Identification of Key Genes Associated with 1,2,6-Tri-O-galloyl-β-D-glucopyranose Accumulation in Camellia sinensis Based on Transcriptome Sequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of 1,2,6-TGGP and Other Metabolite Contents
2.3. RNA Extraction, Library Preparation, and RNA-seq
2.4. Data Quality Control and Mapping of Reads to the Genome
2.5. Identification of DEGs and Functional Enrichment Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR) Validation of DEGs
3. Results
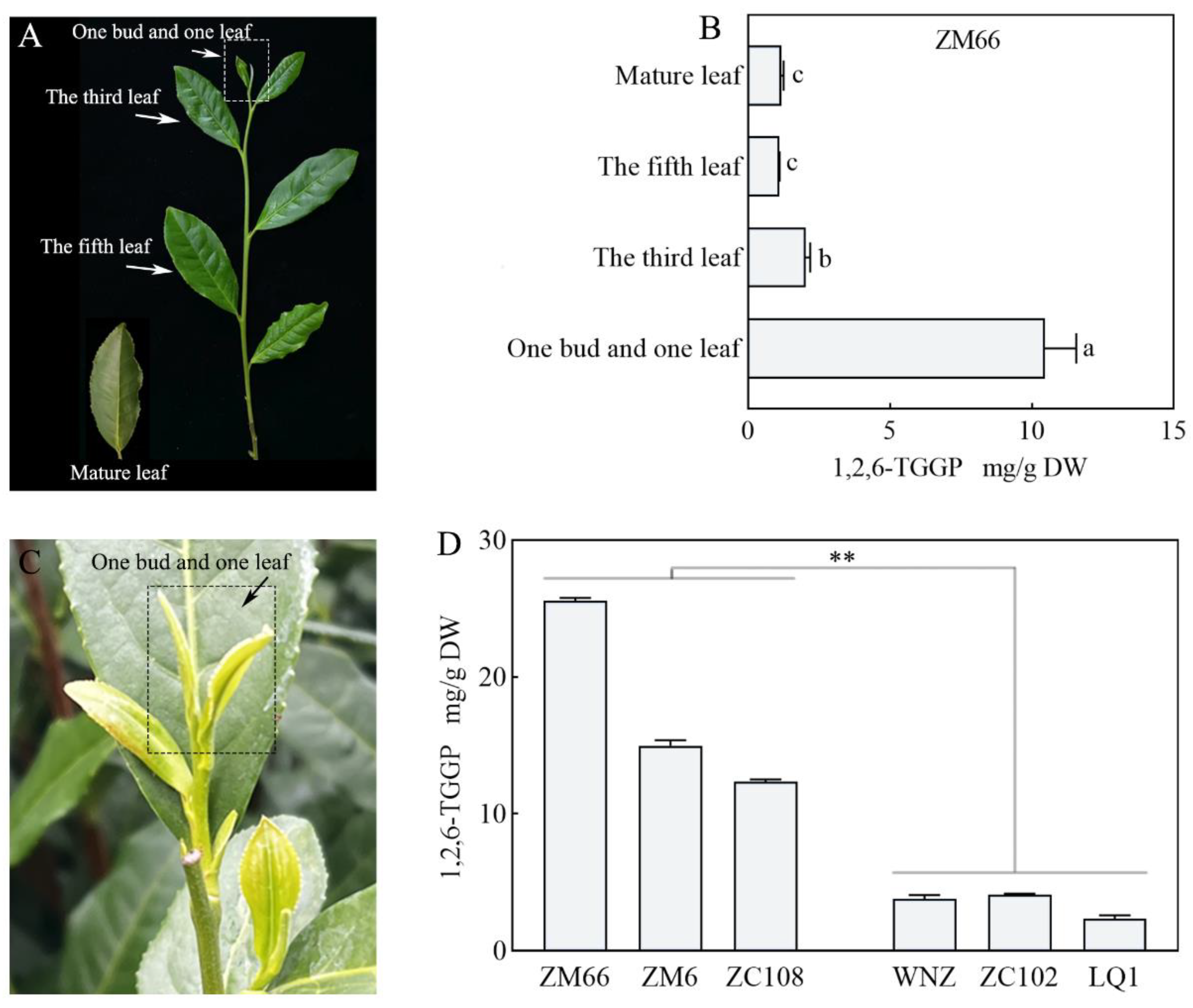
3.1. Comparison of the 1,2,6-TGGP and Related Metabolites Contents in Different Tea Samples
3.2. Transcriptome Sequencing Quality Assessment and Reference Genome Alignment
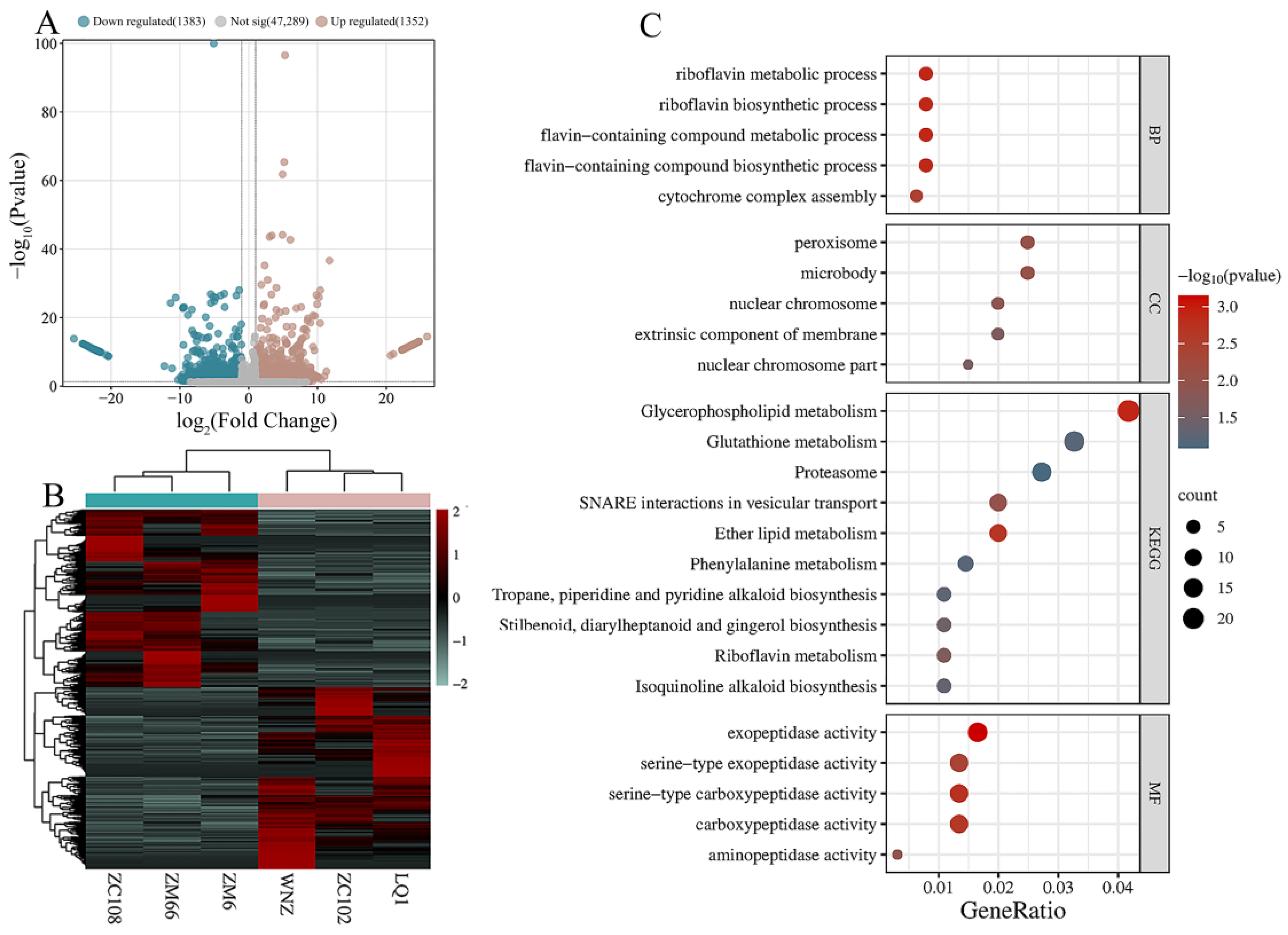
3.3. Screening of Differentially Expressed Genes
3.4. Identification of Key Candidate Genes
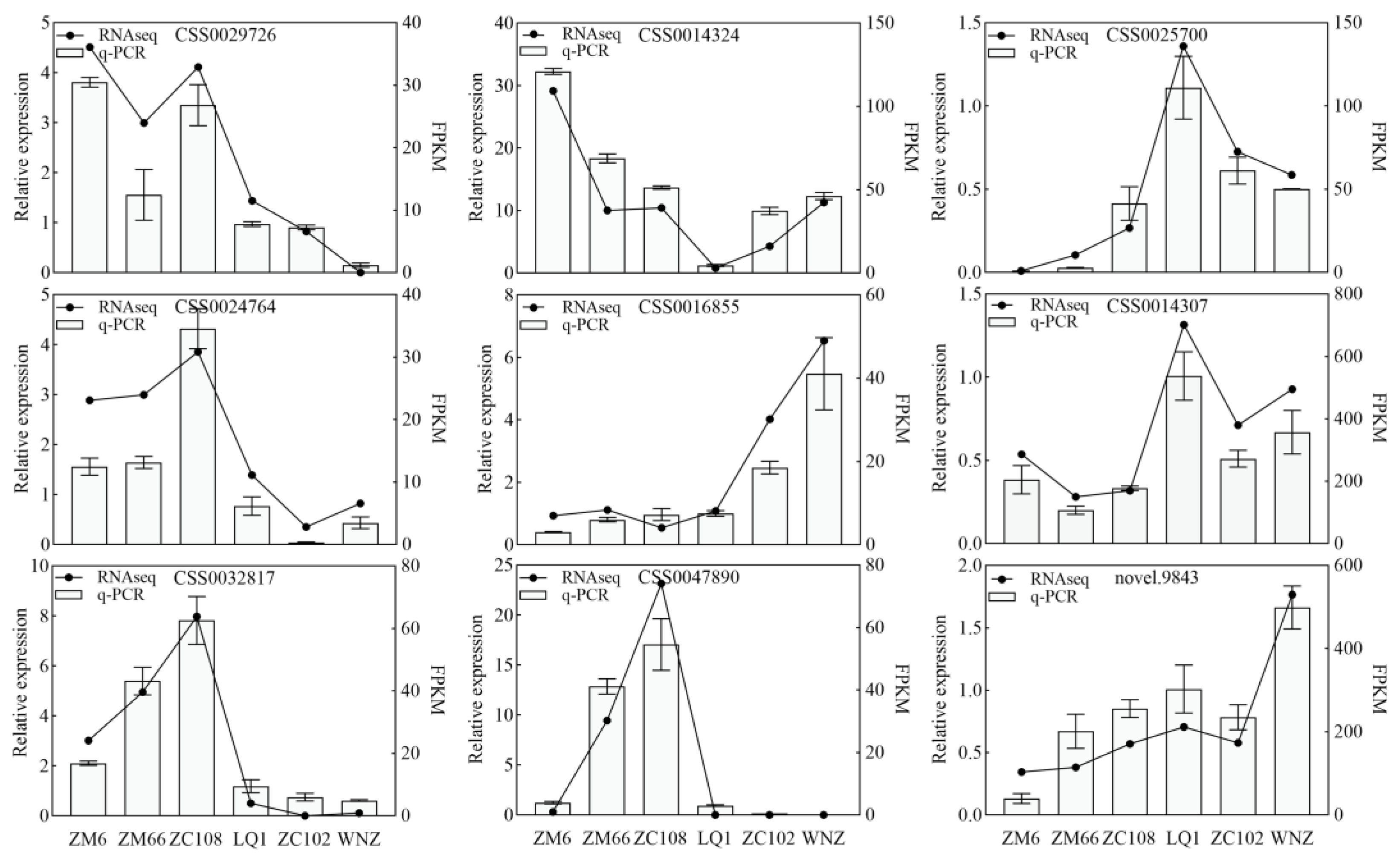
3.5. Quantitative Real-Time PCR Verification of Transcriptome Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazaki, K.; Okuda, T. Gallotannin Production in Cell Cultures of Cornus Officinalis Sieb. et Zucc. Plant Cell Rep. 1989, 8, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Li, Z.G.; Luo, H.P.; Zhang, W.; Chen, L.R.; Xu, X.J. Antiviral Compounds from Traditional Chinese Medicines Gal-la Chinese as Inhibitors of HCV NS3 Protease. Bioorganic Med. Chem. Lett. 2004, 14, 6041–6044. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.B.; Suk, K.; Kim, I.K.; Kin, S.Y.; Kim, J.A.; Lee, S.H.; Kim, S.H. Gallotannin Isolated from Euphorbia Species, 1,2,6-Tri-O-Galloyl-Beta-D-Allose, Decreases Nitric Oxide Production Through Inhibition of Nuclear Factor-Kappa B and Downstream Inducible Nitric Oxide Synthase Expression in Macrophages. Biol. Pharm. Bull. 2009, 32, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. Isolation and Identification of a Gallotannin 1,2,6-Tri-O-Galloyl-Beta-D-Glucopyranose from Hydroalcoholic Extract of Terminalia Chebula Fruits Effective Against Multidrug-Resistant Uropathogens. J. Appl. Microbiol. 2013, 115, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Efflux-Pump Inhibitory Activity of a Gallotannin from Terminalia Chebula Fruit Against Multi-drug-resistant Uropathogenic Escherichia Coli. Nat. Prod. Res. 2014, 28, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Chattopadhyay, R.R. Synergistic Antibiofilm Efficacy of a Gallotannin 1,2,6-Tri-O-Galloyl-Beta-D-Glucopyranose from Terminalia Chebula Fruit in Combination with Gentamicin and Trimethoprim Against Multidrug Resistant Uropathogenic Escherichia Coli Biofilms. PLoS ONE 2017, 12, e0178712. [Google Scholar] [CrossRef]
- Yan, Z.M.; Zhong, Y.Z.; Duan, Y.H.; Chen, Q.H.; Li, F.N. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Huang, K.Y.; Li, M.; Liu, Y.J.; Zhu, M.Q.; Zhao, G.F.; Zhou, Y.H.; Zhang, L.J.; Wu, Y.L.; Dai, X.; Xia, T.; et al. Functional Analysis of 3-Dehydroquinate Dehydratase/Shikimate Dehydrogenases Involved in Shikimate Pathway in Camellia sinensis. Front. Plant Sci. 2019, 10, 1268. [Google Scholar] [CrossRef]
- Lin, L.Z.; Chen, P.; Harnly, J.M. New Phenolic Components and Chromatographic Profiles of Green and Fermented Teas. J. Agric. Food Chem. 2008, 56, 8130–8140. [Google Scholar] [CrossRef]
- Gao, D.F.; Zhang, Y.J.; Yang, C.R.; Chen, K.K.; Jiang, H.J. Phenolic Antioxidants from Green Tea Produced from Camellia taliensis. J. Agric. Food Chem. 2008, 56, 7517–7521. [Google Scholar] [CrossRef]
- Song, M.; Li, Q.; Guan, X.; Wang, T.; Bi, K. Novel HPLC Method to Evaluate the Quality and Identify the Origins of Longjing Green Tea. Anal. Lett. 2013, 46, 60–73. [Google Scholar] [CrossRef]
- Meng, X.H.; Zhu, H.T.; Yan, H.; Wang, D.; Yang, C.R.; Zhang, Y.J. C-8 N-Ethyl-2-Pyrrolidinone-Substituted Flavan-3-ols from the Leaves of Camellia sinensis var. pubilimba. J. Agric. Food Chem. 2018, 66, 7150–7155. [Google Scholar] [CrossRef]
- Wei, K.; He, H.F.; Li, H.L.; Wang, L.Y.; Ruan, L.; Pang, D.D.; Cheng, H. Gallotannin 1,2,6-Tri-O-Galloyl-Beta-D-Glucopyranose: Its Availability and Changing Patterns in Tea (Camellia sinensis). Food Chem. 2019, 296, 40–46. [Google Scholar] [CrossRef]
- Saxe, H.J.; Horibe, T.; Balan, B.; Butterfield, T.S.; Feinberg, N.G.; Zabaneh, C.M.; Jacobson, A.E.; Dandekar, A.M. Two UGT84A Family Glycosyltransferases Regulate Phenol, Flavonoid, and Tannin Metabolism in Juglans regia (English Walnut). Front. Plant Sci. 2021, 12, 626483. [Google Scholar] [CrossRef]
- Mittasch, J.; Böttcher, C.; Frolova, N.; Bönn, M.; Milkowski, C. Identification of UGT84A13 as A Candidate Enzyme for The First Committed Step of Gallotannin Biosynthesis in Pedunculate Oak (Quercus robur). Phytochemistry 2014, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.N.; Qin, X.Q.; Wilson, A.E.; Li, G.; Tan, L. Two UGT84 Family Glycosyltransferases Catalyze a Critical Reaction of Hydrolyzable Tannin Biosynthesis in Pomegranate (Punica granatum). PLoS ONE 2016, 11, e0156319. [Google Scholar] [CrossRef]
- Ye, Q.H.; Zhang, S.Y.; Qiu, N.N.; Liu, L.M.; Wang, W.; Xie, Q.; Chang, Q.; Chen, Q.X. Identification and Characterization of Glucosyltransferase That Forms 1-Galloyl-β-d-Glucogallin in Canarium album L., a Functional Fruit Rich in Hydrolysable Tannins. Molecules 2021, 26, 4650. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Yao, S.B.; Dai, X.L.; Yin, Q.G.; Liu, Y.J.; Jiang, X.L.; Wu, Y.H.; Qian, Y.M.; Pang, Y.Z.; Gao, L.P.; et al. Identification of UDP-Glycosyltransferases Involved in the Biosynthesis of Astringent Taste Compounds in Tea (Camellia sinensis). J. Exp. Bot. 2016, 67, 2285–2297. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wei, K.; Li, H.L.; Wang, L.Y.; Ruan, L.; Pang, D.D.; Cheng, H. Identification of Key Genes Involved in Catechin Metabolism in Tea Seedlings Based on Transcriptomic and HPLC Analysis. Plant Physiol. Biochem. 2018, 133, 107–115. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Li, P.; She, G.; Xia, E.; Benedito, V.A.; Wan, X.C.; Zhao, J. Genome-Wide Analysis of Serine Carboxypeptidase-Like Acyltransferase Gene Family for Evolution and Characterization of Enzymes Involved in the Biosynthesis of Galloylated Catechins in the Tea Plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 848. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Chen, L.B.; Lu, M.Q.; Zhang, J.; Han, J.H.; Deng, W.W.; Zhang, Z.Z. Caffeine Content and Related Gene Expression: Novel Insight into Caffeine Metabolism in Camellia Plants Containing Low, Normal, and High Caffeine Concentrations. J. Agric. Food Chem. 2019, 67, 3400–3411. [Google Scholar] [CrossRef]
- Wei, C.L.; Yang, H.; Wang, S.B.; Zhao, J.; Liu, C.; Gao, L.P.; Xia, E.H.; Lu, Y.; Tai, Y.L.; She, G.B.; et al. Draft Genome Sequence of Camellia sinensis var. sinensis Provides Insights into the Evolution of the Tea Genome and Tea Quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; Mc, C.K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Li, Q.; Xing, H.; Lu, X.F.; Zhao, L.S.; Qu, K.K.; Bi, K.S. Evaluation of Antioxidant Activity of Ten Compounds in Different Tea Samples by Means of an On-Line HPLC–DPPH Assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Le, R.J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a Major Regulator of Phenylpropanoid Availability and Biological Activity in Plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Jadhav, S.K.R.; Patel, K.A.; Dholakia, B.B.; Khan, B.M. Structural Characterization of a Flavonoid Glycosyltransferase from Withania somnifera. Bioinformation 2012, 8, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.H.; Cao, Y.L.; Xing, M.Y.; Guo, Y.; Li, J.J.; Xue, L.; Sun, C.D.; Xu, C.J.; Chen, K.S.; Li, X. Genome-Wide Analysis of UDP-Glycosyltransferase Gene Family and Identification of Members Involved in Flavonoid Glucosylation in Chinese Bayberry (Morella rubra). Front. Plant Sci. 2022, 13, 998985. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, F.; Dossa, K.; Wang, Z.; Ke, T. Genome-Wide Analysis of UDP-Glycosyltransferase Super Family in Brassica Rapa and Brassica Oleracea Reveals Its Evolutionary History and Functional Characterization. BMC Genom. 2017, 18, 474. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, S.B.; Zhang, X.; Wang, Z.H.; Jiang, C.J.; Liu, Y.J.; Jiang, X.L.; Gao, L.P.; Xia, T. Flavan-3-ol Galloylation-Related Functional Gene Cluster and the Functional Diversification of SCPL Paralogs in Camellia sp. J. Agric. Food Chem. 2022, 71, 488–498. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.Q.; Jia, Y.X. The Serine Carboxypeptidase-Like Gene SCPL41 Negatively Regulates Membrane Lipid Metabolism in Arabidopsis thaliana. Plants 2020, 9, 696. [Google Scholar] [CrossRef]
- Jiang, P.H.; Gao, J.S.; Mu, J.Y.; Duan, L.N.; Gu, Y.S.; Han, S.C.; Chen, L.; Li, Y.X.; Yan, Y.M.; Li, X.H. Interaction Between Serine Carboxypeptidase-Like Protein TtGS5 and Annexin D1 in Developing Seeds of Triticum timopheevi. J. Appl. Genet. 2020, 61, 151–162. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, M.Z.; Wang, W. Structure, Function and Application of Serine Carboxypeptidase-like Acyltransferases from Plants. Sheng Wu Gong Cheng Xue Bao 2021, 37, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.B.; Liu, Y.J.; Zhuang, J.H.; Zhao, Y.; Dai, X.L.; Jiang, C.J.; Wang, Z.H.; Jiang, X.L.; Zhang, S.X.; Qian, Y.M.; et al. Insights into Acylation Mechanisms: Co-Expression of Serine Carboxypeptidase-Like Acyltransferases and Their Non-catalytic Companion Paralogs. Plant J. 2022, 111, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, M.; Liu, J.Y.; Cai, J. Metabolomic and Transcriptomic Analyses Reveal the Characteristics of Tea Flavonoids and Caffeine Accumulation and Regulation between Chinese Varieties (Camellia sinensis var. sinensis) and Assam Varieties (C. sinensis var. assamica). Genes 2022, 13, 1194. [Google Scholar] [CrossRef] [PubMed]
- Owatworakit, A.; Townsend, B.; Louveau, T.; Jenner, H.; Rejzek, M.; Hughes, R.K.; Saalbach, G.; Qi, X.Q.; Bakht, S.; Roy, A.D.; et al. Glycosyltransferases from Oat (Avena) Implicated in the Acylation of Avenacins. J. Biol. Chem. 2013, 288, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kaminaga, Y.; Cooper, B.; Pichersky, E.; Dudareva, N.; Chapple, C. Benzoylation and sinapoylation of glucosinolate R-groups in Arabidopsis. Plant J. 2012, 72, 411–422. [Google Scholar] [CrossRef]
- Chang, Y.H.; Gong, W.W.; Xu, J.M.; Gong, H.; Song, Q.L.; Xia, S.X.; Yuan, D.Y. Integration of Semi-in Vivo Assays and Multi-omics Data Reveals the Effect of Galloylated Catechins on Self-pollen Tube Inhibition in Camellia oleifera. Hortic. Res. 2023, 10, uhac248. [Google Scholar] [CrossRef]
- Dhakarey, R.; Yaritz, U.; Tian, L.; Amir, R. A Myb Transcription Factor, PgMyb308-like, Enhances the Level of Shikimate, Aromatic Amino Acids, and Lignins, but Represses the Synthesis of Flavonoids and Hydrolyzable Tannins, in Pomegranate (Punica granatum L.). Hortic. Res. 2022, 9, uhac008. [Google Scholar] [CrossRef]

| ZM6 | ZM66 | ZC108 | ZC102 | WNZ | LQ1 | |
|---|---|---|---|---|---|---|
| GA | 7.54 ± 0.09 e | 8.42 ± 0.10 c | 9.40 ± 0.10 a | 9.07 ± 0.04 b | 7.90 ± 0.12 d | 5.66 ± 0.07 f |
| GC | 3.95 ± 0.15 a | 0.96 ± 0.29 bc | 1.02 ± 0.06 bc | 0.83 ± 0.11 c | 0.45 ± 0.26 c | 1.34 ± 0.23 b |
| EGC | 1.11 ± 0.05 a | 0.87 ± 0.02 b | 0.99 ± 0.02 a | 0.59 ± 0.09 cd | 0.42 ± 0.06 d | 0.61 ± 0.02 c |
| C | 1.60 ± 0.28 e | 5.61 ± 0.10 b | 9.17 ± 0.09 a | 2.41 ± 0.03 c | 2.11 ± 0.08 d | 1.47 ± 0.00 e |
| EC | 6.39 ± 0.42 a | 5.30 ± 0.31 b | 4.68 ± 0.03 c | 4.35 ± 0.05 d | 5.37 ± 0.22 b | 6.92 ± 0.14 a |
| GCG | 0.74 ± 0.03 a | 0.32 ± 0.16 c | 0.65 ± 0.01 b | 0.39 ± 0.02 c | 0.36 ± 0.18 c | 0.72 ± 0.03 ab |
| ECG | 0.58 ± 0.07 b | 0.20 ± 0.03 c | 1.53 ± 0.00 a | 1.54 ± 0.05 a | 1.62 ± 0.28 a | 0.20 ± 0.15 c |
| EGCG | 68.87 ± 0.1.86 a | 60.67 ± 0.52 b | 69.15 ± 0.87 a | 66.19 ± 1.54 a | 55.74 ± 2.19 c | 58.83 ± 1.36 b |
| Caffeine | 25.44 ± 0.40 b | 31.79 ± 0.40 a | 31.87 ± 0.36 a | 31.29 ± 0.16 a | 30.92 ± 0.41 a | 27.62 ± 0.34 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xun, H.; Wang, L.; Aktar, S.; Lei, Y.; Zhang, R.; Wang, L.; Wei, K. Identification of Key Genes Associated with 1,2,6-Tri-O-galloyl-β-D-glucopyranose Accumulation in Camellia sinensis Based on Transcriptome Sequencing. Foods 2024, 13, 495. https://doi.org/10.3390/foods13030495
Wang Y, Xun H, Wang L, Aktar S, Lei Y, Zhang R, Wang L, Wei K. Identification of Key Genes Associated with 1,2,6-Tri-O-galloyl-β-D-glucopyranose Accumulation in Camellia sinensis Based on Transcriptome Sequencing. Foods. 2024; 13(3):495. https://doi.org/10.3390/foods13030495
Chicago/Turabian StyleWang, Yueqi, Hanshuo Xun, Liubin Wang, Shirin Aktar, Yuping Lei, Rui Zhang, Liyuan Wang, and Kang Wei. 2024. "Identification of Key Genes Associated with 1,2,6-Tri-O-galloyl-β-D-glucopyranose Accumulation in Camellia sinensis Based on Transcriptome Sequencing" Foods 13, no. 3: 495. https://doi.org/10.3390/foods13030495





