Aegle marmelos (L.) Leaf Extract Improves Symptoms of Memory Loss Induced by Scopolamine in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aegle marmelos (L.) Correa (AM) Leaf Preparation and Extraction
2.2. Aegle marmelos (L.) High Performance Liquid Chromatograph (HPLC)
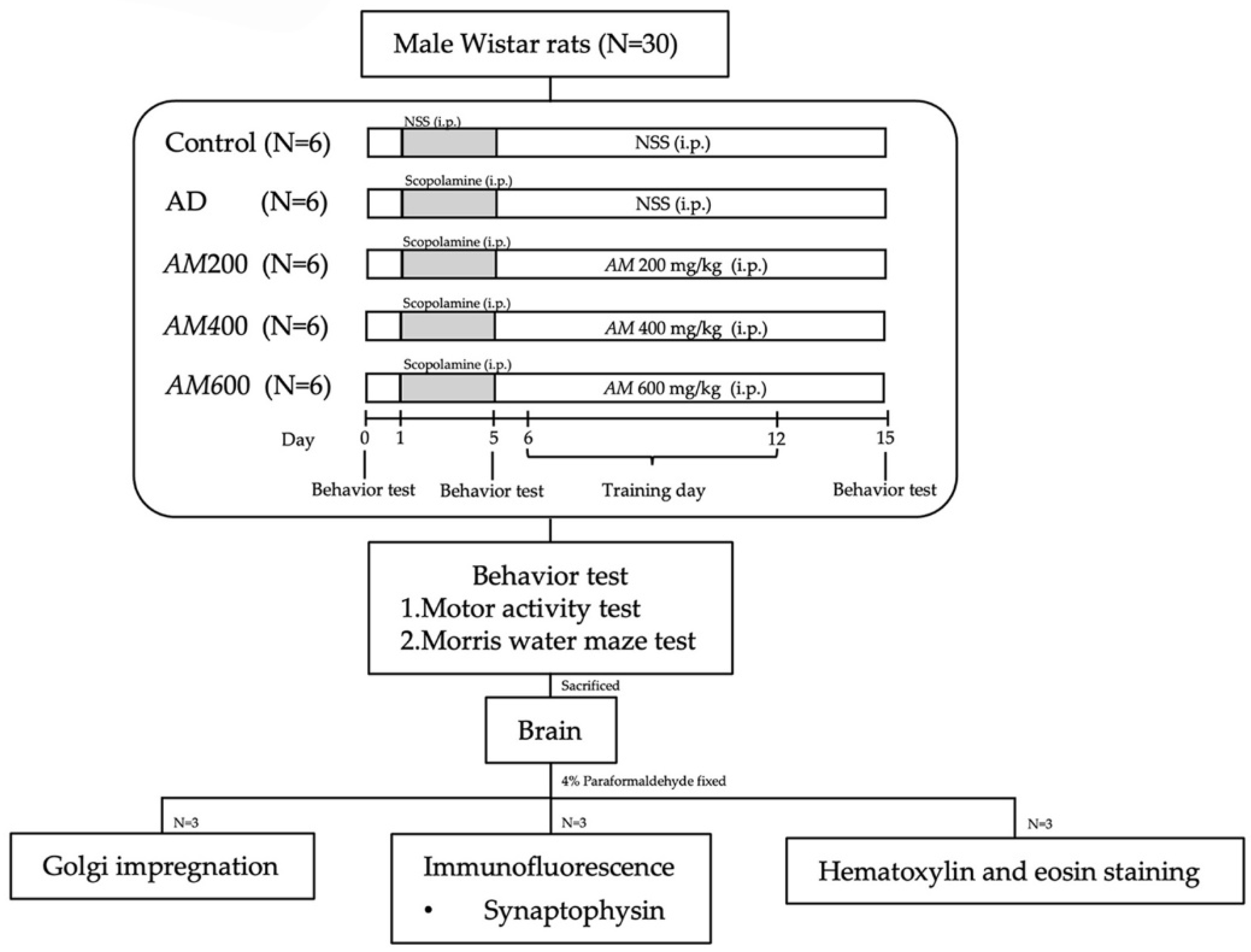
2.3. Animals and the Experimental Protocol
2.4. Motor Activity Test
2.5. Morris Water Maze (MWM) Test
2.6. Animal Perfusion and Brain Sectioning
2.7. Immunofluorescence and Analysis of the Synaptophysin Density
2.8. Golgi Impregnation and Analysis of Dendritic Spine Density
2.9. Hematoxylin and Eosin Staining
2.10. Statistical Analysis
3. Results
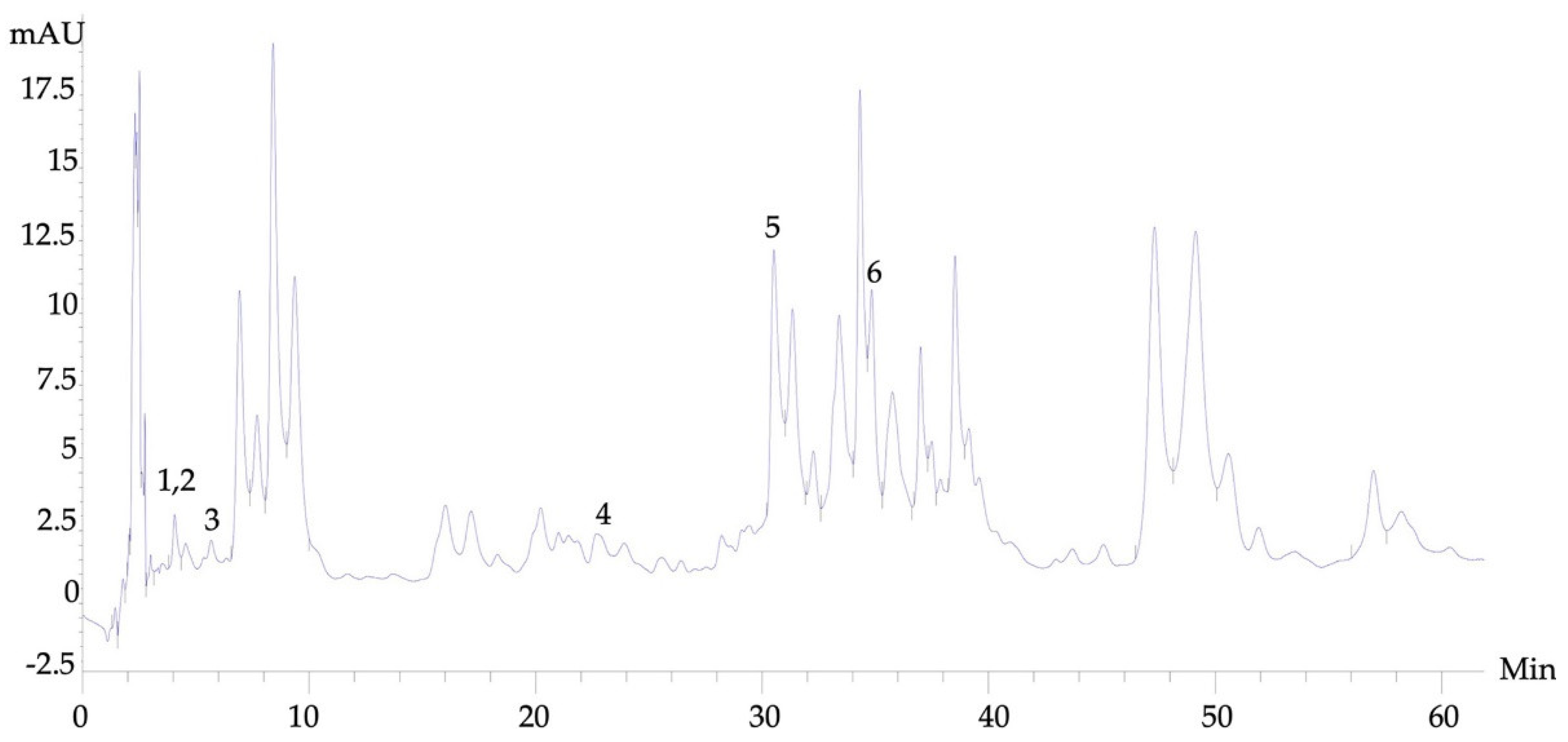
3.1. High-Performance Liquid Chromatography (HPLC) Analysis
3.2. Effects of the AM Leaf Extract on Motor Activity
3.3. Effects of the AM Leaf Extract on Memory Impairment
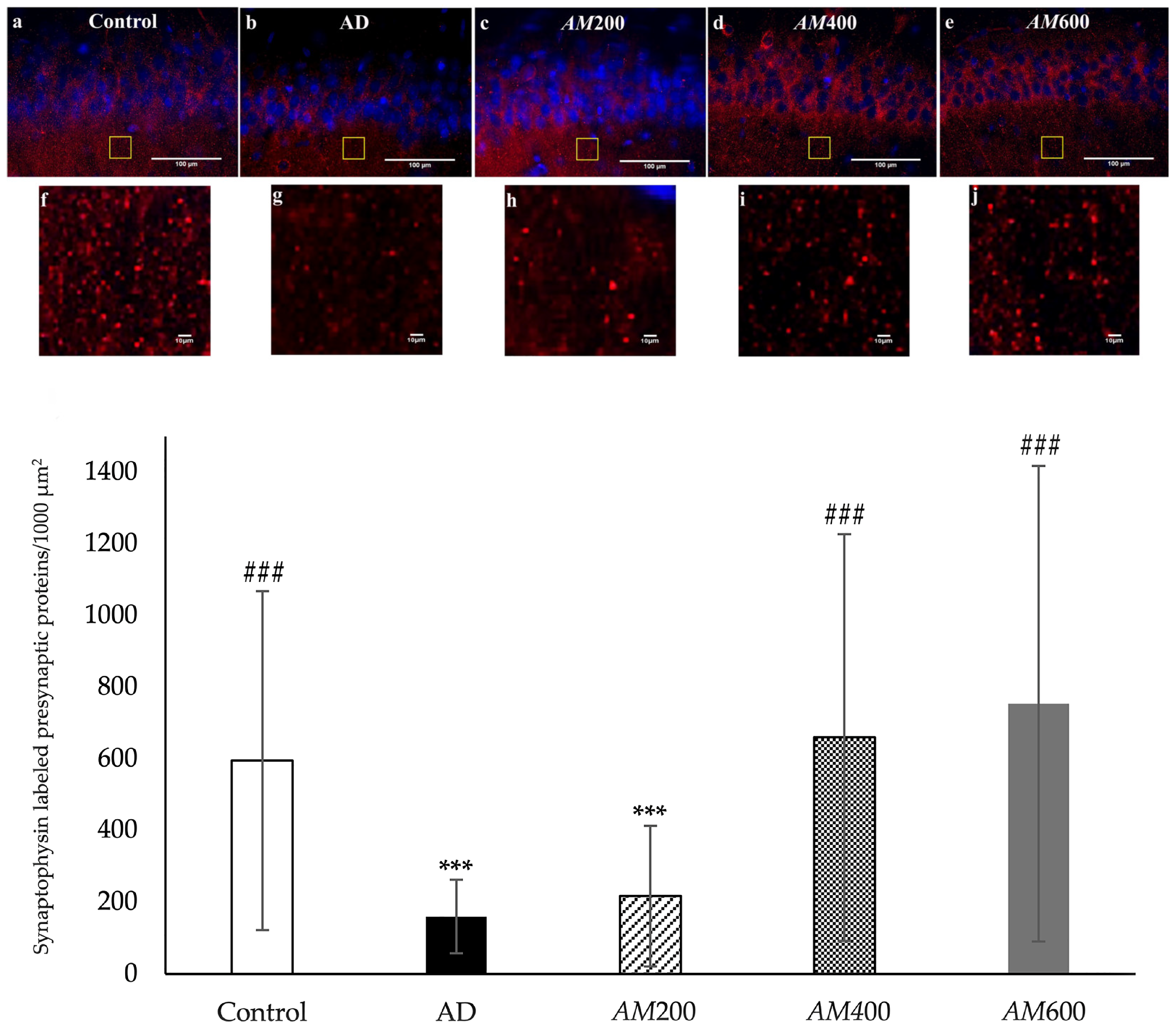
3.4. Effects of the AM Leaf Extract on the Density of the Presynaptic Vesicle Proteins in the Axon Terminals of CA1
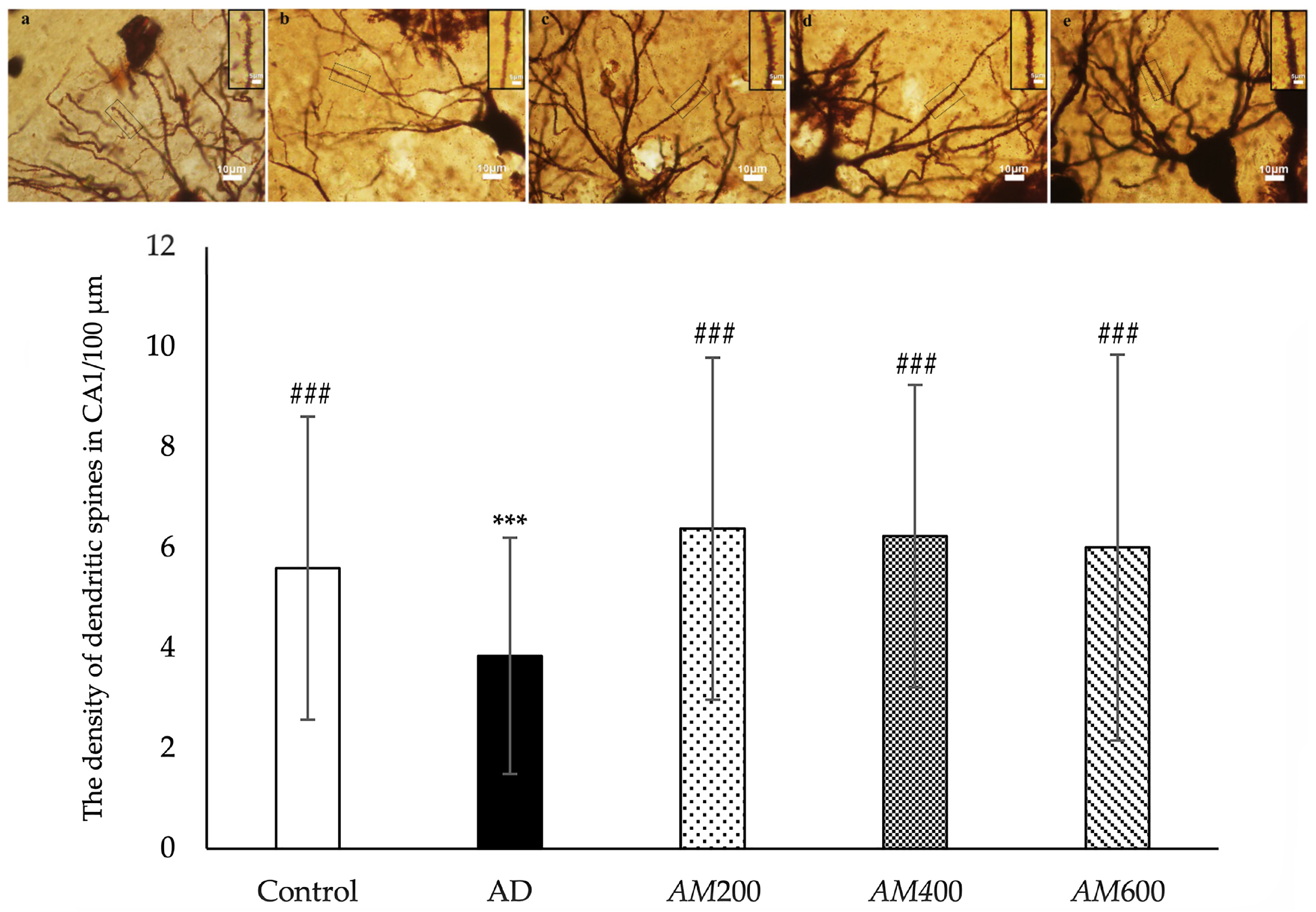
3.5. Effects of the AM Leaf Extract on the Density of the Dendritic Spines in the CA1
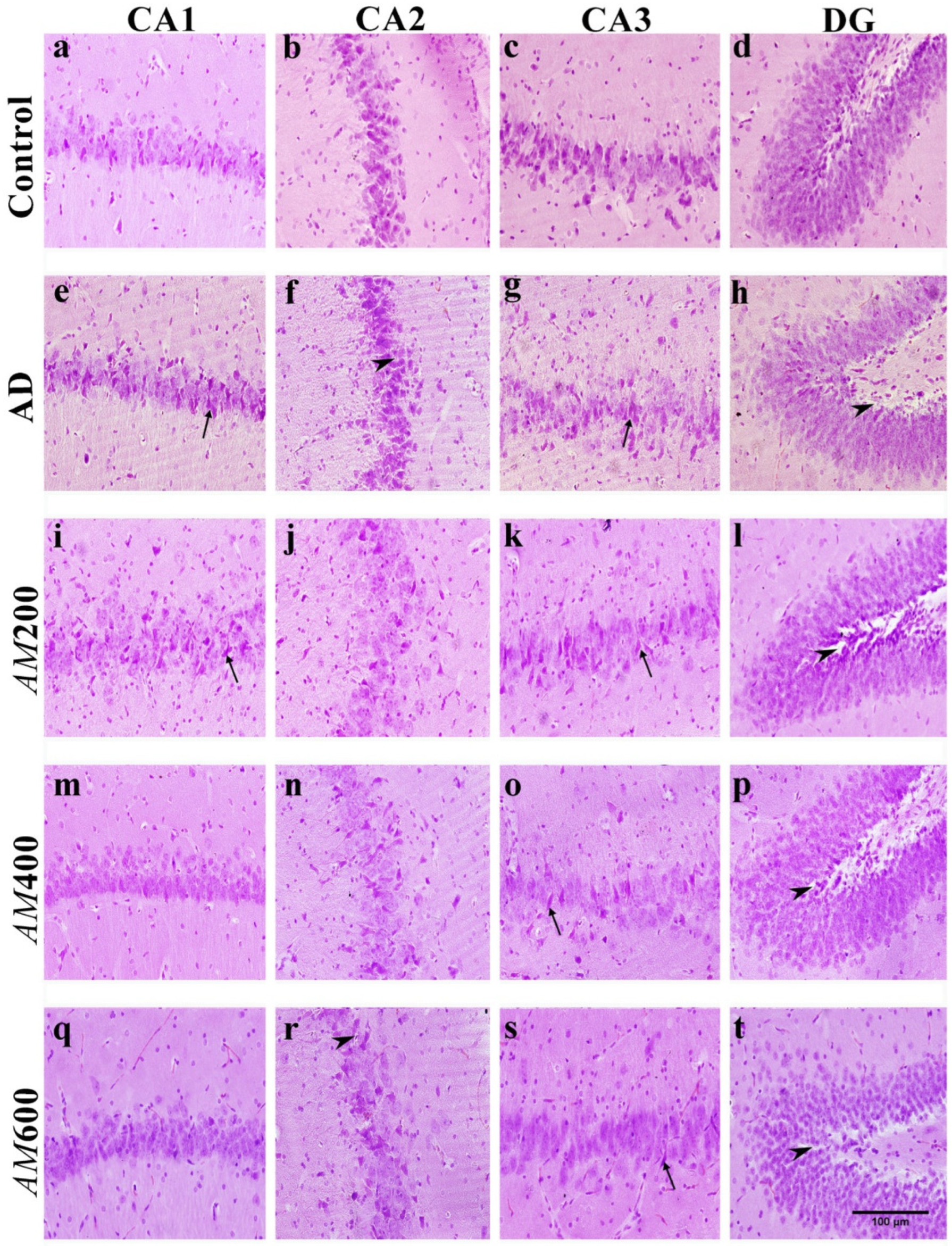
3.6. Effect of the AM Leaf Extract on the Histological Structure of the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Singh, A.; Ekavali, A. Review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Ministry of Public Health. Burden of Disease Thailand. Disability-Adjusted Life Year: DALY 2013 Nonthaburi. Available online: http://bodthai.net/en/research-report/ (accessed on 18 June 2018).
- Association, A. 2018 Alzheimer’s disease facts and figures. Alzheimers. Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Van Dam, D.; De Deyn, P.P. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br. J. Pharmacol. 2011, 164, 1285–1300. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Riberio, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Brown, D.A. Acetylcholine and cholinergic receptors. Brain Neurosci. Adv. 2019, 3, 2398212818820506. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Brunton, L.; Chabner, B.A.; Knollman, B. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 12th ed.; McGraw-Hill Education: New York, NY, USA, 2015; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=1613§ionid=102124003 (accessed on 16 November 2023).
- Uddin, S.; Mamun, A.A.; Hossain, S.; Ashaduzzaman, M.; Noor, A.A.; Hossain, S.; Uddin, J.; Sarker, J. Asaduzzaman, Neuroprotective effect of Phyllanthus acidus L. on learning and memory impairment in scopolamine-induced animal model of dementia and oxidative stress: Natural wonder for regulating the development and progression of Alzheimer’s disease. Adv. Alzheimer’s Dis. 2016, 5, 53–72. [Google Scholar] [CrossRef]
- Safar, M.M.; Arab, H.H.; Rizk, S.M.; El-Maraghy, S.A. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced Alzheimer-like pathological aberrations. Mol. Neurobiol. 2016, 53, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Imam, A.; Ajao, M.S.; Ajibola, M.I.; Amin, A.; Abdulmajeed, W.I.; Lawal, A.Z. Black seed oil ameliorated scopolamine-induced memory dysfunction and cortico-hippocampal neural alterations in male Wistar rats. Bull. Fac. Pharm. Cairo Univ. 2016, 54, 49–57. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.H.; Wu, Y.Y.; Tang, H.; Tan, L.; Wang, X.; Gao, X.Y.; Xiong, Y.S.; Liu, D.; Wang, J.Z.; et al. Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol. Neurobiol. 2013, 47, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Limmatvapirat, C. Treatment of Alzheimer’s disease. Thai Bull. Pharm. Sci. 2005, 2, 29–46. [Google Scholar]
- Rajkumar, S.; Selvamani, P.; Latha, S.; Dhivya, P.S. Role of medicinal plants in management of Alzheimer’s and neurodegenerative disease—Review. World J. Pharm. Res. 2015, 4, 352–366. [Google Scholar]
- Koynova, R.; Tenchov, B. Natural product formulations for the prevention and treatment of Alzheimer’s disease: A Patent Review. Recent Pat. Drug Deliv. Formul. 2018, 12, 23–39. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Reddy, P.H. Can herbs provide a new generation of drugs for treating Alzheimer’s disease? Brain Res. 2005, 50, 361–376. [Google Scholar] [CrossRef]
- Rahnama, S.; Rabiei, Z.; Alibabaei, Z.; Mokhtari, S.; Rafieian-Kopaei, M.; Deris, F. Anti-amnesic activity of Citrus aurantium flowers extract against scopolamine-induced memory impairments in rats. Neurol. Sci. 2015, 36, 553–560. [Google Scholar] [CrossRef]
- Baliga, M.S.; Bhat, H.P.; Joseph, N.; Fazal, F. Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa): A concise review. Food Res. Int. 2011, 44, 1768–1775. [Google Scholar] [CrossRef]
- Adavala, P.D.; Musukula, Y.R.; Puchchakayala, G. Neuroprotective effect of Aegle marmelos leaf extract in scopolamine induced cognitive impairment and oxidative stress in mice. Glob. J. Pharmacol. 2016, 10, 45–53. [Google Scholar]
- Asaduzzaman, M.; Uddin, M.J.; Kader, M.A.; Alam, A.H.M.K.; Rahman, A.A.; Rashid, M.; Kato, K.; Tanaka, T.; Takeda, M.; Sadik, G. In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: Implications for the treatment of Alzheimer’s disease. Psychogeriatrics 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bodla, R.B.; Bansal, H. Antioxidant activity of leaf extract of Aegle marmelos Correa ex Roxb. Pharmacogn. J. 2016, 8, 447–450. [Google Scholar] [CrossRef]
- Kumari, K.D.K.P.; Weerakoon, T.C.S.; Handunnetti, S.M.; Samarasinghe, K.; Suresh, T.S. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 2014, 151, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Raheja, S.; Girdhar, A.; Kamboj, A.; Lather, V.; Pandita, D. Aegle marmelos leaf extract ameliorates the cognitive impairment and oxidative stress induced by intracerebroventricular streptozotocin in male rats. Life Sci. 2019, 221, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Seth, E.; Kaushal, S.; Ahsan, A.U.; Sharma, V.L.; Chopra, M. Neuroprotective effects of Aegle marmelos (L.) Correa against cadmium toxicity by reducing oxidative stress and maintaining the histoarchitecture of neural tissue in BALB/c mice. Indian J. Biochem. Biophys. 2018, 55, 95–104. [Google Scholar]
- Phuaklee, P.; Dechayont, B.; Chunthorng-Orn, J.; Itharat, A. Anti-allergic, anti-inflammatory and antioxidant activities of Aegle marmelos Correa. fruit. Thammasat Med. J. 2018, 18, 349–357. [Google Scholar]
- Dhankhar, S.; Ruhil, S.; Balhara, M.; Dhankhar, S.; Chhillar, A.K. Aegle marmelos (Linn.) Correa: A potential source of phytomedicine. J. Med. Plant Res. 2011, 5, 1497–1507. [Google Scholar]
- Laoung-on, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical Screening, Antioxidant and Sperm Viability of Nelumbo nucifera Petal Extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Jiang, X.; Jia, L.W.; Li, X.H.; Cheng, X.S.; Xie, J.Z.; Ma, Z.W.; Xu, W.J.; Liu, Y.; Yao, Y.; Du, L.L.; et al. Capsaicin ameliorates stress-induced Alzheimer’s disease-like pathological and cognitive impairments in rats. J. Alzheimer’s. Dis. 2013, 35, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Chaiwut, T. Effects of Thunbergia laurifolia Lind. Leave Extract for Preventing Parkinson’s Disease in Mice Induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2018. [Google Scholar]
- Villalba, R.M.; Lee, H.; Smith, Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp. Neurol. 2009, 215, 220–227. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.-P.; Tang, H.; Shan, W.Y.; Wang, X.; Liu, D.; Wu, Y.-Y.; Tain, Q.; Wang, J.-Z.; Zhu, L.-Q. Acetyl-L-carnitine rescues scopolamine-induced memory deficits by restoring insulin-like growth factor II via decreasing p53 oxidation. Neuropharmacology 2014, 76 Pt A, 80–87. [Google Scholar] [CrossRef]
- Joshi, A.; Soni, P.; Malviya, S.; Kharia, A. Memory enhancing activity of Momordica charantia by scopolamine induced amnesia in rats. Int. J. Compr. Adv. Pharmacol. 2017, 2, 11–18. [Google Scholar]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimer’s Dis. 2020, 2020, 6372059. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.K.; Kumar, G.; Karthik, L.; Rao, K.V.B. A review on pharmacological and phytochemical properties of Aegle marmelos (L.) Corr. Serr. (Rutaceae). Asian J. Plant Sci. Res. 2011, 1, 8–17. [Google Scholar]
- Furman, B. Testosterone. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–4. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128012383980450?via%3Dihub (accessed on 5 December 2020).
- Kothari, S.; Minda, M.; Tonpay, S.D. Anxiolytic and antidepressant activities of methanol extract of Aegle marmelos leaves in mice. Indian J. Physiol. Pharmacol. 2010, 54, 318–328. [Google Scholar] [PubMed]
- Chavan, A.; Lakshmikantha, R.Y.; Satwadi, P.R. Evaluation of nootropic activity of Aegle marmelos extract using different experimental models in rats. Int. J. Pharm. Chem. Biol. Sci. 2012, 2, 538–544. [Google Scholar]
- Maiti, P.; Manna, J.; Ilavazhagan, G.; Rossignol, J.; Dunbar, G.K. Molecular regulation of dendritic spine dynamics and their potential impact on synaptic plasticity and neurological diseases. Neurosci. Biobehav. Rev. 2015, 59, 208–237. [Google Scholar] [CrossRef] [PubMed]
- Dietzmann, K.; von Bossanyi, P. Dendritic spines and immunoreactivity of synaptophysin in the frontal cortex of humans with infantile brain damage. A correlative study. Clin. Neuropathol. 1994, 13, 127–133. [Google Scholar] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Koppula, S.; Choi, D.K. Cuminum cyminum extract attenuates scopolamine-induced memory loss and stress-induced urinary biochemical changes in rats: A noninvasive biochemical approach. Pharm. Biol. 2011, 49, 702–708. [Google Scholar] [CrossRef]
- Jafaripour, L.; Esmaeilpour, K.; Maneshian, M.; Bashiri, H.; Rajizadeh, M.A.; Ahmadvan, H.; Asadi-Shekaari, M. The effect of gallic acid on memory and anxiety-like behaviors in rats with bile duct ligation-induced hepatic encephalopathy: Role of AMPK pathway. Avicenna J. Phytomed. 2022, 12, 425–438. [Google Scholar]
- Akbar, L.; Juliandi, B.; Boediono, A.; Batubara, I.; Subangkit, M. Effects of Eugenol on Memory Performance, Neurogenesis, and Dendritic Complexity of Neurons in Mice Analyzed by Behavioral Tests and Golgi Staining of Brain Tissue. J. Stem Cells Regen. Med. 2021, 17, 35–41. [Google Scholar] [PubMed]
- Castro, M.F.V.; Assmann, C.E.; Stefanello, N.; Reichert, K.P.; Palma, T.V.; Silva, A.D.; Miron, V.V.; Mostardeiro, V.M.; Morsch, V.M.M.; Schetinger, M.R.C. Caffeic acid attenuates neuroinflammation and cognitive impairment in streptozotocin-induced diabetic rats: Pivotal role of the cholinergic and purinergic signaling pathways. J. Nutr. Biochem. 2023, 15, 109280. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongsopha, C.; Chaiwut, T.; Thaweekhotr, P.; Sudwan, P.; Phasukdee, N.; Quiggins, R. Aegle marmelos (L.) Leaf Extract Improves Symptoms of Memory Loss Induced by Scopolamine in Rats. Foods 2024, 13, 627. https://doi.org/10.3390/foods13040627
Thongsopha C, Chaiwut T, Thaweekhotr P, Sudwan P, Phasukdee N, Quiggins R. Aegle marmelos (L.) Leaf Extract Improves Symptoms of Memory Loss Induced by Scopolamine in Rats. Foods. 2024; 13(4):627. https://doi.org/10.3390/foods13040627
Chicago/Turabian StyleThongsopha, Chanida, Thanasit Chaiwut, Pornnarez Thaweekhotr, Paiwan Sudwan, Noppadol Phasukdee, and Ranida Quiggins. 2024. "Aegle marmelos (L.) Leaf Extract Improves Symptoms of Memory Loss Induced by Scopolamine in Rats" Foods 13, no. 4: 627. https://doi.org/10.3390/foods13040627




