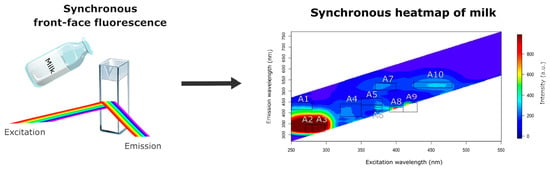
Synchronous Front-Face Fluorescence Spectra: A Review of Milk Fluorophores
Abstract
:1. Introduction
2. General Fluorophores Features
3. Fluorescence Measurements
3.1. Fluorescence Geometry
3.2. Fluorescence Measurements
4. Synchronous Front-Face Fluorescence of Milk
4.1. Area 1 (Exc. 250–280 nm/Em. 420–450 nm)
4.2. Areas 2 and 3 (Exc. 270–280 nm/Em. 323–355 nm and Exc. 280–298 nm/Em. 323–355 nm)
4.3. Area 4 (Exc. 320–350 nm/Em. 390–445 nm)
4.4. Area 5 (Exc. 350–380 nm/Em. 440–470 nm)
4.5. Areas 6, 7, and 8 (Exc. 368–380 nm/Em. 415–430 nm; Exc. 370–400 nm/Em. 505–535 nm; Exc. 390–410 nm/Em. 410–430 nm)
4.6. Area 9 (Exc. 410–430 nm/Em. 410–450 nm)
4.7. Area 10 (Exc. 425–480 nm/Em. 515–540 nm)
4.8. Other Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Massouras, T.; Charmanta, A.-A.; Koutsouli, P.; Masoura, M.; Politis, I.; Hettinga, K. The Effect of Milking Frequency, Breed, and Stage of Lactation on the Milk Fat Globule Size and Fatty Acid Composition in Sheep’s Milk. Foods 2023, 12, 2446. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Heat Treatment of Milk: Heat Stability of Milk. In Encyclopedia of Dairy Sciences; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 744–749. ISBN 978-0-12-374407-4. [Google Scholar]
- Liu, J.; Zamora, A.; Castillo, M.; Saldo, J. Modeling of the Changes in Bovine Milk Caused by Ultra-High Pressure Homogenization Using Front-Face Fluorescence Spectroscopy. J. Food Eng. 2018, 233, 88–97. [Google Scholar] [CrossRef]
- Babu, K.S.; Amamcharla, J.K. Application of Front-Face Fluorescence Spectroscopy as a Tool for Monitoring Changes in Milk Protein Concentrate Powders during Storage. J. Dairy Sci. 2018, 101, 10844–10859. [Google Scholar] [CrossRef]
- Karoui, R.; Blecker, C. Fluorescence Spectroscopy Measurement for Quality Assessment of Food Systems—A Review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Ayala, N.; Zamora, A.; Rinnan, Å.; Saldo, J.; Castillo, M. The Effect of Heat Treatment on the Front-Face Fluorescence Spectrum of Tryptophan in Skim Milk. J. Food Compos. Anal. 2020, 92, 103569. [Google Scholar] [CrossRef]
- Schamberger, G.P.; Labuza, T.P. Evaluation of Front-Face Fluorescence for Assessing Thermal Processing of Milk. J. Food Sci. 2006, 71, C69–C74. [Google Scholar] [CrossRef]
- Karoui, R.; Martin, B.; Dufour, É. Potentiality of Front-Face Fluorescence Spectroscopy to Determine the Geographic Origin of Milks from the Haute-Loire Department (France). Le Lait 2005, 85, 223–236. [Google Scholar] [CrossRef]
- Dufour, E.; Riaublanc, A. Potentiality of Spectroscopic Methods for the Characterisation of Dairy Products. I. Front-Face Fluorescence Study of Raw, Heated and Homogenised Milks. Le Lait 1997, 77, 657–670. [Google Scholar] [CrossRef]
- Teng, Y.T.; Freire, P.; Zamora, A.; Castillo, M. Tryptophan Front-Face Fluorescence and Functional Properties of Whey: A Preliminary Study. LWT 2022, 163, 113589. [Google Scholar] [CrossRef]
- Taterka, H. Optical Prediction Models of Whey Protein Denaturation in Thermally Treated Milk for the Development for an Inline Sensor; Universitat Autònoma de Barcelona: Bellaterra, Spain, 2016. [Google Scholar]
- Ayala, N.; Zamora, A.; González, C.; Saldo, J.; Castillo, M. Predicting Lactulose Concentration in Heat-Treated Reconstituted Skim Milk Powder Using Front-Face Fluorescence. Food Control 2017, 73, 110–116. [Google Scholar] [CrossRef]
- Alvarado, U. Aplicación de Indicadores Nativos de Fluorescencia para la Evaluación Rápida de Daño Térmico en el Procesado de Leche; Veterinary of the Universitat Autònoma de Barcelona: Bellaterra, Spain, 2016. [Google Scholar]
- Alvarado, U.; Zamora, A.; Arango, O.; Saldo, J.; Castillo, M. Prediction of Riboflavin and Ascorbic Acid Concentrations in Skimmed Heat-Treated Milk Using Front-Face Fluorescence Spectroscopy. J. Food Eng. 2022, 318, 110869. [Google Scholar] [CrossRef]
- Ayala, O. Application of Native Fluorescence Tracers for Quick Evaluation of Thermal Damage in Milk; Universitat Autònoma de Barcelona: Bellaterra, Spain, 2018. [Google Scholar]
- Liu, J.; Zamora, A.; Castillo, M.; Saldo, J. Using Front-Face Fluorescence Spectroscopy for Prediction of Retinol Loss in Milk during Thermal Processing. LWT 2018, 87, 151–157. [Google Scholar] [CrossRef]
- Henihan, L.E.; O’Donnell, C.P.; Esquerre, C.; Murphy, E.G.; O’Callaghan, D.J. Quality Assurance of Model Infant Milk Formula Using a Front-Face Fluorescence Process Analytical Tool. Food Bioprocess Technol. 2018, 11, 1402–1411. [Google Scholar] [CrossRef]
- Christensen, J.; Nørgaard, L.; Bro, R.; Engelsen, S.B. Multivariate Autofluorescence of Intact Food Systems. Chem. Rev. 2006, 106, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; O’Donnell, C. Applications of Fluorescence Spectroscopy in Dairy Processing: A Review. Curr. Opin. Food Sci. 2017, 17, 16–24. [Google Scholar] [CrossRef]
- Andersen, C.M.; Mortensen, G. Fluorescence Spectroscopy: A Rapid Tool for Analyzing Dairy Products. J. Agric. Food Chem. 2008, 56, 720–729. [Google Scholar] [CrossRef]
- Lopez, C.; Dufour, E. The Composition of the Milk Fat Globule Surface Alters the Structural Characteristics of the Coagulum. J. Colloid Interface Sci. 2001, 233, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Karoui, R. Monitoring of Mild Heat Treatment of Camel Milk by Front-Face Fluorescence Spectroscopy. LWT—Food Sci. Technol. 2017, 79, 586–593. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Li, X.-Y.; Shindi, A.A.F.; Zou, Z.-X.; Liu, Q.; Lin, L.-R.; Li, N. Synchronous Fluorescence Spectroscopy and Its Applications in Clinical Analysis and Food Safety Evaluation. In Reviews in Fluorescence 2010; Geddes, C.D., Ed.; Springer: New York, NY, USA, 2012; pp. 95–117. ISBN 978-1-4419-9828-6. [Google Scholar]
- Radotić, K.; Stanković, M.; Bartolić, D.; Natić, M. Intrinsic Fluorescence Markers for Food Characteristics, Shelf Life, and Safety Estimation: Advanced Analytical Approach. Foods 2023, 12, 3023. [Google Scholar] [CrossRef]
- Boubellouta, T.; Galtier, V.; Dufour, É. Structural Changes of Milk Components during Acid-Induced Coagulation Kinetics as Studied by Synchronous Fluorescence and Mid-Infrared Spectroscopy. Appl. Spectrosc. 2011, 65, 284–292. [Google Scholar] [CrossRef]
- Durakli Velioglu, S.; Ercioglu, E.; Boyaci, I.H. Rapid Discrimination between Buffalo and Cow Milk and Detection of Adulteration of Buffalo Milk with Cow Milk Using Synchronous Fluorescence Spectroscopy in Combination with Multivariate Methods. J. Dairy Res. 2017, 84, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Freire, P.; Freire, D.; Licon, C.C. A Comprehensive Review of Machine Learning and Its Application to Dairy Products. Crit. Rev. Food Sci. Nutr. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Genis, D.O.; Sezer, B.; Bilge, G.; Durna, S.; Boyaci, I.H. Development of Synchronous Fluorescence Method for Identification of Cow, Goat, Ewe and Buffalo Milk Species. Food Control 2020, 108, 106808. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Han, D.; Wang, S. Detection of the Presence of Reconstituted Milk in Raw Milk and in Pasteurized Milk Using Synchronous Fluorescence Spectroscopy. Food Anal. Methods 2017, 10, 2078–2084. [Google Scholar] [CrossRef]
- Ma, Y.B.; Amamcharla, J.K. Front-Face Fluorescence Spectroscopy Combined with Chemometrics to Detect High Proteinaceous Matter in Milk and Whey Ultrafiltration Permeate. J. Dairy Sci. 2019, 102, 8756–8767. [Google Scholar] [CrossRef] [PubMed]
- Blecker, C.; Habib-Jiwan, J.M.; Karoui, R. Effect of Heat Treatment of Rennet Skim Milk Induced Coagulation on the Rheological Properties and Molecular Structure Determined by Synchronous Fluorescence Spectroscopy and Turbiscan. Food Chem. 2012, 135, 1809–1817. [Google Scholar] [CrossRef]
- Elgarhi, H.-E.; El-Aidie, S.; Hamdy, S.; Abbas, K. Identification of Milk Types Using Two Different Fluorescence Spectroscopy Techniques. Egypt. J. Food Sci. 2020, 48, 73–80. [Google Scholar] [CrossRef]
- Fotakis, C.; Mousdis, G.; Langi, P.; Kalantzi, K.; Hatzigeorgiou, A.; Proestos, C. Front Face Synchronous Fluorescence as a Tool for the Quality Assurance of Greek Milk. Arab. J. Chem. 2020, 13, 7875–7885. [Google Scholar] [CrossRef]
- Albani, J.R. Principles and Applications of Fluorescence Spectroscopy; Blackwell Science: Oxford, UK, 2007. [Google Scholar]
- Chatterjee, D.P.; Pakhira, M.; Nandi, A.K. Fluorescence in “Nonfluorescent” Polymers. ACS Omega 2020, 5, 30747–30766. [Google Scholar] [CrossRef]
- Hofmann, A. Spectroscopic Techniques. In Principles and Techniques of Biochemistry and Molecular Biology; Wilson, K., Walker, J., Eds.; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780521516358. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Kulmyrzaev, A.A.; Levieux, D.; Dufour, É. Front-Face Fluorescence Spectroscopy Allows the Characterization of Mild Heat Treatments Applied to Milk. Relations with the Denaturation of Milk Proteins. J. Agric. Food Chem. 2005, 53, 502–507. [Google Scholar] [CrossRef]
- Hammami, M.; Rouissi, H.; Salah, N.; Selmi, H.; Al-Otaibi, M.; Blecker, C.; Karoui, R. Fluorescence Spectroscopy Coupled with Factorial Discriminant Analysis Technique to Identify Sheep Milk from Different Feeding Systems. Food Chem. 2010, 122, 1344–1350. [Google Scholar] [CrossRef]
- Zaïdi, F.; Rouissi, H.; Dridi, S.; Kammoun, M.; de Baerdemaeker, J.; Karoui, R. Front-Face Fluorescence Spectroscopy as a Rapid and Non-Destructive Tool for Differentiating between Sicilo-Sarde and Comisana Ewe’s Milk during Lactation Period: A Preliminary Study. Food Bioprocess Technol. 2008, 1, 143–151. [Google Scholar] [CrossRef]
- Brandao, M.P.; Anjos, V.d.C.d.; Bell, M.J.V. Time resolved fluorescence of cow and goat milk powder. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Dufour, É. Recent Advances in the Analysis of Dairy Product Quality Using Methods Based on the Interactions of Light with Matter. Int. J. Dairy Technol. 2011, 64, 153–165. [Google Scholar] [CrossRef]
- Sugiyama, J.; Fujita, K. Detection of Food Safety Using Fluorescence Fingerprint. Hitachi Sci. Instrum. News 2014, 5, 17–22. [Google Scholar]
- Yu, J.; Xiao, K.; Xue, W.; Shen, Y.; Tan, J.; Liang, S. Excitation-emission Matrix (EEM) Fluorescence Spectroscopy for Characterization of Organic Matter in Membrane Bioreactors Principles Methods and Applications. Front. Front. Environ. Sci. Eng. 2020, 14, 31. [Google Scholar] [CrossRef]
- Dankowska, A. Advances in Fluorescence Emission Spectroscopy for Food Authenticity Testing. In Advances in Food Authenticity Testing; Downey, G., Ed.; Woodhead Publishing: Amsterdam, The Netherlands, 2016; pp. 117–145. ISBN 9780081002209. [Google Scholar]
- Bouvellouta, T.; Dufour, É. Effects of Mild Heating and Acidification on the Molecular Structure of Milk Components as Investigated by Synchronous Front-Face Fluorescence Spectroscopy Coupled with Parallel Factor Analysis. Appl. Spectrosc. 2008, 62, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, A.B.; Lawaetz, A.J.; Ipsen, R.H. Front Face Fluorescence Spectroscopy and Multi-Way Data Analysis for Characterization of Milk Pasteurized Using Instant Infusion. LWT—Food Sci. Technol. 2013, 53, 331–337. [Google Scholar] [CrossRef]
- Fragkoulis, N.; Samartzis, P.C.; Velegrakis, M. Commercial Milk Discrimination by Fat Content and Animal Origin Using Optical Absorption and Fluorescence Spectroscopy. Int. Dairy J. 2021, 123, 105181. [Google Scholar] [CrossRef]
- Ali, H.; Saleem, M.; Ullah, R.; Khan, S.; Atta, B.M.; Bilal, M. Thermal Effects on Biochemical Signatures of UHT, Pasteurized and Domestically Boiled Buffalo Milk Detected by Synchronous Fluorescence Spectroscopy. J. Fluoresc. 2019, 29, 485–493. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.; Ali, H.; Bilal, M. Potentiality of Using Front Face Fluorescence Spectroscopy for Quantitative Analysis of Cow Milk Adulteration in Buffalo Milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117518. [Google Scholar] [CrossRef]
- Ozer, D.; Bilge, G.; Sezer, B.; Durna, S.; Hakki, I. Identification of Cow, Buffalo, Goat and Ewe Milk Species in Fermented Dairy Products Using Synchronous Fluorescence Spectroscopy. Food Chem. 2019, 284, 60–66. [Google Scholar] [CrossRef]
- Combs, G.F.; McClung, J.P. Vitamin A. In The Vitamins, 5th ed.; Combs, G.F., McClung, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 109–159. ISBN 978-0-12-802965-7. [Google Scholar]
- MacGibbon, A.K.H. General Characteristics of Milk Lipids☆. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 814–820. ISBN 978-0-12-818767-8. [Google Scholar]
- Futterman, S.; Heller, J. The Enhancement of Fluorescence and the Decreased Susceptibility to Enzymatic Oxidation of Retinol Complexed with Bovine Serum Albumin, β-Lactoglobulin, and the Retinol-Binding Protein of Human Plasma. J. Biol. Chem. 1972, 247, 5168–5172. [Google Scholar] [CrossRef]
- Le, T.T. Thermal Denaturation and Aggregation of Whey Proteins. In Encyclopedia of Dairy Sciences; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier: Oxford, UK, 2022; pp. 623–628. ISBN 978-0-12-818767-8. [Google Scholar]
- Teale, F.W. The Ultraviolet Fluorescence of Proteins in Neutral Solution. Biochem. J. 1960, 76, 381–388. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Mukhopadhyay, S. Studying Protein Misfolding and Aggregation by Fluorescence Spectroscopy. In Reviews in Fluorescence 2015; Geddes, C., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–27. ISBN 9783319246093. [Google Scholar]
- Lelis, C.A.; Galvan, D.; Tessaro, L.; de Andrade, J.C.; Mutz, Y.S.; Conte-Junior, C.A. Fluorescence Spectroscopy in Tandem with Chemometric Tools Applied to Milk Quality Control. J. Food Compos. Anal. 2022, 109, 104515. [Google Scholar] [CrossRef]
- Singh, R.; Amamcharla, J.K. Effect of PH on Heat-Induced Interactions in High-Protein Milk Dispersions and Application of Fluorescence Spectroscopy in Characterizing These Changes. J. Dairy Sci. 2021, 104, 3899–3915. [Google Scholar] [CrossRef]
- Grigoryan, K.R.; Shilajyan, H.A. Fluorescence 2D and 3D Spectra Analysis of Tryptophan, Tyrosine and Phenylalanine. Chem. Biol. 2017, 51, 3–7. [Google Scholar]
- Murillo Pulgarín, J.; Alañón, A.; Alañón, M.T. Fluorescence Characteristics of Several Whey Samples Subjected to Different Treatments and Conditions. Anal. Chim. Acta 2005, 536, 153–158. [Google Scholar] [CrossRef]
- Matiacevich, S.B.; Santagapita, P.R.; Buera, M.P. Fluorescence from the Maillard Reaction and Its Potential Applications in Food Science. Crit. Rev. Food Sci. Nutr. 2005, 45, 483–495. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food Protein-Polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Nursten, H. Maillard Reactions. In Encyclopedia of Dairy Sciences; Roginski, H., Ed.; Elsevier: Oxford, UK, 2002; pp. 1657–1672. ISBN 978-0-12-227235-6. [Google Scholar]
- Sell, D.R.; Monnier, V.M. Structure Elucidation of a Senescence Cross-Link from Human Extracellular Matrix: Implication of Pentoses in the Aging Process. J. Biol. Chem. 1989, 264, 21597–21602. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Sun, D.W. Advanced Glycation End-Products (AGEs) in Foods and Their Detecting Techniques and Methods: A Review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Henle, T.; Schwarzenbolz, U.; Klostermeyer, H. Detection and Quantification of Pentosidine in Foods. Eur. Food Res. Technol. 1997, 204, 95–98. [Google Scholar] [CrossRef]
- Harms, G.S.; Pauls, S.W.; Hedstrom, J.F.; Johnson, C.K. Fluorescence and Rotational Dynamics of Dityrosine. J. Fluoresc. 1997, 7, 283–292. [Google Scholar] [CrossRef]
- Schmidt, A.; Schreiner, M.G.; Mayer, H.K. Rapid Determination of the Various Native Forms of Vitamin B6 and B2 in Cow’s Milk Using Ultra-High Performance Liquid Chromatography. J. Chromatogr. A 2017, 1500, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, H.; Nakano, K.; Shigeta, H.; Yamaguchi, M.; Yoshimori, K.; Fukui, M.; Fujii, M.; Kitagawa, Y.; Nakamura, N.; Nakamura, K.; et al. Formation of Crossline as a Fluorescent Advanced Glycation End Productin Vitroandin Vivo. Biochem. Biophys. Res. Commun. 1996, 226, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Stender, I.-M.; Henriksen, M.; Wulf, H.C. Autofluorescence of Human Skin Is Age-Related After Correction for Skin Pigmentation and Redness. J. Investig. Dermatol. 2001, 116, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.; Mackenzie, T.; Corstjens, H.; Muizzuddin, N.; Matsui, M.S. Two Fluorescent Wavelengths, 440ex/520em Nm and 370ex/440em Nm, Reflect Advanced Glycation and Oxidation End Products in Human Skin without Diabetes. Diabetes Technol. Ther. 2012, 14, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Tu, M.-C.; Zhung, P. Advanced Glycation End Product (AGE): Characterization of the Products from the Reaction between D-Glucose and Serum Albumin. J. Clin. Lab. Anal. 1996, 10, 21–34. [Google Scholar] [CrossRef]
- Graham, L.; Peters, R.; Nagaraj, R.H.; Sayre, L.M.; Monnier, V.M. Structure and Biological Significance of Pentodilysine, a Novel Fluorescent Advanced Maillard Reaction Protein Crosslink. In The Maillard Reaction in Foods and Medicine; O’Brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; Woodhead Publishing: Cambrige, UK, 2005; p. 410. ISBN 978-1-85573-791-4. [Google Scholar]
- Birlouez-Aragon, I.; Nicolas, M.; Metais, A.; Marchond, N.; Grenier, J.; Calvo, D. A Rapid Fluorimetric Method to Estimate the Heat Treatment of Liquid Milk. Int. Dairy J. 1998, 8, 771–777. [Google Scholar] [CrossRef]
- Ferrer, E.; Alegría, A.; Farré, R.; Clemente, G.; Calvo, C. Fluorescence, Browning Index, and Color in Infant Formulas during Storage. J. Agric. Food Chem. 2005, 53, 4911–4917. [Google Scholar] [CrossRef]
- Morales, F.J.; Boekel, M.A.J.S. Van A Study on Advanced Maillard Reaction in Heated Casein/Sugar Solutions: Colour Formation. J. Agric. Food Chem. 1997, 6946, 907–915. [Google Scholar]
- Yang, H.; Xiao, X.; Zhao, X.S.; Hu, L.; Xue, X.F.; Ye, J.S. Study on Fluorescence Spectra of Thiamine and Riboflavin. MATEC Web Conf. 2016, 63, 03013. [Google Scholar] [CrossRef]
- Karoui, R.; Mazerolles, G.; Dufour, É. Spectroscopic Techniques Coupled with Chemometric Tools for Structure and Texture Determinations in Dairy Products. Int. Dairy J. 2003, 13, 607–620. [Google Scholar] [CrossRef]
- Wold, J.P.; Jørgensen, K.; Lundby, F. Nondestructive Measurement of Light-Induced Oxidation in Dairy Products by Fluorescence Spectroscopy and Imaging. J. Dairy Sci. 2002, 85, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Koziol, J. Fluorometric Analyses of Riboflavin and Its Coenzymes. In Methods Enzymol; Academic Press: Cambridge, MA, USA, 1971; Volume 18, pp. 253–285. [Google Scholar]
- Bhattacharjee, U.; Jarashow, D.; Casey, T.A.; Petrich, J.W.; Rasmussen, M.A. Using Fluorescence Spectroscopy to Identify Milk from Grass-Fed Dairy Cows and to Monitor Its Photodegradation. J. Agric. Food Chem. 2018, 66, 2168–2173. [Google Scholar] [CrossRef]
- Wold, J.P.; Veberg, A.; Nilsen, A.; Iani, V.; Juzenas, P.; Moan, J. The Role of Naturally Occurring Chlorophyll and Porphyrins in Light-Induced Oxidation of Dairy Products. A Study Based on Fluorescence Spectroscopy and Sensory Analysis. Int. Dairy J. 2005, 15, 343–353. [Google Scholar] [CrossRef]






| Area | Fluorophore | Excitation Wavelength (nm) | Emission Wavelength (nm) | Reference |
|---|---|---|---|---|
| A1 | Carotenoids | >390 | 300–550 | [51] |
| Vit A | 321 shoulders 292/308 | 412 | [9] | |
| Vit A | 307/320 | 410 | [22] | |
| A2 | Tyr | 276 | 302 | [18] |
| Tyr | 280 | 305 | [60] | |
| A3 | Trp | 290 | 340 | [6,8,9,17] |
| A4 | AGEs | 310–350 | 380–430 | [30] |
| Pentosidine | 335 | 385 | [65] | |
| Dityrosine | 315 | 400 | [68] | |
| Vit B6 | 333 | 393 | [31] | |
| Vit B6 | 328 | 393 | [18] | |
| Vit A | 321 | 412 | [9] | |
| A5 | AGEs | 340–370 | 420–440 | [70] |
| AGEs | 340–370 | 420–470 | [62] | |
| AGEs | 370 | 440 | [71,72,73] | |
| Pentodilysine | 366 | 440 | [77] | |
| NADH | 360 | 460 | [38] | |
| NADH | 340 | 450 | [22] | |
| NADH | 340 | 460 | [37] | |
| A6 | AGEs | 340–370 | 420–440 | [70] |
| AGEs | 350 | 415 | [76] | |
| pyrropyridine | 379 | 455 | [77] | |
| A7 | Rb | 370 | 525 | [78] |
| Rb | 370 | 507 | [13] | |
| A8 | Rb | 380 | 420/520 | [8] |
| Rb | 370 | 490/507 | [13] | |
| A9 | Lumichrome | 450 | 444–479 | [20] |
| A10 | Rb | 450 | 525 | [78] |
| Rb | 450 | 522 | [13] | |
| FADH2 | 450 | 515 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, P.; Zamora, A.; Castillo, M. Synchronous Front-Face Fluorescence Spectra: A Review of Milk Fluorophores. Foods 2024, 13, 812. https://doi.org/10.3390/foods13050812
Freire P, Zamora A, Castillo M. Synchronous Front-Face Fluorescence Spectra: A Review of Milk Fluorophores. Foods. 2024; 13(5):812. https://doi.org/10.3390/foods13050812
Chicago/Turabian StyleFreire, Paulina, Anna Zamora, and Manuel Castillo. 2024. "Synchronous Front-Face Fluorescence Spectra: A Review of Milk Fluorophores" Foods 13, no. 5: 812. https://doi.org/10.3390/foods13050812