± 0.02 e | 5.06 | 169 |
| Lipid Class (% Total Lipids) | Quinoa (Boiled) | Raw Chub Mackerel | Raw Hamburger | Steamed Hamburger | Roasted Hamburger | Grilled Hamburger | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Initial | Bioacc. | Initial | Bioacc. | Initial | Bioacc. | Initial | Bioacc. | Initial | Bioacc. | |
| PL | 5.3 ± 0.7 | 9.2 ± 1.0 ab | 13.7 ± 0.5 AB | 8.9 ± 1.1 ab | 13.2 ± 0.7 AB | 10.2 ± 0.8 a | 12.4 ± 0.6 A | 8.4 ± 0.4 b | 16.1 ± 1.2 B | 8.0 ± 0.8 b | 14.2 ± 1.6 AB |
| MAG | --- | --- | 25.0 ± 0.7 A | --- | 30.1 ± 0.3 B | --- | 28.0 ± 1.0 B | --- | 28.8 ± 1.2 B | --- | 28.7 ± 0.4 B |
| ST + 1,2-DAG | 4.2 ± 0.5 | 18.3 ± 0.6 a | 9.6 ± 0.0 A | 12.6 ± 1.2 b | 8.4 ± 0.9 A | 11.4 ± 0.8 b | 8.1 ± 0.7 A | 15.3 ± 1.8 a | 9.3 ± 1.0 A | 15.8 ± 2.1 a | 8.4 ± 0.5 A |
| FFA | 23.9 ± 2.7 | 18.9 ± 1.0 a | 51.7 ± 0.8 A | 12.6 ± 1.9 b | 48.3 ± 0.6 AB | 11.2 ± 1.0 bc | 51.5 ± 2.1 A | 12.7 ± 1.7 b | 45.7 ± 1.2 B | 8.9 ± 0.7 c | 48.7 ± 0.3 AB |
| TAG | 43.9 ± 3.0 | 53.6 ± 2.1 a | --- | 65.9 ± 1.7 bc | --- | 67.2 ± 1.0 c | --- | 63.6 ± 2.5 b | --- | 67.4 ± 0.9 c | --- |
| Others | 22.7 ± 1.7 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| Fatty Acid | Raw Chub Mackerel | Quinoa (Boiled) | Raw Hamburger | Steamed Hamburger | Roasted Hamburger | Grilled Hamburger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (mg/100 g) | (%) | (mg/100 g) | (%) | (mg/100 g) | (%) | (mg/100 g) | (%) | (mg/100 g) | (%) | (mg/100 g) | |
| 14:0 | 1.4 ± 0.3 a | 95 ± 16 A | --- | --- | 1.4 ± 0.2 a | 58 ± 10 A | 1.7 ± 0.1 a | 82 ± 5 A | 1.3 ± 0.1 a | 66 ± 6 A | 1.5 ± 0.5 a | 81 ± 27 A |
| 15:0 | 0.5 ± 0.1 a | 33 ± 5 A | --- | --- | 0.5 ± 0.1 a | 21 ± 3 B | 0.6 ± 0.0 a | 28 ± 1 AB | 0.5 ± 0.0 a | 25 ± 2 AB | 0.5 ± 0.1 a | 28 ± 6 AB |
| 16:0 | 11.9 ± 1.2 a | 784 ± 79 A | 8.5 ± 0.4 b | 21 ± 1 C | 12.2 ± 0.9 a | 510 ± 38 B | 13.6 ± 0.3 a | 635 ± 16 B | 11.6 ± 0.5 ab | 598 ± 28 B | 12.1 ± 1.4 a | 643 ± 77 AB |
| 17:0 | 0.7 ± 0.1 a | 47 ± 4 A | --- | --- | 0.8 ± 0.0 a | 32 ± 1 C | 0.8 ± 0.0 a | 37 ± 1 BC | 0.8 ± 0.0 a | 39 ± 1 B | 0.7 ± 0.0 a | 39 ± 2 B |
| 18:0 | 6.2 ± 0.3 a | 407 ± 20 A | 1.4 ± 0.5 b | 3.5 ± 1.2 E | 6.4 ± 0.2 a | 267 ± 6 D | 6.5 ± 0.1 a | 301 ± 4 C | 6.4 ± 0.0 a | 328 ± 1 BC | 6.3 ± 0.1 a | 335 ± 5 B |
| ΣSFA | 22.0 ± 2.0 a | 1453 ± 134 A | 11.1 ± 0.7 b | 27 ± 2 D | 22.7 ± 1.1 a | 950 ± 45 C | 24.8 ± 0.5 a | 1157 ± 24 BC | 22.0 ± 0.6 a | 1135 ± 33 BC | 22.8 ± 2.1 a | 1208 ± 111 B |
| 16:1n-9 | 0.9 ± 0.1 a | 61 ± 6 A | --- | --- | 0.9 ± 0.1 a | 37 ± 4 C | 1.1 ± 0.1 a | 49 ± 2 B | 0.9 ± 0.0 a | 48 ± 1 BC | 0.9 ± 0.1 a | 47 ± 6 BC |
| 16:1n-7 | 1.6 ± 0.1 a | 108 ± 8 A | --- | --- | 1.6 ± 0.1 a | 67 ± 6 B | 1.8 ± 0.0 a | 84 ± 2 B | 1.5 ± 0.1 a | 75 ± 7 B | 1.6 ± 0.3 a | 86 ± 14 B |
| 17:1 | 0.5 ± 0.0 a | 31 ± 3 A | 0.5 ± 0.0 a | 1.3 ± 0.1 D | 0.5 ± 0.0 a | 21 ± 1 C | 0.5 ± 0.0 a | 23 ± 0 BC | 0.5 ± 0.0 a | 24 ± 1 BC | 0.5 ± 0.0 a | 25 ± 1 B |
| 18:1n-9 | 12.3 ± 0.5 a | 813 ± 30 A | 20.8 ± 0.7 b | 51 ± 2 D | 13.0 ± 0.8 a | 543 ± 35 C | 13.7 ± 0.0 a | 639 ± 1 B | 13.2 ± 0.0 a | 678 ± 1 B | 13.0 ± 0.3 a | 687 ± 14 B |
| 18:1n-7 | 3.1 ± 0.1 a | 204 ± 6 A | 1.1 ± 0.1 b | 2.7 ± 0.2 E | 3.1 ± 0.1 a | 129 ± 4 D | 3.2 ± 0.0 a | 147 ± 1 C | 3.1 ± 0.0 a | 157 ± 1 BC | 3.0 ± 0.0 a | 161 ± 1 B |
| 20:1n-9 | 1.6 ± 0.1 a | 106 ± 4 A | 1.9 ± 0.1 b | 4.6 ± 0.3 D | 1.6 ± 0.1 a | 67 ± 4 C | 1.7 ± 0.0 ab | 77 ± 1 BC | 1.7 ± 0.2 ab | 88 ± 8 B | 1.7 ± 0.1 ab | 88 ± 4 B |
| ΣMUFA | 21.2 ± 0.7 a | 1401 ± 46 A | 26.0 ± 0.5 b | 64 ± 1 D | 21.9 ± 1.3 a | 917 ± 53 C | 23.1 ± 0.0 a | 1080 ± 2 B | 22.1 ± 0.2 a | 1139 ± 8 B | 21.9 ± 0.6 a | 1163 ± 30 B |
| 18:2n-6 | 2.2 ± 0.8 a | 147 ± 51 A | 52.6 ± 3.4 b | 129 ± 8 A | 2.7 ± 0.1 a | 113 ± 3 A | 2.6 ± 0.1 a | 120 ± 3 A | 2.5 ± 0.1 a | 126 ± 3 A | 2.5 ± 0.1 a | 132 ± 4 A |
| 18:3n-3 | 1.1 ± 0.2 a | 70 ± 16 A | 6.0 ± 0.4 b | 15 ± 1 B | 1.3 ± 0.0 a | 56 ± 1 A | 1.3 ± 0.0 a | 59 ± 1 A | 1.2 ± 0.0 a | 63 ± 2 A | 1.2 ± 0.1 a | 65 ± 3 A |
| 18:4n-3 | 1.3 ± 0.1 a | 84 ± 6 A | --- | --- | 1.2 ± 0.1 a | 51 ± 3 C | 1.3 ± 0.0 a | 59 ± 1 BC | 1.2 ± 0.0 a | 60 ± 2 BC | 1.2 ± 0.1 a | 63 ± 4 B |
| 20:2n-6 | 0.5 ± 0.0 a | 34 ± 2 A | --- | --- | 0.5 ± 0.0 a | 20 ± 0 D | 0.5 ± 0.0 a | 22 ± 1 CD | 0.5 ± 0.0 a | 25 ± 1 BC | 0.5 ± 0.0 a | 26 ± 2 B |
| 20:4n-6 | 2.4 ± 0.1 a | 159 ± 7 A | --- | --- | 2.4 ± 0.1 a | 101 ± 6 C | 2.3 ± 0.0 a | 105 ± 1 C | 2.4 ± 0.1 a | 126 ± 3 B | 2.4 ± 0.1 a | 126 ± 6 B |
| 20:4n-3 | 0.7 ± 0.1 a | 47 ± 3 A | --- | --- | 0.6 ± 0.0 ab | 26 ± 1 C | 0.6 ± 0.0 b | 28 ± 1 BC | 0.6 ± 0.1 b | 29 ± 4 BC | 0.6 ± 0.0 b | 32 ± 2 B |
| 20:5n-3 | 10.3 ± 0.6 a | 681 ± 42 A | --- | --- | 9.8 ± 0.2 ab | 411 ± 7 D | 9.3 ± 0.1 b | 435 ± 4 CD | 9.6 ± 0.1 ab | 494 ± 7 BC | 9.7 ± 0.4 ab | 513 ± 20 B |
| 21:5n-3 | 0.3 ± 0.0 a | 21 ± 1 A | --- | --- | 0.3 ± 0.0 a | 14 ± 0 C | 0.3 ± 0.0 a | 15 ± 0 C | 0.4 ± 0.1 b | 20 ± 3 AB | 0.3 ± 0.0 ab | 18 ± 1 B |
| 22:4n-6 | 0.5 ± 0.1 a | 30 ± 7 A | --- | --- | 0.3 ± 0.0 ab | 14 ± 1 B | 0.3 ± 0.0 b | 14 ± 0 B | 0.4 ± 0.1 ab | 18 ± 3 B | 0.3 ± 0.0 ab | 17 ± 1 B |
| 22:5n-6 | 1.0 ± 0.1 a | 63 ± 5 A | --- | --- | 1.0 ± 0.1 a | 40 ± 3 C | 0.9 ± 0.0 a | 40 ± 1 C | 1.0 ± 0.0 a | 52 ± 0 B | 1.0 ± 0.1 a | 50 ± 3 B |
| 22:5n-3 | 2.7 ± 0.2 a | 177 ± 15 A | --- | --- | 2.7 ± 0.1 a | 111 ± 4 C | 2.5 ± 0.0 a | 116 ± 2 C | 2.9 ± 0.1 a | 147 ± 5 B | 2.7 ± 0.2 a | 143 ± 11 B |
| 22:6n-3 | 29.7 ± 2.3 a | 1957 ± 154 A | --- | --- | 29.3 ± 2.2 a | 1227 ± 92 D | 26.4 ± 0.3 a | 1234 ± 14 CD | 30.0 ± 0.4 a | 1544 ± 20 BC | 29.3 ± 2.3 a | 1552 ± 121 B |
| ΣPUFA | 54.0 ± 3.1 ab | 3565 ± 202 A | 58.6 ± 3.8 a | 144 ± 9 D | 53.2 ± 2.5 ab | 2229 ± 105 C | 49.4 ± 0.5 b | 2307 ± 22 C | 53.9 ± 0.3 ab | 2777 ± 17 B | 52.9 ± 3.0 ab | 2805 ± 158 B |
| Σn-3 PUFA | 46.4 ± 3.1 a | 3060 ± 206 A | 6.0 ± 0.4 b | 14.9 ± 1.0 D | 45.5 ± 2.4 a | 1909 ± 99 C | 42.0 ± 0.4 a | 1960 ± 20 C | 46.1 ± 0.3 a | 2374 ± 17 B | 45.4 ± 2.8 a | 2489 ± 43 B |
| Σn-6 PUFA | 6.7 ± 0.7 a | 441 ± 47 A | 52.6 ± 3.4 b | 129 ± 8 D | 6.9 ± 0.2 a | 291 ± 7 C | 6.6 ± 0.1 a | 307 ± 5 BC | 6.9 ± 0.0 a | 354 ± 1 BC | 6.8 ± 0.2 a | 365 ± 4 B |
| Σn-3/Σn-6 | 7.0 ± 0.9 a ± a | 0.1 ± 0.0 b ± a | 6.6 ± 0.2 a ± a | 6.4 ± 0.1 a ± a | 6.7 ± 0.1 a ± a | 6.7 ± 0.2 a ± a | ||||||
| IA | 0.2 ± 0.0 ab ± a | 0.1 ± 0.0 c ± a | 0.2 ± 0.0 ab ± a | 0.3 ± 0.0 a ± a | 0.2 ± 0.0 b ± a | 0.2 ± 0.0 b ± a | ||||||
| IT | 0.1 ± 0.0 ab ± a | 0.2 ± 0.0 a ± a | 0.1 ± 0.0 ab ± a | 0.2 ± 0.0 a ± a | 0.1 ± 0.0 b ± a | 0.1 ± 0.0 b ± a | ||||||
| Raw Chub Mackerel | Raw Hamburger | Steamed Hamburger | Roasted Hamburger | Grilled Hamburger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/100 g) | % Bioac. | (g/100 g) | % Bioac. | (g/100 g) | % Bioac. | (g/100 g) | % Bioac. | (g/100 g) | % Bioac. | |
| Total Lipids | 5.3 ± 1.0 a | 73 ± 8 A | 4.9 ± 0.6 a | 95 ± 5 B | 5.4 ± 0.2 a | 96 ± 3 B | 5.4 ± 0.3 a | 84 ± 5 AB | 5.8 ± 0.3 a | 87 ± 1 AB |
| Fatty Acid | (mg/100 g) | % Bioac. | (mg/100 g) | % Bioac. | (mg/100 g) | % Bioac. | (mg/100 g) | % Bioac. | (mg/100 g) | % Bioac. |
| 16:0 | 731 ± 74 ab | 93 ± 9 A | 539 ± 43 b | 113 ± 7 AB | 743 ± 18 a | 117 ± 2 AB | 691 ± 32 ab | 116 ± 5 AB | 752 ± 90 a | 130 ± 15 B |
| 17:0 | 43 ± 4 a | 91 ± 8 A | 34 ± 1 b | 108 ± 2 BC | 40 ± 1 ab | 108 ± 2 BC | 36 ± 1 ab | 93 ± 3 AB | 42 ± 2 ab | 117 ± 6 C |
| 18:0 | 258 ± 13 a | 63 ± 3 A | 197 ± 4 c | 72 ± 2 B | 241 ± 3 b | 80 ± 1 C | 196 ± 1 c | 60 ± 0 A | 268 ± 4 a | 87 ± 1 D |
| 17:1 | 24 ± 2 b | 76 ± 6 A | 21 ± 1 a | 99 ± 3 B | 26 ± 0 bc | 110 ± 1 C | 23 ± 1 ab | 96 ± 3 B | 28 ± 1 c | 113 ± 3 C |
| 18:1n-9 | 646 ± 24 a | 79 ± 3 A | 530 ± 36 b | 104 ± 5 BC | 647 ± 1 a | 101 ± 0 BC | 651 ± 1 a | 96 ± 0 B | 695 ± 15 a | 108 ± 2 C |
| 18:1n-7 | 156 ± 5 a | 76 ± 2 A | 125 ± 4 c | 100 ± 3 C | 144 ± 1 b | 98 ± 1 C | 143 ± 1 b | 91 ± 1 B | 158 ± 1 a | 103 ± 1 C |
| 20:1n-9 | 15 ± 1 bc | 14 ± 1 A | 11 ± 1 a | 18 ± 1 B | 14 ± 0 abc | 18 ± 0 B | 13 ± 1 ab | 15 ± 1 A | 16 ± 1 c | 18 ± 1 B |
| 18:2n-6 | 7 ± 2 a | 5 ± 1 A | 16 ± 0 d | 14 ± 0 C | 10 ± 0 bc | 8 ± 0 B | 8 ± 0 ab | 7 ± 0 AB | 11 ± 0 c | 13 ± 0 C |
| 18:3n-3 | 43 ± 10 a | 62 ± 12 A | 43 ± 1 ab | 78 ± 1 AB | 50 ± 1 ab | 85 ± 1 BC | 50 ± 1 ab | 79 ± 2 AB | 55 ± 3 b | 86 ± 5 C |
| 18:4n-3 | 48 ± 3 a | 57 ± 4 A | 49 ± 3 ab | 100 ± 6 B | 59 ± 0 c | 100 ± 1 B | 57 ± 2 bc | 95 ± 3 B | 64 ± 4 c | 100 ± 3 B |
| 20:2n-6 | 22 ± 1 bc | 65 ± 4 A | 18 ± 0 a | 91 ± 0 B | 20 ± 0 ab | 94 ± 2 B | 19 ± 0 ab | 75 ± 2 A | 24 ± 2 c | 91 ± 6 B |
| 20:4n-6 | 47 ± 2 a | 30 ± 1 A | 50 ± 3 a | 48 ± 3 C | 46 ± 0 a | 44 ± 0 C | 45 ± 1 a | 36 ± 1 B | 55 ± 3 b | 44 ± 2 C |
| 20:4n-3 | 23 ± 2 a | 50 ± 3 A | 22 ± 1 a | 82 ± 3 B | 24 ± 1 ab | 87 ± 2 B | 24 ± 3 a | 82 ± 10 B | 28 ± 2 b | 86 ± 5 B |
| 20:5n-3 | 323 ± 20 a | 47 ± 3 A | 336 ± 6 ab | 81 ± 1 BC | 374 ± 3 c | 86 ± 1 C | 365 ± 5 bc | 74 ± 1 B | 441 ± 17 d | 82 ± 3 C |
| 21:5n-3 | 12 ± 0 a | 59 ± 2 A | 13 ± 0 a | 94 ± 1 B | 14 ± 0 a | 98 ± 2 B | 14 ± 2 a | 72 ± 10 A | 18 ± 1 b | 91 ± 6 B |
| 22:4n-6 | 13 ± 3 a | 44 ± 9 A | 11 ± 1 a | 74 ± 5 B | 11 ± 0 a | 75 ± 1 B | 11 ± 2 a | 60 ± 9 AB | 13 ± 1 a | 76 ± 0 B |
| 22:5n-6 | 31 ± 2 a | 50 ± 4 A | 32 ± 2 a | 76 ± 6 C | 33 ± 0 a | 82 ± 1 C | 33 ± 0 a | 63 ± 0 B | 42 ± 3 b | 73 ± 1 BC |
| 22:5n-3 | 95 ± 8 a | 53 ± 4 A | 102 ± 3 a | 88 ± 3 C | 106 ± 2 a | 91 ± 1 C | 107 ± 4 a | 73 ± 3 B | 131 ± 10 b | 81 ± 4 BC |
| 22:6n-3 | 809 ± 64 a | 41 ± 3 A | 938 ± 66 ab | 72 ± 5 BC | 969 ± 11 b | 79 ± 1 C | 961 ± 13 ab | 62 ± 1 B | 1219 ± 95 c | 69 ± 2 BC |
| Element | Ingredient | Hamburger | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw Chub Mackerel | Quinoa (Boiled) | Seaweed Saccorhiza polyschides (Freeze-Dried) * | Raw | Steamed | Roasted | Grilled | ||
| Se | Initial (µg/100 g) | 59 ± 4 A | 1.1 ± 0.1 A | 116 ± 0 | 48 ± 2 aA | 50 ± 4 aA | 54 ± 3 abA | 61 ± 5 bA |
| Bioacc. (µg/100 g) | 47 ± 3 B | 0.08 ± 0.01 B | <LOQ | 38 ± 1 aB | 41 ± 3 aB | 46 ± 4 aB | 43 ± 2 aB | |
| Bioaacessibility (%) | 80 ± 0 ¥ | 7 ± 0 § | <LOQ | 80 ± 10 ¥ | 82 ± 13 ¥ | 85 ± 5 ¥ | 70 ± 0 ¥ | |
| I | Initial (µg/100 g) | 25 ± 1 A | <LOQ | 36,720 ± 603 A | 224 ± 7 aA | 221 ± 2 aA | 232 ± 16 abA | 255 ± 6 bA |
| Bioacc. (µg/100 g) | 19 ± 1 B | ND | 17,586 ± 289 B | 127 ± 4 aB | 153 ± 1 bB | 163 ± 11 bB | 153 ± 4 bB | |
| Bioaacessibility (%) | 78 ± 0 ¥ | ND | 48 ± 3 £ | 57 ± 1 Ϫ | 69 ± 2 § | 70 ± 1 § | 60 ± 2 Ϫ | |
| Property | Ingredient | Hamburger | ||||
|---|---|---|---|---|---|---|
| Seaweed Saccorhiza polyschides (Freeze-Dried) | Raw | Steamed | Roasted | Grilled | ||
| Polyphenol content | (mg GAE/g) | 1.25 ± 0.06 | 0.06 ± 0.02 b | 0.08 ± 0.01 ab | 0.12 ± 0.03 a | 0.02 ± 0.02 c |
| FRAP | (µmol Fe2+ eq./g) | 9.5 ± 0.8 | 1.1 ± 0.2 ab | 0.9 ± 0.3 b | 1.5 ± 0.5 a | 0.4 ± 0.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, C.; Valentim, J.; Gomes, R.; Matos, J.; Rego, A.; Coelho, I.; Delgado, I.; Motta, C.; Castanheira, I.; Prates, J.A.M.; et al. Mackerel and Seaweed Burger as a Functional Product for Brain and Cognitive Aging Prevention. Foods 2024, 13, 1332. https://doi.org/10.3390/foods13091332
Cardoso C, Valentim J, Gomes R, Matos J, Rego A, Coelho I, Delgado I, Motta C, Castanheira I, Prates JAM, et al. Mackerel and Seaweed Burger as a Functional Product for Brain and Cognitive Aging Prevention. Foods. 2024; 13(9):1332. https://doi.org/10.3390/foods13091332
Chicago/Turabian StyleCardoso, Carlos, Jorge Valentim, Romina Gomes, Joana Matos, Andreia Rego, Inês Coelho, Inês Delgado, Carla Motta, Isabel Castanheira, José A. M. Prates, and et al. 2024. "Mackerel and Seaweed Burger as a Functional Product for Brain and Cognitive Aging Prevention" Foods 13, no. 9: 1332. https://doi.org/10.3390/foods13091332