Understanding the Interaction between Nanomaterials Originated from High-Temperature Processed Starch/Myristic Acid and Human Monocyte Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Nanomaterials from Starch/Myristic Acid
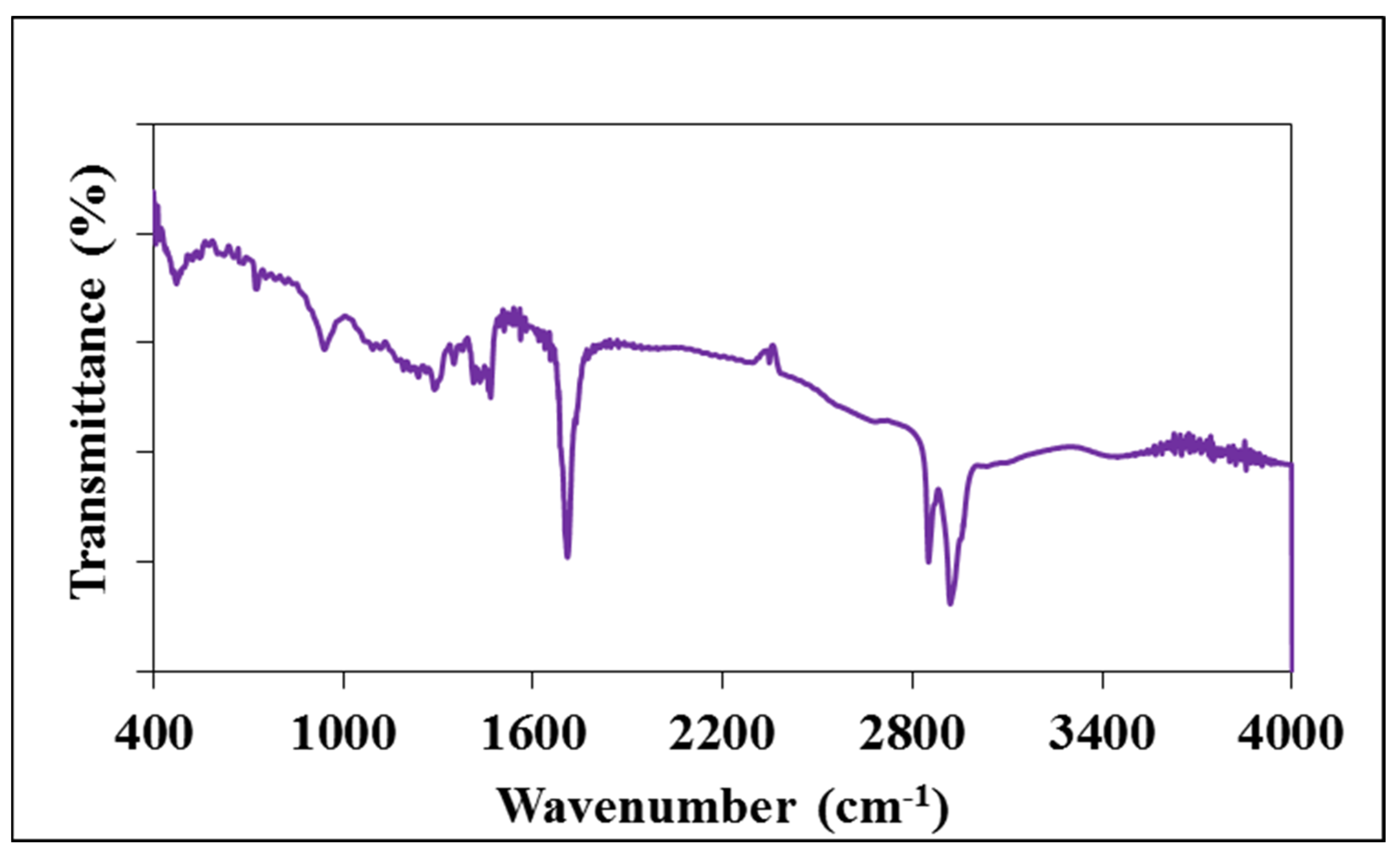
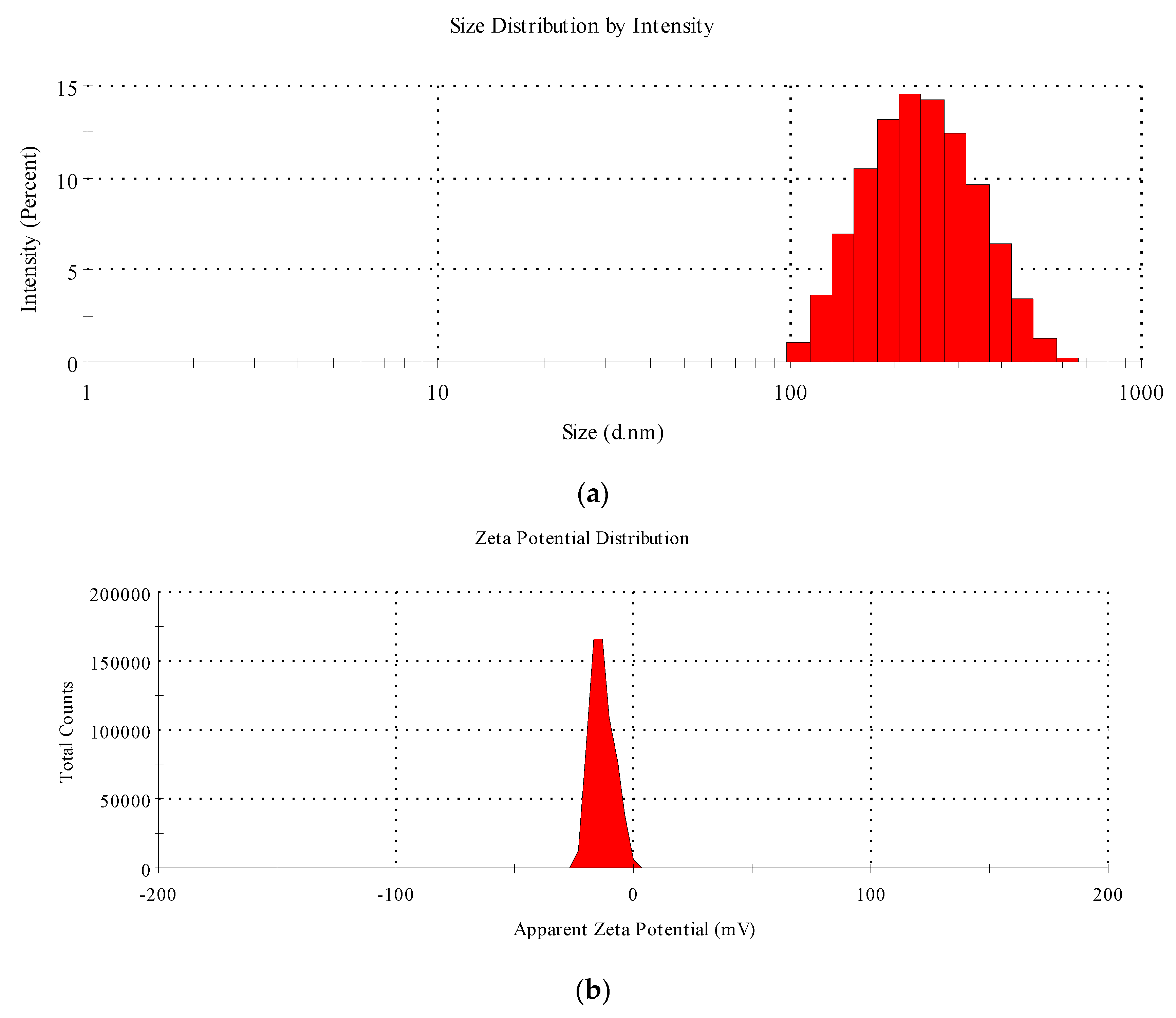
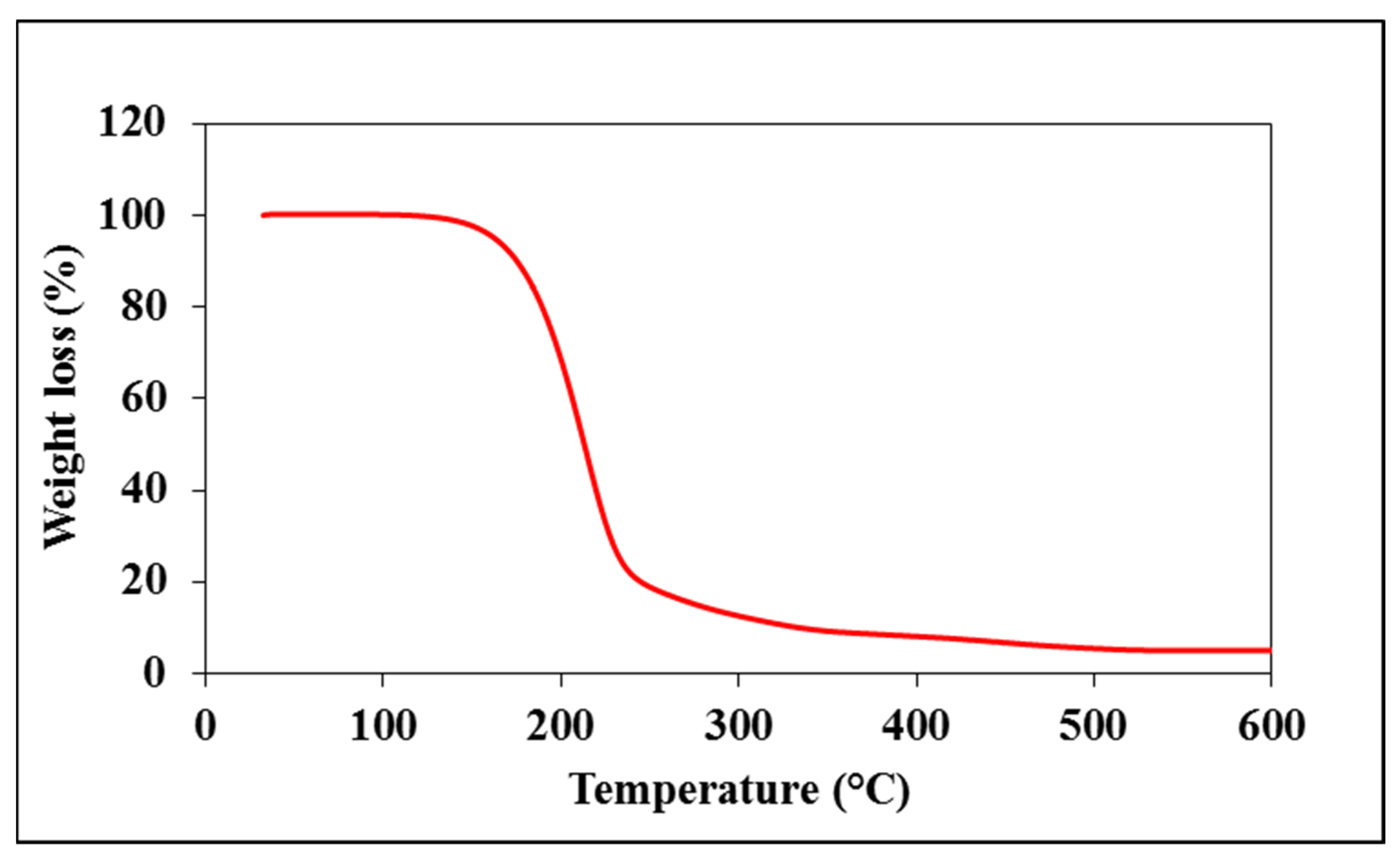
2.3. Characterization of Starch—Myristic Acid Complex
2.4. Cell Culture
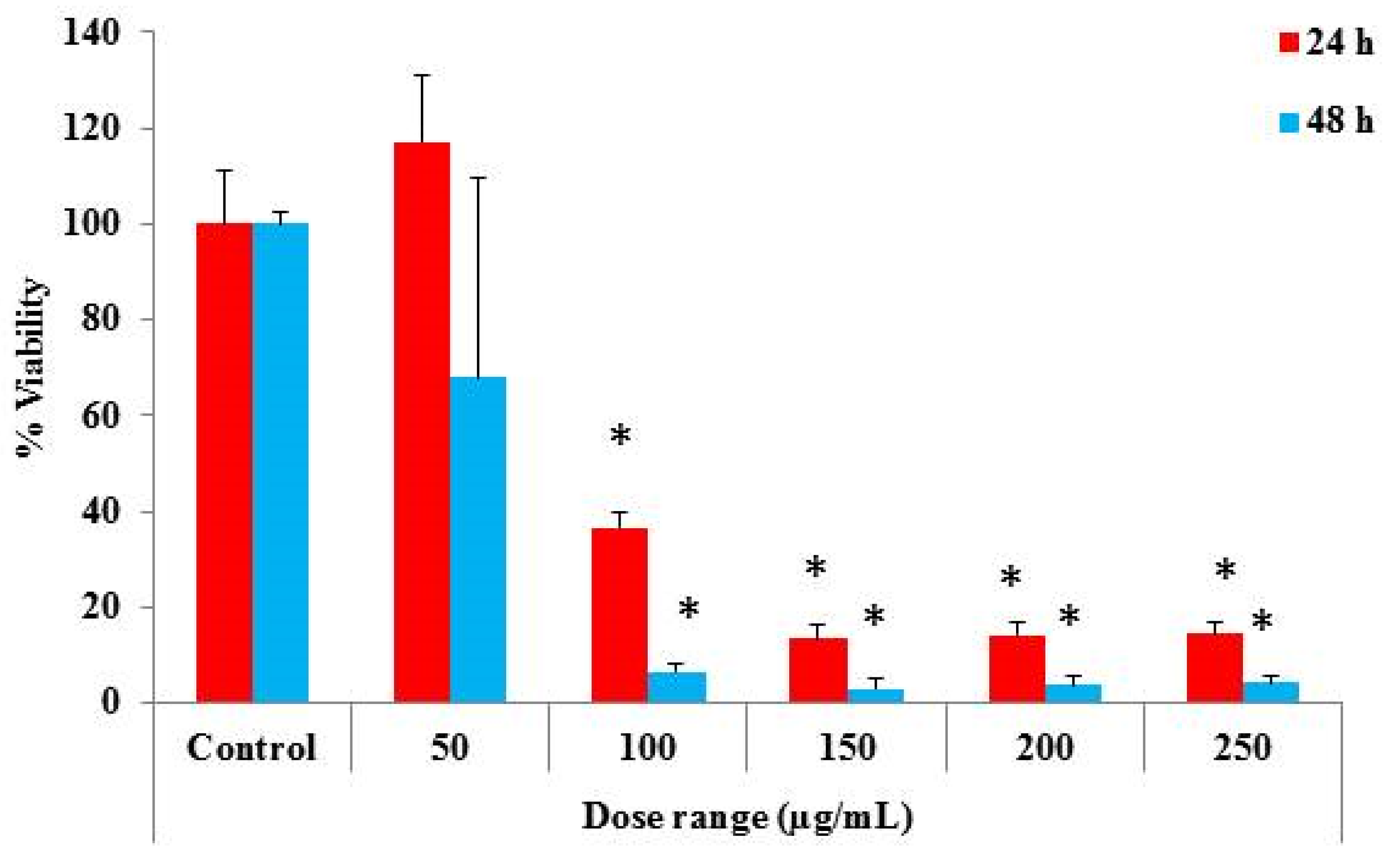
2.5. Determination of Cytotoxic Properties
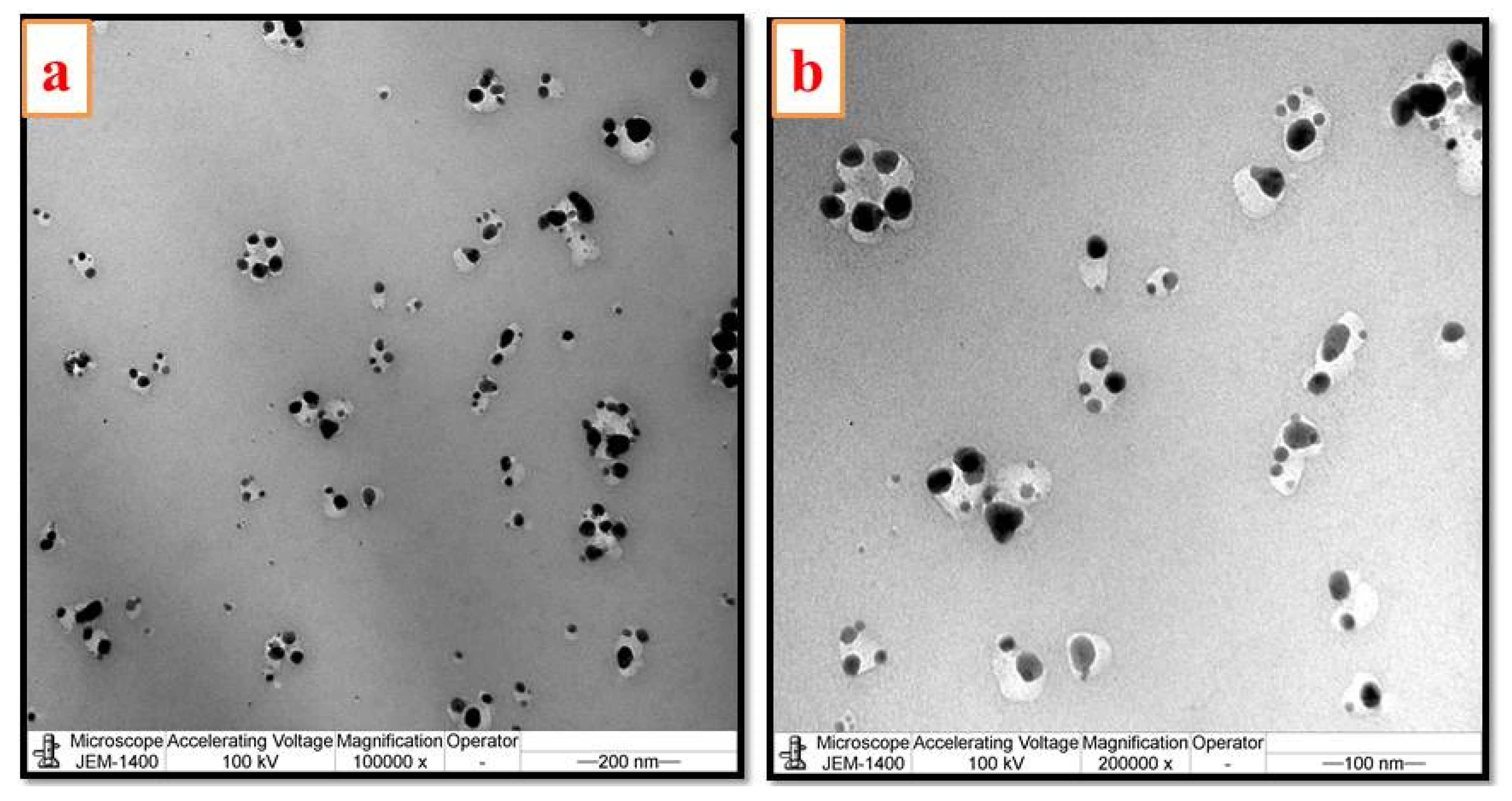
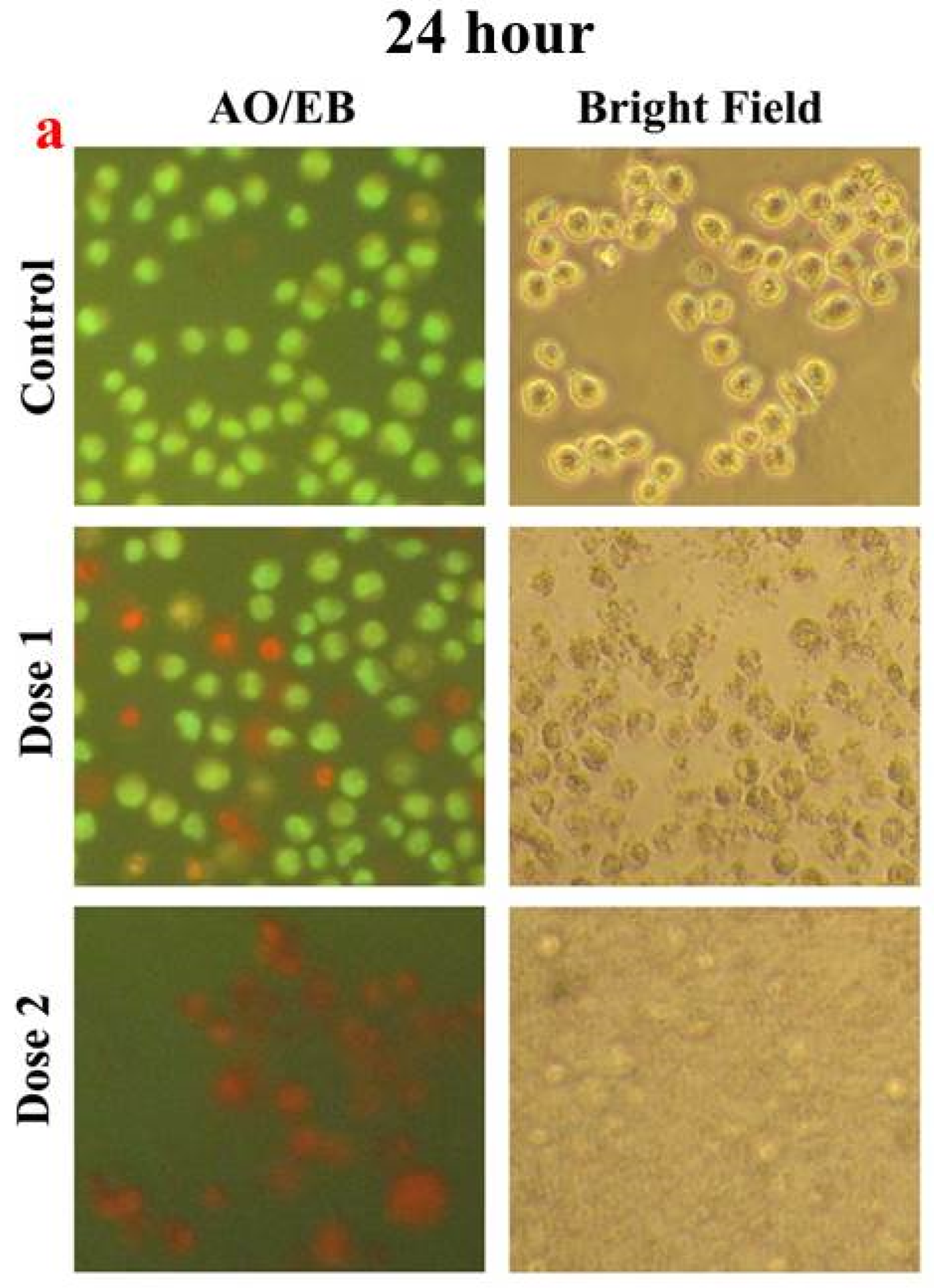
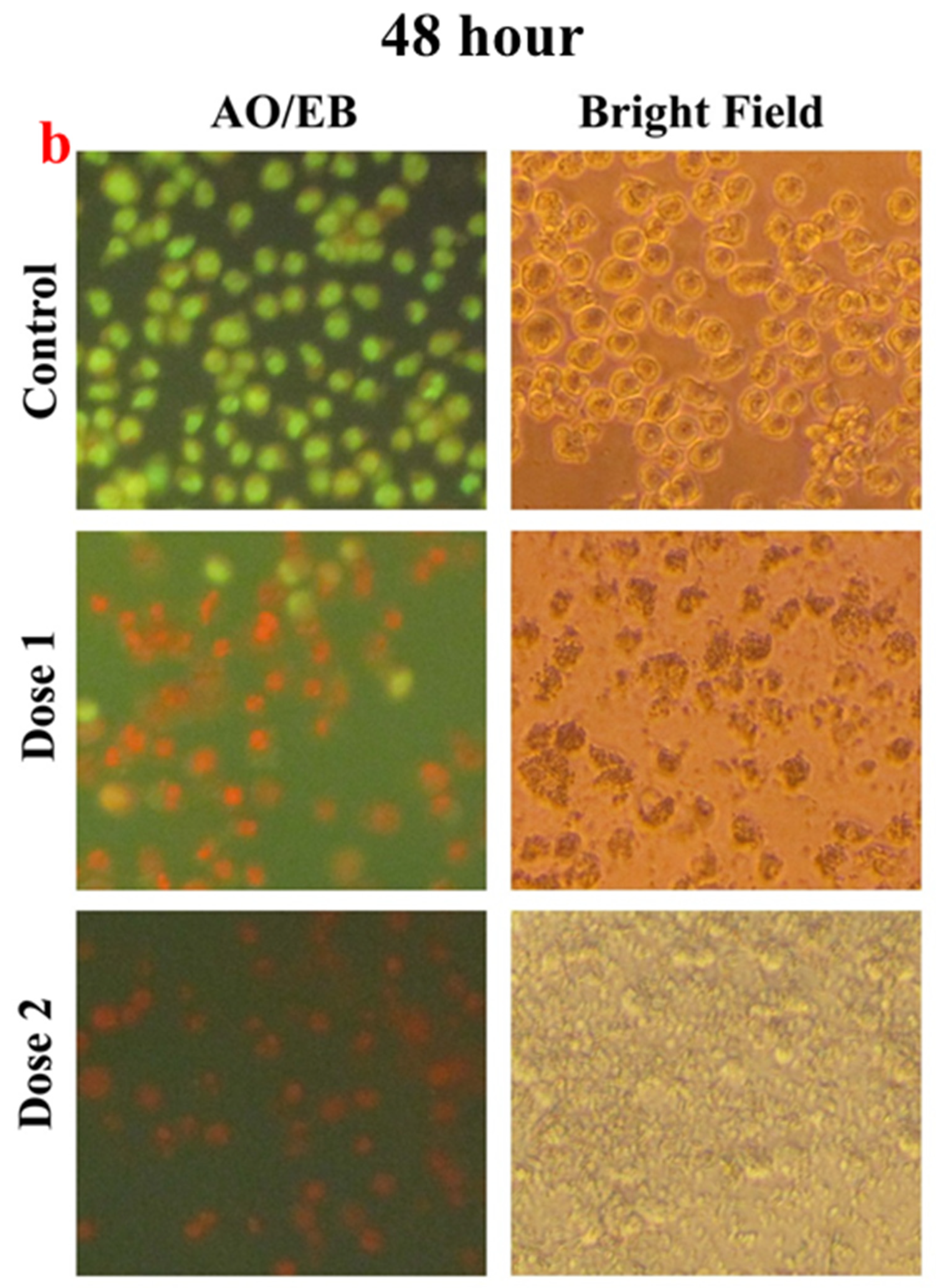
2.6. Microscopic Studies
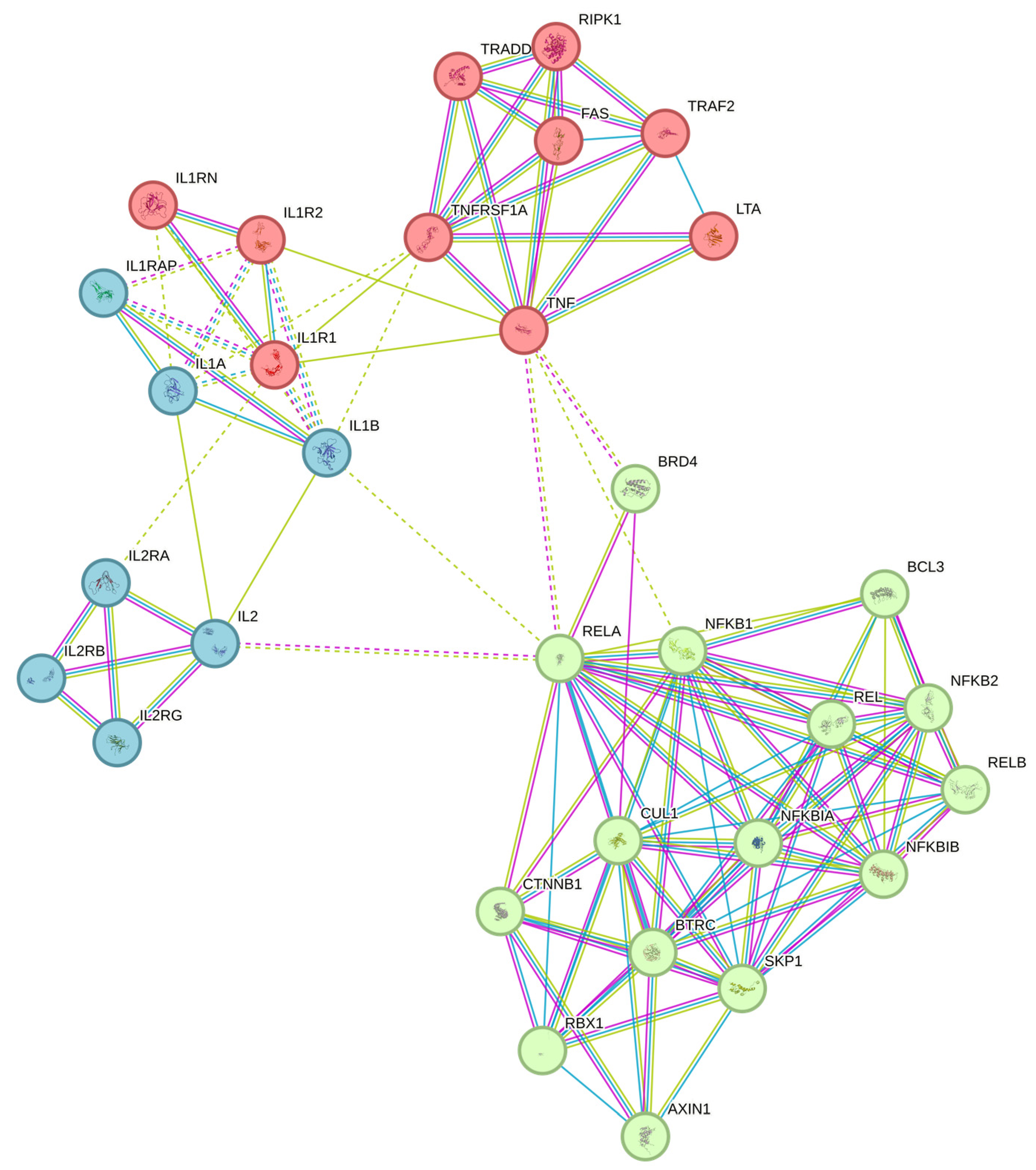
2.7. Gene Expression
2.8. Gene Marker Selection
2.9. Statistical Analysis
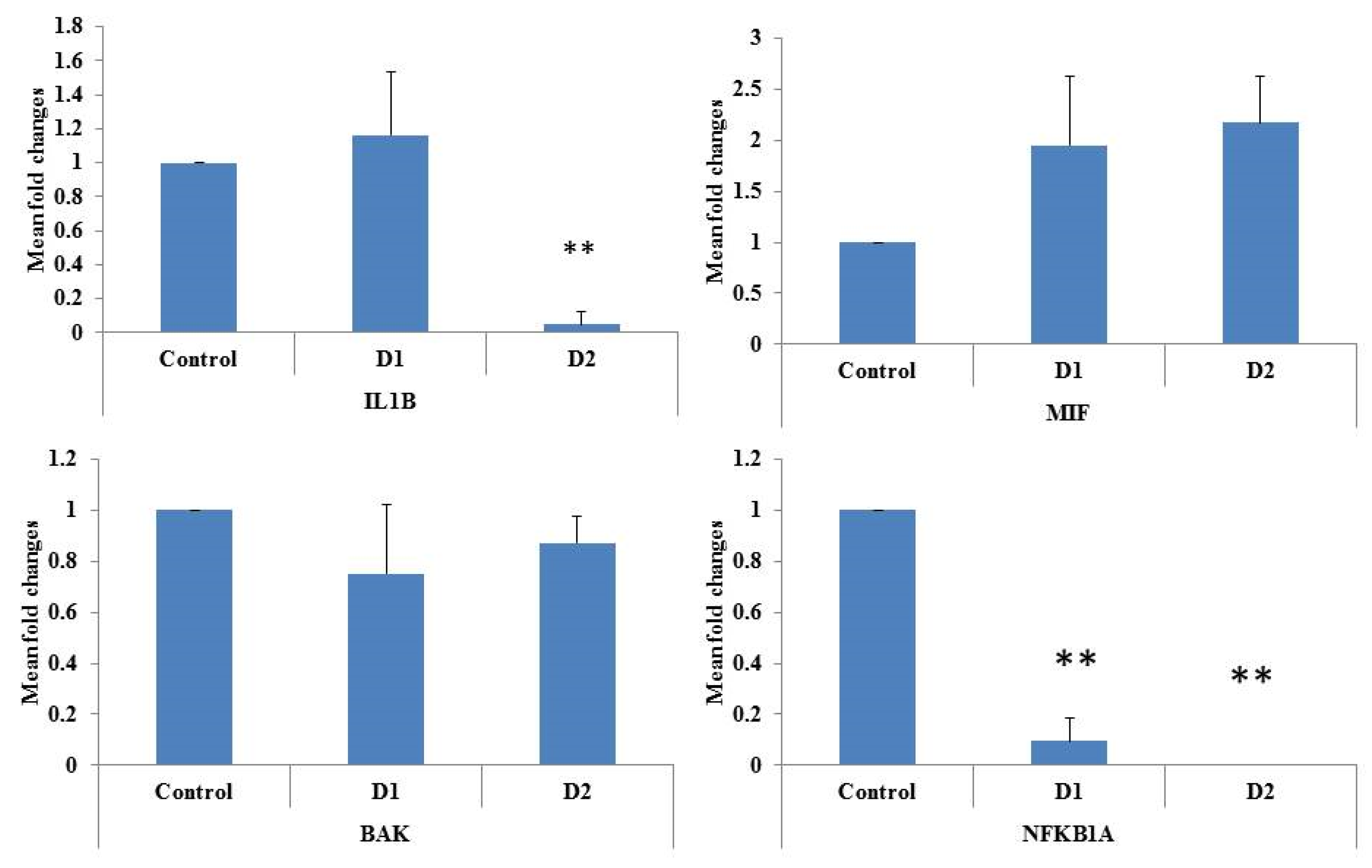
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taş, N.G.; Gökmen, V. Maillard reaction and caramelization during hazelnut roasting: A multiresponse kinetic study. Food Chem. 2017, 221, 1911–1922. [Google Scholar]
- Jiang, S.; Shi, Y.; Li, M.; Xiong, L.; Sun, Q. Characterization of Maillard reaction products micro/nano-particles present in fermented soybean sauce and vinegar. Sci. Rep. 2019, 9, 11285. [Google Scholar] [CrossRef]
- Wen, P.; Zhang, L.; Kang, Y.; Xia, C.; Jiang, J.; Xu, H.; Cui, G.; Wang, J. Effect of Baking Temperature and Time on Advanced Glycation End Products and Polycyclic Aromatic Hydrocarbons in Beef. J. Food Prot. 2022, 85, 1726–1736. [Google Scholar] [CrossRef]
- Birch, C.S.; Bonwick, G.A. Advanced Glycation Endproducts (AGEs) in Food: Health Implications and Mitigation Strategies. In Mitigating Contamination from Food Processing; The Royal Society of Chemistry: London, UK, 2019; pp. 191–220. [Google Scholar]
- Ali, A.; Waly, M.I.; Devarajan, S. Impact of Processing Meat on the Formation of Heterocyclic Amines and Risk of Cancer; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Adeyeye, S.A.O.; Ashaolu, T.J. Heterocyclic amine formation and mitigation in processed meat and meat products: A Mini-Review. J. Food Prot. 2021, 84, 1868–1877. [Google Scholar] [CrossRef]
- Geng, Y.; Xie, Y.; Li, W.; Ji, J.; Chen, F.; Liao, X.; Hu, X.; Ma, L. Heterocyclic Amines in Meat and Meat Products: Occurrence, Formation, Mitigation, Health Risks and Intervention. Food Rev. Int. 2023, 1–17. [Google Scholar] [CrossRef]
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Tan, M. Endogenous fluorescence carbon dots derived from food items. Innovation 2020, 1, 100009. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, Y.; Wang, H.; Song, Y.; Cong, S.; Yu, C.; Zhu, B.-W.; Tan, M. Presence and formation mechanism of foodborne carbonaceous nanostructures from roasted pike eel (Muraenesox cinereus). J. Agric. Food Chem. 2017, 66, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shan, S.; Li, J.; Cao, L.; Lv, J.; Tan, M. Assessment of potential toxicity of foodborne fluorescent nanoparticles from roasted pork. Nanotoxicology 2019, 13, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadi, A.M.; Periasamy, V.S.; Athinarayanan, J.; Al-Khalifa, A.S.; Alshatwi, A.A. Extraction of ultrafine carbon nanoparticles from samooli Bread and evaluation of their in vitro cytotoxicity in human mesenchymal stem cells. Process Biochem. 2017, 52, 250–258. [Google Scholar] [CrossRef]
- Al-Hadi, A.M.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. The presence of carbon nanostructures in bakery products induces metabolic stress in human mesenchymal stem cells through CYP1A and p53 gene expression. Environ. Toxicol. Pharmacol. 2016, 41, 103–112. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.-H.; Gu, M.; Xu, J. Carbon nanomaterials: Fundamental concepts, biological interactions, and clinical applications. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 223–242. [Google Scholar]
- Almushawah, M.A.; Athinarayanan, J.; Periasamy, V.S. Fabrication of Myristic acid–Potato Starch Complex Nanostructures and Assessment of Their Cytotoxic Behavior. J. Sci. Food Agric. 2023, 104, 1813–1823. [Google Scholar] [CrossRef]
- Almushawah, M.A.; Periasamy, V.S.; Athinarayanan, J.; Alhussain, M.; Alwarthan, A.; Alshatwi, A.A. Morphological, Thermal, and Cytotoxicity Features Assessment of Starch-Myristic Acid Complex-Derived Nanostructured Materials. Starch-Stärke 2023, 76, 2200239. [Google Scholar] [CrossRef]
- Xi, J.; Yang, G.; Cai, J.; Gu, Z. A review of recent research results on soot: The formation of a kind of carbon-based material in flames. Front. Mater. 2021, 8, 695485. [Google Scholar] [CrossRef]
- Hoheisel, T.N.; Schrettl, S.; Szilluweit, R.; Frauenrath, H. Nanostructured carbonaceous materials from molecular precursors. Angew. Chem. Int. Ed. 2010, 49, 6496–6515. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rauch, J.; Kolch, W.; Laurent, S.; Mahmoudi, M. Big signals from small particles: Regulation of cell signaling pathways by nanoparticles. Chem. Rev. 2013, 113, 3391–3406. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. 2014, 6, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Battigelli, A.; Ménard-Moyon, C.; Bianco, A. Carbon nanomaterials as new tools for immunotherapeutic applications. J. Mater. Chem. B 2014, 2, 6144–6156. [Google Scholar] [CrossRef]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of carbon nanotubes and graphene on immune cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The interactions between nanoparticles and the innate immune system from a nanotechnologist perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef]
- Li, Y.; Italiani, P.; Casals, E.; Valkenborg, D.; Mertens, I.; Baggerman, G.; Nelissen, I.; Puntes, V.F.; Boraschi, D. Assessing the immunosafety of engineered nanoparticles with a novel in vitro model based on human primary monocytes. ACS Appl. Mater. Interfaces 2016, 8, 28437–28447. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.; Vijayaraghavan, R. Carbon black particle exhibits size dependent toxicity in human monocytes. Int. J. Inflamm. 2014, 2014, 827019. [Google Scholar] [CrossRef]
- Waldman, W.J.; Kristovich, R.; Knight, D.A.; Dutta, P.K. Inflammatory properties of iron-containing carbon nanoparticles. Chem. Res. Toxicol. 2007, 20, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Ghosn, E.E.B.; Rallapalli, H.; Prescher, J.A.; Larson, T.; Herzenberg, L.A.; Gambhir, S.S. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 2014, 9, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, V.S.; Athinarayanan, J.; Alfawaz, M.A.; Alshatwi, A.A. Carbon nanoparticle induced cytotoxicity in human mesenchymal stem cells through upregulation of TNF3, NFKBIA and BCL2L1 genes. Chemosphere 2016, 144, 275–284. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of myristic acid as a food ingredient. Food Chem. Toxicol. 2007, 45, 517–529. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-chemical changes during repeated frying of cooked oil: A Review. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Márquez-Ruiz, G. Formation and analysis of oxidized monomeric, dimeric, and higher oligomeric triglycerides. In Deep Frying; Elsevier: Amsterdam, The Netherlands, 2007; pp. 87–110. [Google Scholar]
- Dobarganes, C.; Márquez-Ruiz, G.; Velasco, J. Interactions between fat and food during deep-frying. Eur. J. Lipid Sci. Technol. 2000, 102, 521–528. [Google Scholar] [CrossRef]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of amorphous carbon nanoparticles in food caramels. Sci. Rep. 2012, 2, 383. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, Y.; Liu, S.; Cong, S.; Song, Y.; Xu, X.; Tan, M. Presence of fluorescent carbon nanoparticles in baked lamb: Their properties and potential application for sensors. J. Agric. Food Chem. 2017, 65, 7553–7559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bi, J.; Liu, S.; Wang, H.; Yu, C.; Li, D.; Zhu, B.-W.; Tan, M. Presence and formation of fluorescence carbon dots in a grilled hamburger. Food Funct. 2017, 8, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sahu, S.; Sonkar, S.K.; Tackett II, K.N.; Sun, K.W.; Liu, Y.; Maimaiti, H.; Anilkumar, P.; Sun, Y.-P. Versatility with carbon dots–from overcooked BBQ to brightly fluorescent agents and photocatalysts. Rsc Adv. 2013, 3, 15604–15607. [Google Scholar] [CrossRef]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem.-Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef]
- Lécuyer, T.; Seguin, J.; Balfourier, A.; Delagrange, M.; Burckel, P.; Lai-Kuen, R.; Mignon, V.; Ducos, B.; Tharaud, M.; Saubaméa, B. Fate and biological impact of persistent luminescence nanoparticles after injection in mice: A one-year follow-up. Nanoscale 2022, 14, 15760–15771. [Google Scholar] [CrossRef]
- Dowding, J.M.; Das, S.; Kumar, A.; Dosani, T.; McCormack, R.; Gupta, A.; Sayle, T.X.; Sayle, D.C.; von Kalm, L.; Seal, S. Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano 2013, 7, 4855–4868. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 2012, 5361–5374. [Google Scholar] [CrossRef]
- Kinaret, P.A.S.; Scala, G.; Federico, A.; Sund, J.; Greco, D. Carbon nanomaterials promote M1/M2 macrophage activation. Small 2020, 16, 1907609. [Google Scholar] [CrossRef] [PubMed]
- Hamilton Jr, R.F.; Buford, M.C.; Wood, M.B.; Arnone, B.; Morandi, M.; Holian, A. Engineered carbon nanoparticles alter macrophage immune function and initiate airway hyper-responsiveness in the BALB/c mouse model. Nanotoxicology 2007, 1, 104–117. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular toxicity and immunological effects of carbon-based nanomaterials. Part. Fibre Toxicol. 2019, 16, 1–27. [Google Scholar] [CrossRef]
- Holmes, D. Physiologic role of IL-1β in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Doroudian, M.; O’Neill, A.; O’Reilly, C.; Tynan, A.; Mawhinney, L.; McElroy, A.; Webster, S.S.; MacLoughlin, R.; Volkov, Y.; E Armstrong, M. Aerosolized drug-loaded nanoparticles targeting migration inhibitory factors inhibit Pseudomonas aeruginosa-induced inflammation and biofilm formation. Nanomedicine 2020, 15, 2933–2953. [Google Scholar] [CrossRef]
- Hirano, S.; Fujitani, Y.; Furuyama, A.; Kanno, S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2010, 249, 8–15. [Google Scholar] [CrossRef]
- Roger, T.; David, J.; Glauser, M.P.; Calandra, T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 2001, 414, 920–924. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages–an inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef] [PubMed]
- Franiak-Pietryga, I.; Ostrowska, K.; Maciejewski, H.; Ziemba, B.; Appelhans, D.; Voit, B.; Jander, M.; Treliński, J.; Bryszewska, M.; Borowiec, M. Affecting NF-κB cell signaling pathway in chronic lymphocytic leukemia by dendrimers-based nanoparticles. Toxicol. Appl. Pharmacol. 2018, 357, 33–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Understanding the Interaction between Nanomaterials Originated from High-Temperature Processed Starch/Myristic Acid and Human Monocyte Cells. Foods 2024, 13, 554. https://doi.org/10.3390/foods13040554
Periasamy VS, Athinarayanan J, Alshatwi AA. Understanding the Interaction between Nanomaterials Originated from High-Temperature Processed Starch/Myristic Acid and Human Monocyte Cells. Foods. 2024; 13(4):554. https://doi.org/10.3390/foods13040554
Chicago/Turabian StylePeriasamy, Vaiyapuri Subbarayan, Jegan Athinarayanan, and Ali A. Alshatwi. 2024. "Understanding the Interaction between Nanomaterials Originated from High-Temperature Processed Starch/Myristic Acid and Human Monocyte Cells" Foods 13, no. 4: 554. https://doi.org/10.3390/foods13040554




