Antibacterial Activity of Modified Sesbania Gum Composite Film and Its Preservation Effect on Wampee Fruit (Clausena lansium (Lour.) Skeels)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oxidized SG
2.3. Preparation of OSG Composite Film
2.4. Determination of Thickness
2.5. Determination of Mechanical Properties
2.6. Determination of WATER Vapor Permeability (WVP)
2.7. Calculation of Comprehensive Score
2.8. Antibacterial Activity
2.9. Application of Composite Films in Wampee Fruit Preservation
2.9.1. Wampee Fruit Processing
2.9.2. Weight Loss Rate
2.9.3. Rate of Rotting
2.9.4. Determination of Total Soluble Solid Content
2.9.5. Determination of Titrable Acid
2.10. Statistical Analysis
3. Results and discussion
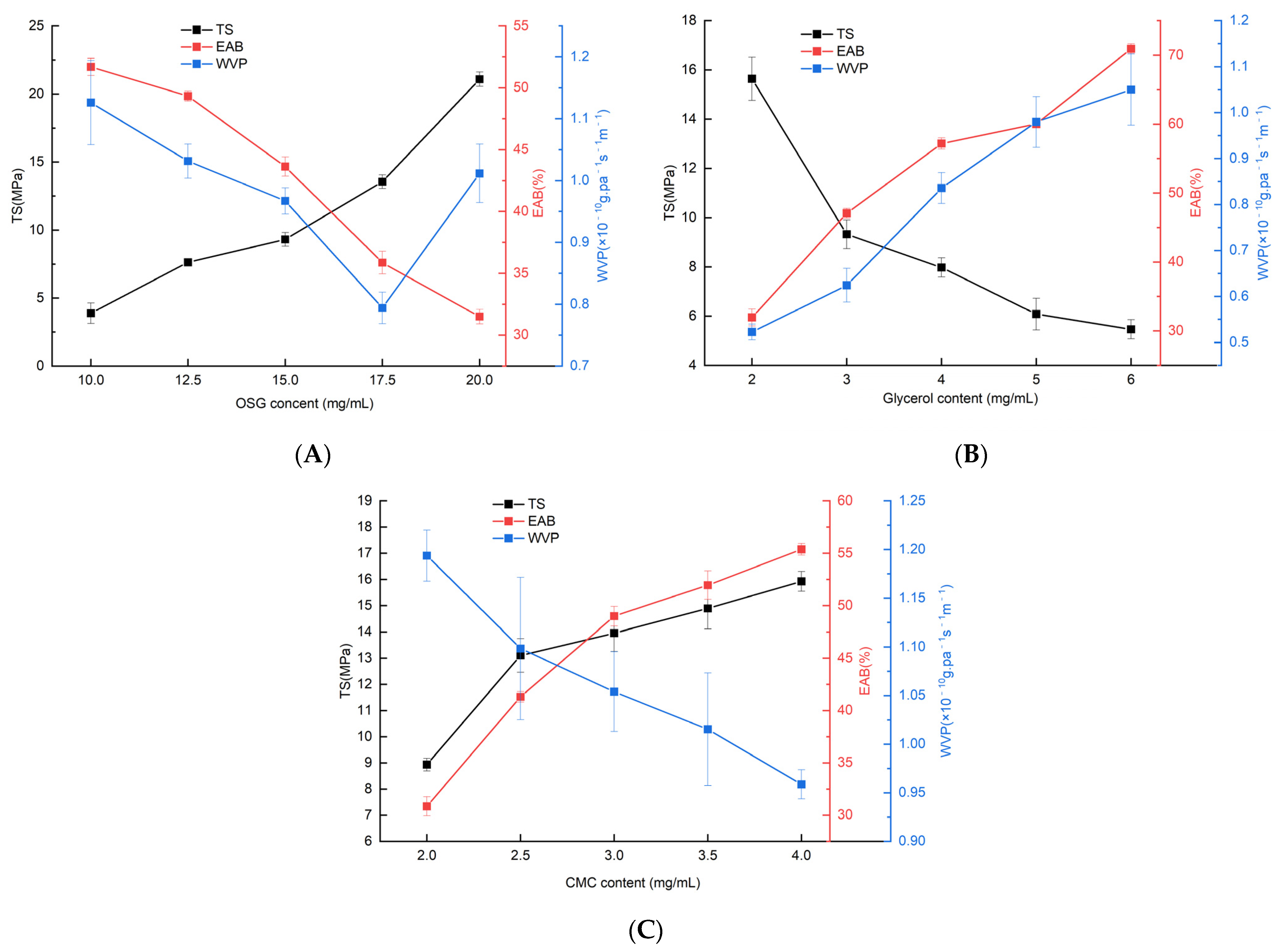
3.1. Effects of OSG, CMC, and Glycerol Additions on TS, EAB and WVP of Composite Films
3.2. Analysis of Box–Behnken Response Surface Optimization Results
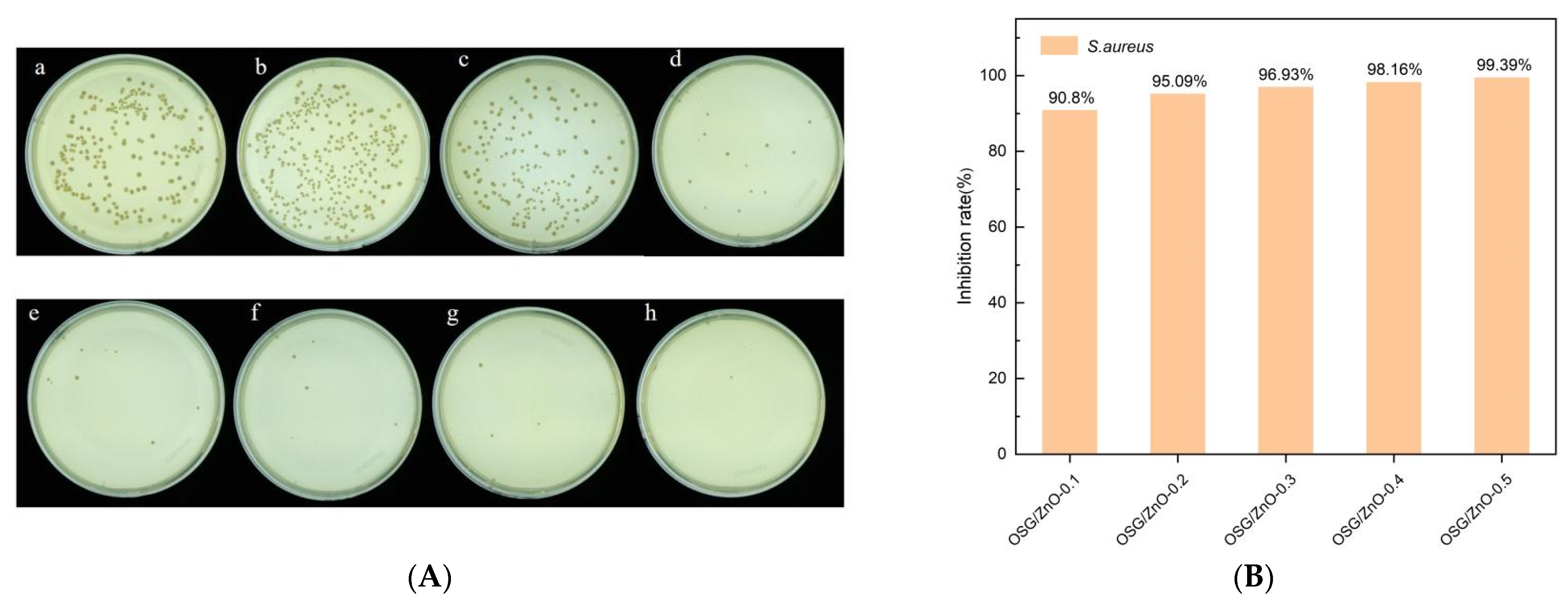
3.3. Determination of Antibacterial Activity
3.4. Effect Test of the Composite Film on the Wampee Fruit Preservation Period
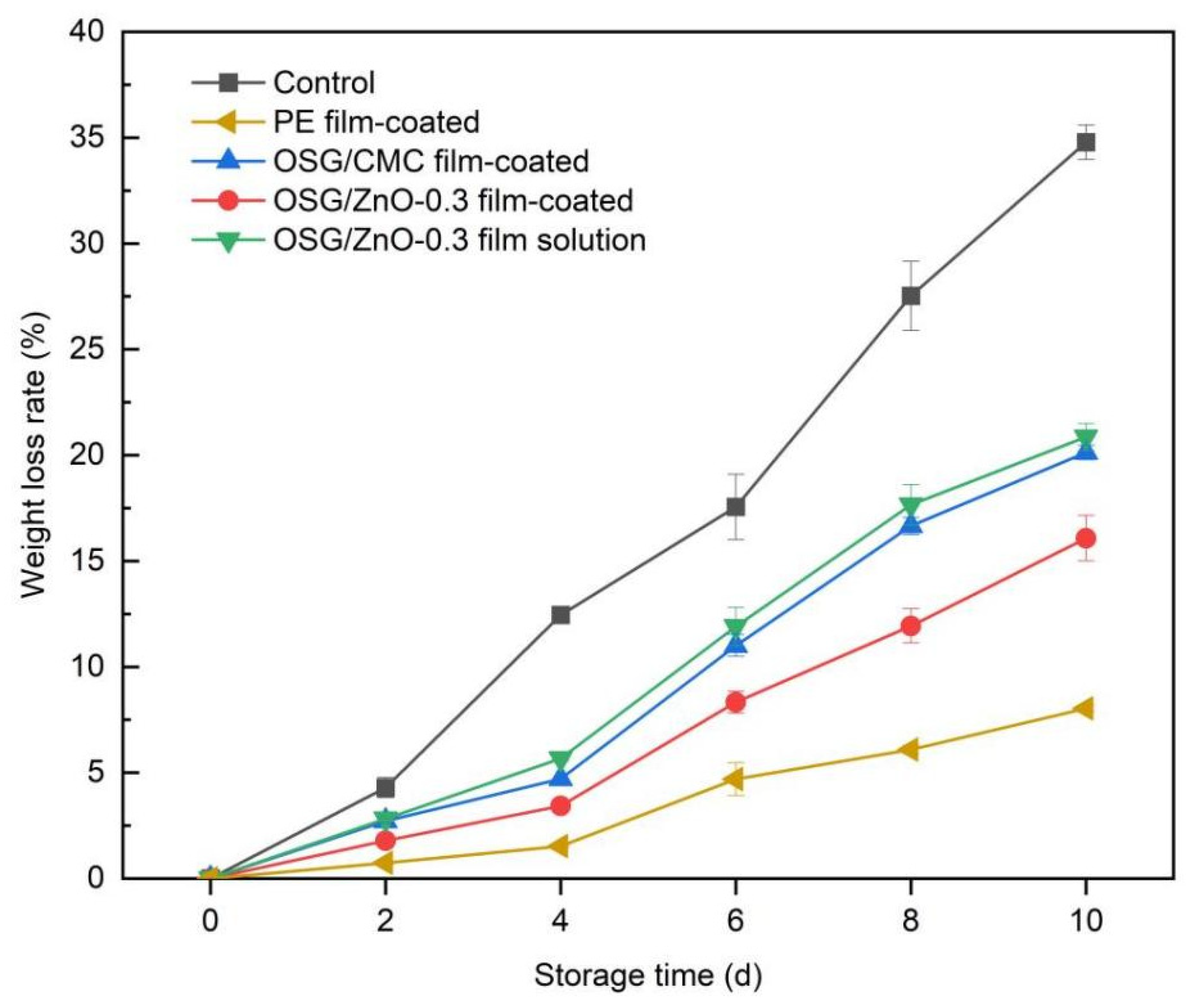
3.4.1. Weight Loss Rate
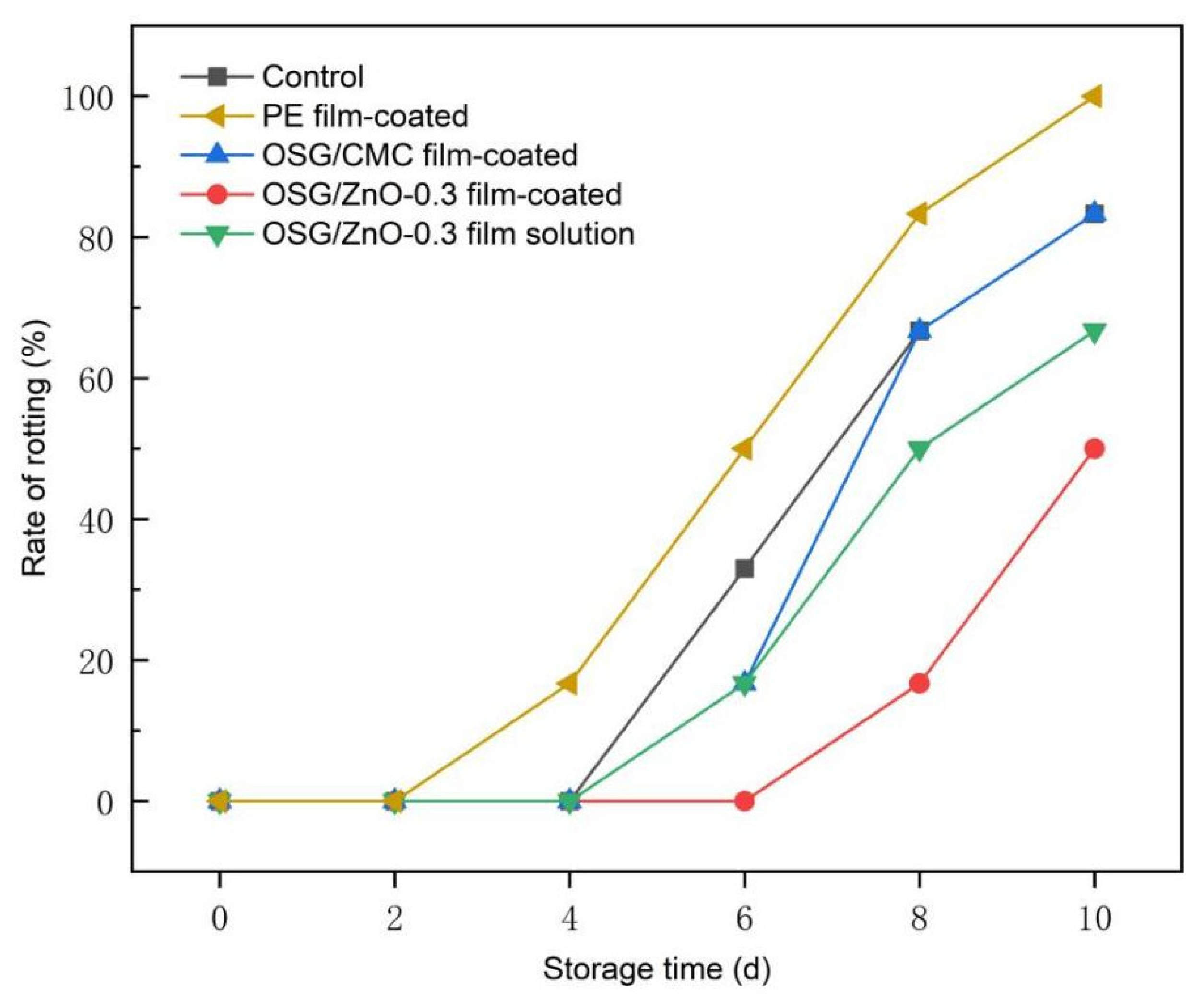
3.4.2. Rate of Rotting
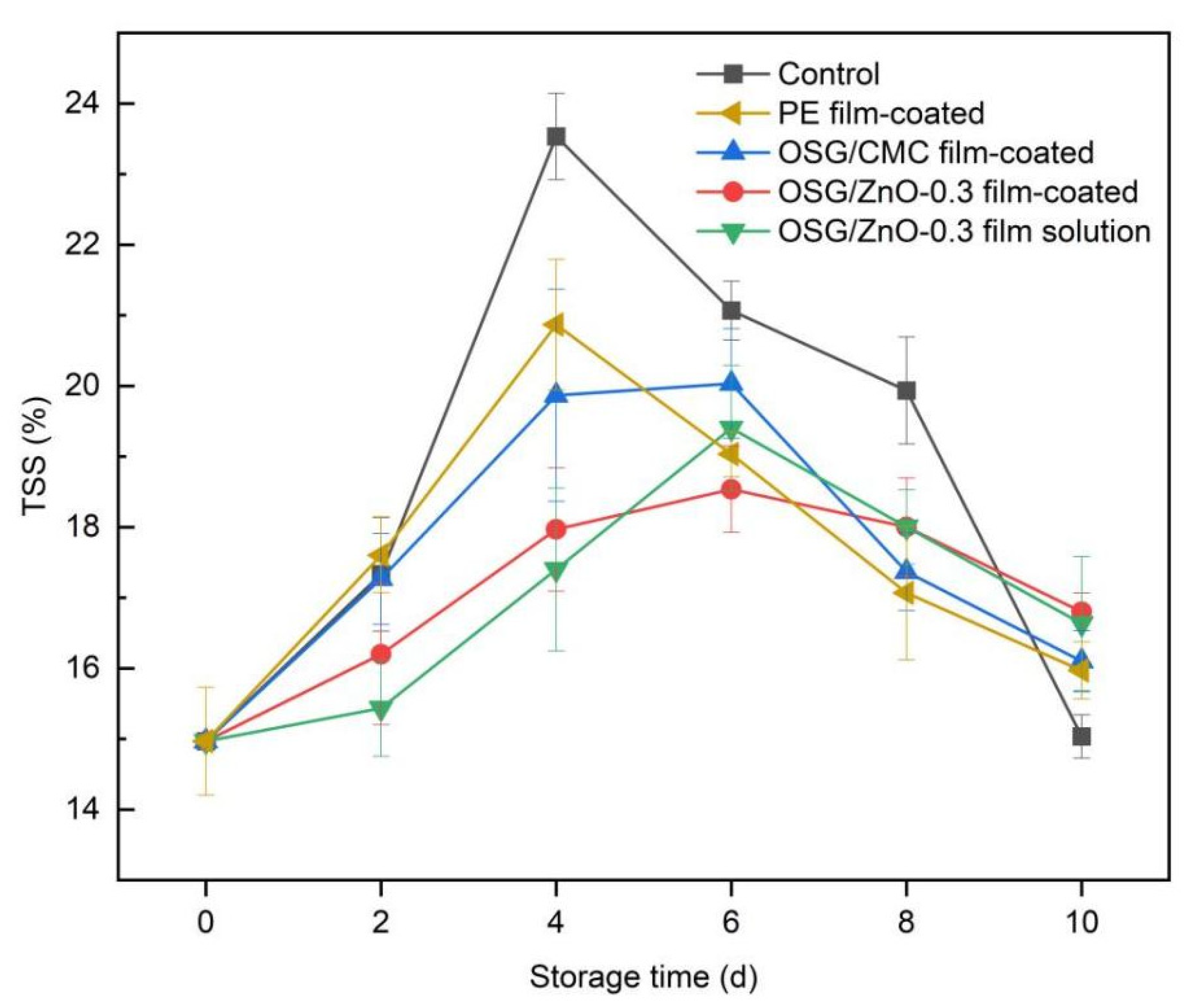
3.4.3. Total Soluble Solid Content
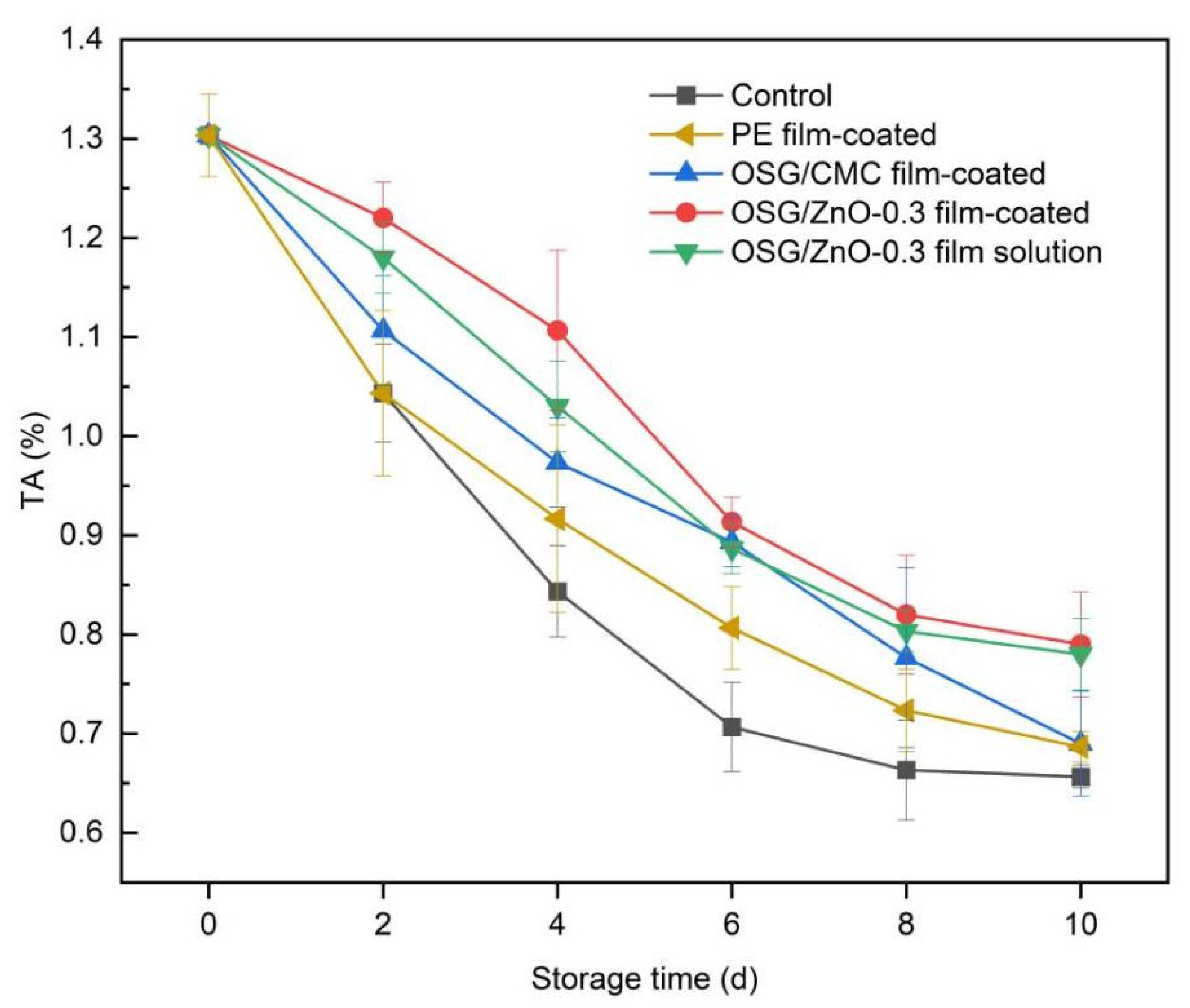
3.4.4. Titratable Acidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, S.; Xue, Z.; Zhang, S.; Wu, C.; Feng, Y.; Kou, X. The characterization of antimicrobial nanocomposites based on chitosan, cinnamon essential oil, and TiO2 for fruits preservation. Food Chem. 2023, 413, 135446. [Google Scholar] [CrossRef]
- Li, T.; Chi, W.; Ning, Y.; Xu, S.; Wang, L. Locust bean gum/carboxycellulose nanocrystal coating incorporating ZnO clusters built by the accretion of micro spindles or sheets for strawberries preservation. Int. J. Biol. Macromol. 2023, 226, 267–278. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Zhang, C.; Zhai, X.; Zhang, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Chitosan-cinnamon essential oil/sodium alginate-TiO2 bilayer films with enhanced bioactive retention property: Application for mango preservation. Int. J. Biol. Macromol. 2023, 222, 2843–2854. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, C.; Zhou, C.; Pan, Q.; He, Z.; Wang, C.; Liu, Y.; Song, S.; Yu, L.; Qu, Y.; et al. Amphiphilic chitosan/carboxymethyl gellan gum composite films enriched with mustard essential oil for mango preservation. Carbohydr. Polym. 2023, 300, 120290. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT-Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Li, Y.; Li, Q.; Liu, X. Hydroxypropylation of cross-linked sesbania gum, characterization and properties. Int. J. Biol. Macromol. 2020, 152, 1010–1019. [Google Scholar] [CrossRef]
- Tang, H.; Gao, S.; Li, Y.; Dong, S. Modification mechanism of sesbania gum, and preparation, property, adsorption of dialdehyde cross-linked sesbania gum. Carbohydr. Polym. 2016, 149, 151–162. [Google Scholar] [CrossRef]
- Shen, D.; Xue, M.; Zhang, L.; Liu, H.; Gao, L.; Cui, Y. Preparation and characterization of oxidized sesbania gum and evaluation of its warp sizing performance for fine cotton yarns. Polym. Degrad. Stab. 2011, 96, 2181–2188. [Google Scholar] [CrossRef]
- Tang, H.; Yao, Y.; Li, Y.; Dong, S. Effect of cross-linking and oxidization on structure and properties of sesbania gum. Int. J. Biol. Macromol. 2018, 114, 640–648. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Liu, S.; Qiao, C.; Wang, E.; Chen, H.; Zhang, C.; Yang, X.; Li, T. Effects of Chitosan and Cellulose Derivatives on Sodium Carboxymethyl Cellulose-Based Films: A Study of Rheological Properties of Film-Forming Solutions. Molecules 2023, 28, 5211. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef]
- Yunusov, K.E.; Sarymsakov, A.A.; Rashidova, S.S. Structure and properties of biodegradable carboxymethyl cellulose films containing silver nanoparticles. Polym. Sci. Ser. A 2014, 56, 283–288. [Google Scholar] [CrossRef]
- Kim, N.; Seo, E.; Kim, Y. Physical, mechanical and water barrier properties of yuba films incorporated with various types of additives. J. Sci. Food Agric. 2019, 99, 2808–2817. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Zhu, Y.; Zeng, Y.; Fang, C.; Liu, Y.; Hu, S.; Ge, Y.; Jiang, W. Preparation and application of a colorimetric film based on sodium alginate/sodium carboxymethyl cellulose incorporated with rose anthocyanins. Food Chem. 2022, 393, 133342. [Google Scholar] [CrossRef]
- Li, S.-C.; Li, B.; Qin, Z.-J. The Effect of the Nano-ZnO Concentration on the Mechanical, Antibacterial and Melt Rheological Properties of LLDPE/Modified Nano-ZnO Composite Films. Polym. Plast. Technol. Eng. 2010, 49, 1334–1338. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, W.; Yang, J.; Yang, Q. Preparation and characterization of chitosan/Nano-ZnO composite film with antimicrobial activity. Bioprocess Biosyst. Eng. 2021, 44, 1193–1199. [Google Scholar] [CrossRef]
- Fan, R.Y.; Peng, C.; Zhang, X.X.; Qiu, D.Y.; Mao, G.L.; Lu, Y.S.; Zeng, J.W. A comparative UPLC-Q-Orbitrap-MS untargeted metabolomics investigation of different parts of Clausena lansium (Lour.) Skeels. Food Sci. Nutr. 2020, 8, 5811–5822. [Google Scholar] [CrossRef]
- Ye, Y.; Chang, X.; Brennan, M.A.; Brennan, C.S.; Guo, X. Comparison of phytochemical profiles, cellular antioxidant and anti-proliferative activities in five varieties of wampee (Clausena lansium) fruits. Int. J. Food Sci. Technol. 2019, 54, 2487–2493. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zeng, J.; Li, W.; Dong, Y. Effect of ethanol fumigation on pericarp browning associated with phenol metabolism, storage quality, and antioxidant systems of wampee fruit during cold storage. Food Sci. Nutr. 2020, 8, 3380–3388. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, A.; Huang, J.; Luo, R.; Yu, K. Screening of sweet wampee Clausena lansium (Lour.) Skeels progenies in the early growth stage based on chloroplast genome analysis. Genet. Resour. Crop Evol. 2021, 68, 1747–1750. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Lin, X.; Li, W.; Zeng, J. Effects of different postharvest treatments on quality of wampee fruits during shelf life after cold storage. Food Sci. Technol. 2019, 44, 33–38. (In Chinese) [Google Scholar]
- Zeng, J.K.; Jiang, Z.T.; Li, W.; Zhang, L.B.; Shao, Y.Z. Effects of UV-C irradiation on postharvest quality and antioxidant properties of wampee fruit (Clausena lansium (Lour.) Skeels) during cold storage. Fruits 2020, 75, 36–43. [Google Scholar] [CrossRef]
- Su, X.; Wu, X.; Nong, H.; Chen, Y. Research on the Application of Chitosan Coating Package in the Preservation of Clausenaanisum-olens. China Fruit Veg. 2023, 43, 4–11. (In Chinese) [Google Scholar]
- Lin, Y.; Feng, M.; Chen, H.; Jiang, X.; Zhen, J.; Wu, J. Effects of oxyresveratrol treatment on the storage behavior and postharvest quality of wampee fruit. Chin. J. Trop. Crops 2023, 1–13. (In Chinese) [Google Scholar]
- Mingyan, W.; Yun-chi, Z.; Hengfu, H.; Yue, S.; Jian, C.; Guanglong, Y. Preparation, properties, and characterization of modified sesbania gum composite film. Ind. Crops Prod. 2023, 206, 117640. [Google Scholar]
- Li, R.; Chen, C.; Chen, M.; Wu, R.; Sun, Y.; Zhu, B.; Yao, Z. Fabrication of carboxymethyl chitosan/oxidized carboxymethyl cellulose composite film and its assessment for coating preservation of strawberry. J. Food Sci. 2023, 88, 1865–1878. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; He, B.; Yong, Y.; Zhu, J. Composite films of sodium alginate and konjac glucomannan incorporated with tea polyphenols for food preservation. Int. J. Biol. Macromol. 2023, 242, 124732. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Tang, J.; Zhang, H.; Dai, J.; Li, S.; Yan, J.; Qin, W.; Liu, Y. Preparation of sodium alginate/konjac glucomannan active films containing lycopene microcapsules and the effects of these films on sweet cherry preservation. Int. J. Biol. Macromol. 2022, 215, 67–78. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, H.; Fu, Y.; Chang, C.; Wu, J. Sodium alginate/gum arabic/glycerol multicomponent edible films loaded with natamycin: Study on physicochemical, antibacterial, and sweet potatoes preservation properties. Int. J. Biol. Macromol. 2022, 213, 1068–1077. [Google Scholar] [CrossRef]
- He, X.; Li, M.; Gong, X.; Niu, B.; Li, W. Biodegradable and antimicrobial CSC films containing cinnamon essential oil for preservation applications. Food Packag. Shelf Life 2021, 29, 100697. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Chai, Y.; Guo, C.; Luo, C.; Chen, D.; Cheng, X.; Wang, F.; Huang, C. Chitosan-pullulan films enriched with Artemisia annua essential oil: Characterization and application in grape preservation. Int. J. Biol. Macromol. 2023, 243, 125216. [Google Scholar] [CrossRef]
- Dong, Z.; Du, Z.; Wu, X.; Zhai, K.; Wei, Z.; Rashed, M.M.A. Fabrication and characterization of ZnO nanofilms using extracted pectin of Premna microphylla Turcz leaves and carboxymethyl cellulose. Int. J. Biol. Macromol. 2022, 209, 525–532. [Google Scholar] [CrossRef]
- Dilimeiheri, D.; Yang, M.; Chen, L.; Li, X.; Zhao, F.; Zhang, X. Optimization of extraction technology of Erchen Sangbei Zhike Granules based on entropy weight method and Box-Behnken response surface methodology. Asia-Pac. Tradit. Med. 2022, 18, 53–58. (In Chinese) [Google Scholar]
- Chang, L.; Xu, L.; Yang, Z.; Liu, L.; Qiu, D. Antibacterial and antioxidative biogenic films for room-temperature strawberry preservation. Food Chem. 2023, 405, 134893. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Ni, Y.; Yang, C.; Jin, X.; Wang, Y.; Yang, Y.; Jin, Y.; Sun, J.; Wang, J. ZnO/C-mediated k-carrageenan based pseudo-pasteurization films for kumquat preservation. Food Hydrocoll. 2022, 128, 107582. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.; Liu, Y. Developing poly(vinyl alcohol)/chitosan films incorporate with D-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, J.; Xie, G.; Xiong, T.; Xu, H. Preparation and characterization of sodium alginate/gelatin/Ag nanocomposite antibacterial film and its application in the preservation of tangerine. Food Packag. Shelf Life 2022, 33, 100928. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Ji, R.; Li, K.; Zhang, W. Preparation and Characterization of Pullulan/Carboxymethyl Cellulose/Nano-TiO2 Composite Films for Strawberry Preservation. Food Biophys. 2021, 16, 460–473. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Yue, T.; Cui, L. Preparation and Characterization of Chitosan-Nano-ZnO Composite Films for Preservation of Cherry Tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, W.; Niu, B.; Fang, X.; Chen, H.; Wang, Y.; Gao, H. Characterization of Zizania latifolia polysaccharide-corn starch composite films and their application in the postharvest preservation of strawberries. LWT-Food Sci. Technol. 2023, 173, 114332. [Google Scholar] [CrossRef]
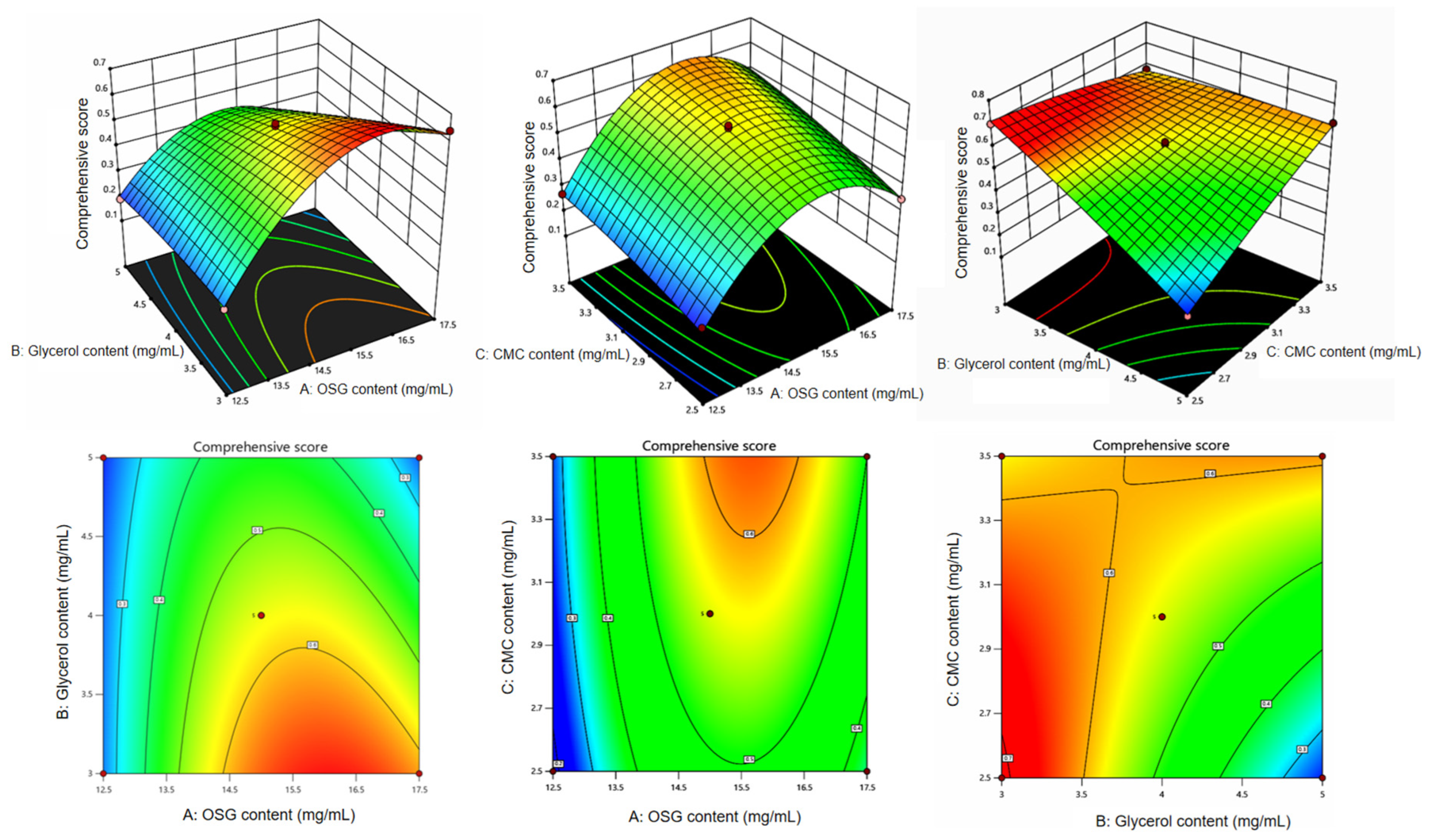


| Run | OSG Content | Glycerol Content | CMC Content | Comprehensive Score |
|---|---|---|---|---|
| (A)/mg mL−1 | (B)/mg mL−1 | (A)/mg mL−1 | ||
| 1 | 12.5 | 3 | 3 | 0.24 |
| 2 | 17.5 | 5 | 3 | 0.24 |
| 3 | 15 | 5 | 2.5 | 0.23 |
| 4 | 15 | 4 | 3 | 0.58 |
| 5 | 17.5 | 4 | 2.5 | 0.35 |
| 6 | 12.5 | 4 | 2.5 | 0.2 |
| 7 | 15 | 5 | 3.5 | 0.62 |
| 8 | 15 | 4 | 3 | 0.57 |
| 9 | 12.5 | 5 | 3 | 0.19 |
| 10 | 15 | 3 | 2.5 | 0.7 |
| 11 | 15 | 4 | 3 | 0.55 |
| 12 | 12.5 | 4 | 3.5 | 0.27 |
| 13 | 15 | 4 | 3 | 0.55 |
| 14 | 17.5 | 4 | 3.5 | 0.48 |
| 15 | 17.5 | 3 | 3 | 0.64 |
| 16 | 15 | 3 | 3.5 | 0.58 |
| 17 | 15 | 4 | 3 | 0.56 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.5149 | 9 | 0.0572 | 170.04 | <0.0001 | significant |
| A-OSG content | 0.082 | 1 | 0.082 | 243.77 | <0.0001 | ** |
| B-Glycerol content | 0.0968 | 1 | 0.0968 | 287.73 | <0.0001 | ** |
| C-CMC content | 0.0276 | 1 | 0.0276 | 82.08 | <0.0001 | ** |
| AB | 0.0306 | 1 | 0.0306 | 91.03 | <0.0001 | ** |
| AC | 0.0009 | 1 | 0.0009 | 2.68 | 0.1459 | |
| BC | 0.065 | 1 | 0.065 | 193.28 | <0.0001 | ** |
| A2 | 0.2056 | 1 | 0.2056 | 611.26 | <0.0001 | ** |
| B2 | 0.0008 | 1 | 0.0008 | 2.28 | 0.1747 | |
| C2 | 0.0011 | 1 | 0.0011 | 3.2 | 0.1166 | |
| Residual | 0.0024 | 7 | 0.0003 | |||
| Lack of Fit | 0.0017 | 3 | 0.0006 | 3.28 | 0.1404 | not significant |
| Pure Error | 0.0007 | 4 | 0.0002 | |||
| Cor Total | 0.5172 | 16 |
| Analysis | Data | Analysis | Data |
|---|---|---|---|
| Std. Dev. | 0.01834 | R2 | 0.9954 |
| Mean | 0.4441 | Adjusted R2 | 0.9896 |
| C.V.% | 4.13 | Predicted R2 | 0.9461 |
| Adeq Precision | 37.7639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Huang, D.; Sun, Y.; Yao, G.; Huan, H.; Chen, J. Antibacterial Activity of Modified Sesbania Gum Composite Film and Its Preservation Effect on Wampee Fruit (Clausena lansium (Lour.) Skeels). Foods 2024, 13, 639. https://doi.org/10.3390/foods13050639
Wang M, Huang D, Sun Y, Yao G, Huan H, Chen J. Antibacterial Activity of Modified Sesbania Gum Composite Film and Its Preservation Effect on Wampee Fruit (Clausena lansium (Lour.) Skeels). Foods. 2024; 13(5):639. https://doi.org/10.3390/foods13050639
Chicago/Turabian StyleWang, Mingyan, Dongfen Huang, Yue Sun, Guanglong Yao, Hengfu Huan, and Jian Chen. 2024. "Antibacterial Activity of Modified Sesbania Gum Composite Film and Its Preservation Effect on Wampee Fruit (Clausena lansium (Lour.) Skeels)" Foods 13, no. 5: 639. https://doi.org/10.3390/foods13050639





