Yeast Starter Culture Identification to Produce of Red Wines with Enhanced Antioxidant Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Vinifications
2.3. Total Polyphenols Content
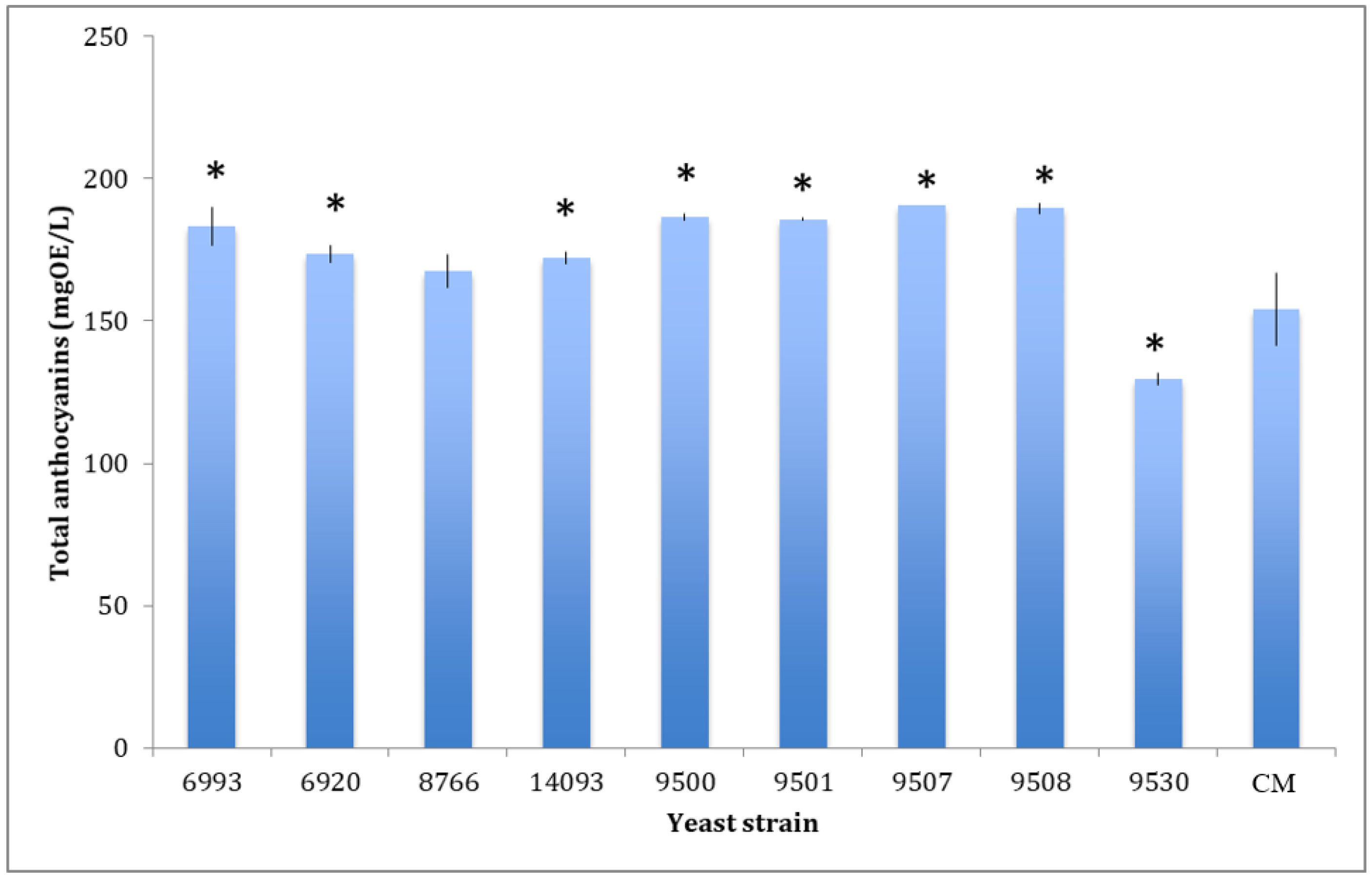
2.4. Total Anthocyanin Content
2.5. TEAC Antioxidant Capacity Determination
2.6. Determination of Polyphenolic Profile of Wines
2.7. Volatile Profile
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 52. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive Oil and Red Wine Antioxidant Polyphenols Inhibit Endothelial Activation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.M.; Guasch, V.; Castillo, O.; Irribarra, V.; Mizon, C.; Martin, A.S.; Strobel, P.; Perez, D.; Germain, A.M.; Leighton, F. A High-Fat Diet Induces and Red Wine Counteracts Endothelial Dysfunction in Human Volunteers. Lipids 2000, 35, 143–148. [Google Scholar] [CrossRef]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol Attenuates TNF-α-Induced Activation of Coronary Arterial Endothelial Cells: Role of NF-κB Inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1694–H1699. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, B.M.; Steinhilber, D.; Stein, J.M.; Ulrich, S. Phytochemicals Resveratrol and Sulforaphane as Potential Agents for Enhancing the Anti-Tumor Activities of Conventional Cancer Therapies. Curr. Pharm. Biotechnol. 2012, 13, 137–146. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple Anti-Inflammatory and Anti-Atherosclerotic Properties of Red Wine Polyphenolic Extracts: Differential Role of Hydroxycinnamic Acids, Flavonols and Stilbenes on Endothelial Inflammatory Gene Expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring Wine Yeast for the New Millennium: Novel Approaches to the Ancient Art of Winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, W.; Meng, Y.; Zhang, Y.; Jin, G.; Fang, Z. Wine Phenolic Profile Altered by Yeast: Mechanisms and Influences. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3579–3619. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of Anthocyanins by Yeast Cell Walls during the Fermentation of Red Wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Villena, M.A.; Iranzo, J.F.Ú.; Pérez, A.I.B. β-Glucosidase Activity in Wine Yeasts: Application in Enology. Enzym. Microb. Technol. 2007, 40, 420–425. [Google Scholar] [CrossRef]
- Manzanares, P.; Rojas, V.; Genovés, S.; Vallés, S. A Preliminary Search for anthocyanin-β-D-glucosidase Activity in non-Saccharomyces Wine Yeasts. Int. J. Food Sci. Technol. 2000, 35, 95–103. [Google Scholar] [CrossRef]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast Effects on Pinot Noir Wine Phenolics, Color, and Tannin Composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nogales, J.M.; Fernández-Fernández, E.; Gómez, M.; Vila-Crespo, J. Antioxidant Properties of Sparkling Wines Produced with β-Glucanases and Commercial Yeast Preparations. J. Food Sci. 2012, 77, C1005–C1010. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of Indigenous Yeast Strains for the Production of Sparkling Wines from Native Apulian Grape Varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial Terroir and Food Innovation: The Case of Yeast Biodiversity in Wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, M.; Bleve, G.; Grieco, F.; Tristezza, M.; Tufariello, M.; Grieco, F. An Optimized Procedure for the Enological Selection of Non-Saccharomyces Starter Cultures. Antonie van Leeuwenhoek 2011, 99, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Vetrano, C.; Bleve, G.; Grieco, F.; Tufariello, M.; Quarta, A.; Mita, G.; Spano, G.; Grieco, F. Autochthonous Fermentation Starters for the Industrial Production of Negroamaro Wines. J. Ind. Microbiol. Biotechnol. 2012, 39, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Vetrano, C.; Bleve, G.; Spano, G.; Capozzi, V.; Logrieco, A.; Mita, G.; Grieco, F. Biodiversity and Safety Aspects of Yeast Strains Characterized from Vineyards and Spontaneous Fermentations in the Apulia Region, Italy. Food Microbiol. 2013, 36, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Fantastico, L.; Vetrano, C.; Bleve, G.; Corallo, D.; Grieco, F.; Mita, G.; Grieco, F. Molecular and Technological Characterization of Saccharomyces cerevisiae Strains Isolated from Natural Fermentation of Susumaniello Grape Must in Apulia, Southern Italy. Int. J. Microbiol. 2014, 2014, 897428. [Google Scholar] [CrossRef]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G. Influence of Autochthonous Saccharomyces cerevisiae Strains on Volatile Profile of Negroamaro Wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar]
- Garofalo, C.; El Khoury, M.; Lucas, P.; Bely, M.; Russo, P.; Spano, G.; Capozzi, V. Autochthonous Starter Cultures and Indigenous Grape Variety for Regional Wine Production. J. Appl. Microbiol. 2015, 118, 1395–1408. [Google Scholar] [CrossRef]
- Garofalo, C.; Russo, P.; Beneduce, L.; Massa, S.; Spano, G.; Capozzi, V. Non-Saccharomyces Biodiversity in Wine and the ‘Microbial Terroir’: A Survey on Nero Di Troia Wine from the Apulian Region, Italy. Ann. Microbiol. 2016, 66, 143–150. [Google Scholar] [CrossRef]
- Roscini, L.; Tristezza, M.; Corte, L.; Colabella, C.; Perrotta, C.; Rampino, P.; Robert, V.; Vu, D.; Cardinali, G.; Grieco, F. Early Ongoing Speciation of Ogataea Uvarum Sp. Nov. Within the Grape Ecosystem Revealed by the Internal Variability Among the rDNA Operon Repeats. Front. Microbiol. 2018, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Maiorano, G.; Rampino, P.; Spano, G.; Grieco, F.; Perrotta, C.; Capozzi, V.; Grieco, F. Selection of an Autochthonous Yeast Starter Culture for Industrial Production of Primitivo “Gioia Del Colle” PDO/DOC in Apulia (Southern Italy). LWT 2019, 99, 188–196. [Google Scholar] [CrossRef]
- Tristezza, M.; Gerardi, C.; Logrieco, A.; Grieco, F. An Optimized Protocol for the Production of Interdelta Markers in Saccharomyces cerevisiae by Using Capillary Electrophoresis. J. Microbiol. Methods 2009, 78, 286–291. [Google Scholar] [CrossRef]
- Grieco, F.; Tristezza, M.; Vetrano, C.; Bleve, G.; Panico, E.; Mita, G.; Logrieco, A. Exploitation of Autochthonous Micro-Organism Potential to Enhance the Quality of Apulian Wines. Ann. Microbiol. 2011, 61, 67–73. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Gerardi, C.; D’Amico, L.; Accogli, R.; Santino, A. Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots. Agriculture 2018, 8, 102. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gerardi, C.; D’amico, L.; Migoni, D.; Santino, A.; Salomone, A.; Carluccio, M.A.; Giovinazzo, G. Strategies for Reuse of Skins Separated from Grape Pomace as Ingredient of Functional Beverages. Front. Bioeng. Biotechnol. 2020, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Palombi, L.; Tufariello, M.; Durante, M.; Fiore, A.; Baiano, A.; Grieco, F. Assessment of the Impact of Unmalted Cereals, Hops, and Yeast Strains on Volatolomic and Olfactory Profiles of Blanche Craft Beers: A Chemometric Approach. Food Chem. 2023, 416, 135783. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Pati, S.; D’Amico, L.; Bleve, G.; Losito, I.; Grieco, F. Quantitative Issues Related to the Headspace-SPME-GC/MS Analysis of Volatile Compounds in Wines: The Case of Maresco Sparkling Wine. LWT 2019, 108, 268–276. [Google Scholar] [CrossRef]
- Kang, W.; Xu, Y.; Qin, L.; Wang, Y. Effects of Different β-D-Glycosidases on Bound Aroma Compounds in Muscat Grape Determined by HS-SPME and GC-MS. J. Inst. Brew. 2010, 116, 70–77. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Silva, P.; Câmara, J.S. Unraveling Vitis Vinifera L. Grape Maturity Markers Based on Integration of Terpenic Pattern and Chemometric Methods. Microchem. J. 2018, 142, 367–376. [Google Scholar] [CrossRef]
- Caridi, A. Improved Screening Method for the Selection of Wine Yeasts Based on Their Pigment Adsorption Activity. Food Technol. Biotechnol. 2013, 51, 137–144. [Google Scholar]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Heredia, F.J.; Hernández-Hierro, J.M. Phenolic Compounds Extraction in Enzymatic Macerations of Grape Skins Identified as Low-Level Extractable Total Anthocyanin Content. J. Food Sci. 2020, 85, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, V.; Fiore, C.; Maietti, A.; Tedeschi, P.; Romano, P. Influence of Saccharomyces cerevisiae Strains on Wine Total Antioxidant Capacity Evaluated by Photochemiluminescence. World J. Microbiol. Biotechnol. 2007, 23, 581–586. [Google Scholar] [CrossRef]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene Levels and Antioxidant Activity of Vranec and Merlot Wines from Macedonia: Effect of Variety and Enological Practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.V.; Bartolome, B.; Robredo, S.; Leon, A.; Cebollero, E.; Juega, M.; Nunez, Y.P.; Martinez, M.C.; Martinez-Rodriguez, A.J. Influence of Locally-Selected Yeast on the Chemical and Sensorial Properties of Albariño White Wines. LWT Food Sci. Technol. 2012, 46, 319–325. [Google Scholar] [CrossRef]
- Caridi, A.; De Bruno, A.; Salvo, E.; Piscopo, A.; Poiana, M.; Sidari, R. Selected Yeasts to Enhance Phenolic Content and Quality in Red Wine from Low Pigmented Grapes. Eur. Food Res. Technol. 2017, 243, 367–378. [Google Scholar] [CrossRef]
- Grieco, F.; Carluccio, M.A.; Giovinazzo, G. Autochthonous Saccharomyces cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines. Foods 2019, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Heras, J.M.; Callejo, M.J.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Yeast Influence on the Formation of Stable Pigments in Red Winemaking. Food Chem. 2016, 197, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Vernhet, A.; Carrillo, S.; Rattier, A.; Verbaere, A.; Cheynier, V.; Nguela, J.M. Fate of Anthocyanins and Proanthocyanidins during the Alcoholic Fermentation of Thermovinified Red Musts by Different Saccharomyces cerevisiae Strains. J. Agric. Food Chem. 2020, 68, 3615–3625. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, A.; De Vero, L.; Blaiotta, G.; Sidari, R.; Iosca, G.; Gullo, M.; Caridi, A. Selection of Wine Saccharomyces cerevisiae Strains and Their Screening for the Adsorption Activity of Pigments, Phenolics and Ochratoxin A. Fermentation 2020, 6, 80. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A. Natural Flavours of Wine: Correlation between Instrumental Analysis and Sensory Perception. Fresenius’ J. Anal. Chem. 1990, 337, 777–785. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2005; Volume 57, pp. 131–175. ISBN 978-0-12-002659-3. [Google Scholar]
- Swiegers, J.H.; Bartowski, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Moreira, N.; Pina, C.; Mendes, F.; Couto, J.A.; Hogg, T.; Vasconcelos, I. Volatile Compounds Contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during Red Wine Vinifications. Food Control 2011, 22, 662–667. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy Sulphur Compounds, Higher Alcohols and Esters Production Profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii Grown as Pure and Mixed Cultures in Grape Must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Madžgalj, V.; Petrović, A.; Čakar, U.; Maraš, V.; Sofrenić, I.; Tešević, V. The influence of different enzymatic preparations and skin contact time on aromatic profile of wines produced from autochthonous grape varieties Krstač and Žižak. J. Serb. Chem. Soc. 2023, 88, 11–23. [Google Scholar] [CrossRef]
| ID | Strain | TPC (µgGAE/mL) | %TPC Reduction | TEAC (nmolTE/mL) | %TEAC Reduction |
|---|---|---|---|---|---|
| 20 | 6978 | 607.60 ± 5.26 | −48% | 47.63 ± 0.97 | −46% |
| 63 | 8754 | 634.99 ± 5.43 | −46% | 63.70 ± 2.43 | −27% |
| 64 | 8744 | 623.57 ± 7.46 | −47% | 54.24 ± 3.01 | −38% |
| 69 | 8766 | 601.61 ± 5.76 | −48% | 63.50 ± 2.09 | −27% |
| 70 | 8767 | 612.87 ± 8.09 | −48% | 59.98 ± 1.54 | −31% |
| 71 | 8769 | 620.08 ± 5.73 | −47% | 72.75 ± 2.22 | −17% |
| 74 | 8772 | 627.44 ± 5.90 | −46% | 74.66 ± 2.00 | −15% |
| 77 | 8775 | 609.70 ± 8.78 | −48% | 72.22 ± 1.76 | −17% |
| 78 | 8776 | 651.59 ± 4.94 | −44% | 73.20 ± 1.05 | −16% |
| 80 | 8778 | 605.66 ± 3.76 | −48% | 61.80 ± 0.97 | −29% |
| 81 | 8779 | 616.54 ± 3.87 | −47% | 55.76 ± 0.90 | −36% |
| 82 | 8780 | 639.91 ± 5.85 | −45% | 63.57 ± 1.02 | −27% |
| 83 | 6993 | 785.14 ± 8.47 | −33% | 64.04 ± 1.00 | −27% |
| 84 | 6920 | 789.55 ± 9.76 | −32% | 64.81 ± 0.99 | −26% |
| 86 | 8766 | 699.95 ± 6.73 | −40% | 61.60 ± 2.37 | −30% |
| 87 | 6977 | 607.68 ± 2.38 | −48% | 63.23 ± 1.76 | −28% |
| 89 | 8795 | 612.56 ± 5.77 | −48% | 59.79 ± 1.43 | −32% |
| 90 | 17,292 | 626.68 ± 4.38 | −46% | 56.06 ± 0.98 | −36% |
| 91 | 17,293 | 636.65 ± 5.65 | −45% | 66.38 ± 1.76 | −24% |
| 92 | 9502 | 608.41 ± 2.90 | −48% | 64.86 ± 2.02 | −26% |
| 96 | 9531 | 643.67 ± 3.35 | −45% | 64.81 ± 1.74 | −26% |
| 98 | 1407 | 629.36 ± 4.84 | −46% | 62.40 ± 1.64 | −29% |
| 99 | 14,093 | 746.44 ± 2.37 | −36% | 64.79 ± 1.23 | −26% |
| 105 | 9500 | 786.23 ± 5.74 | −33% | 62.95 ± 2.09 | −28% |
| 106 | 9501 | 755.92 ± 6.68 | −35% | 57.90 ± 0.98 | −34% |
| 107 | 9502 | 607.47 ± 2.64 | −48% | 59.17 ± 0.78 | −32% |
| 112 | 9507 | 766.47 ± 5.89 | −34% | 68.38 ± 2.20 | −22% |
| 113 | 9508 | 779.70 ± 3.65 | −33% | 65.78 ± 1.43 | −25% |
| 135 | 9530 | 729.32 ± 5.74 | −38% | 58.66 ± 0.98 | −33% |
| Must | 1167.45 ± 8.46 | 87.49 ± 3.01 |
| Strain | Ethanol | Sugars | TA | VA | Malic Acid | Lactic Acic | Glycerol |
|---|---|---|---|---|---|---|---|
| 6993 | 11.45 ± 0.023 | 1.6 ± 0.034 | 7.11 ± 0.008 | 0.14 ± 0.013 | 2.58 ± 0.005 | 0.15 ± 0.004 | 6.38 ± 0.055 |
| 6920 | 11.58 ± 0.004 | 1.34 ± 0.059 | 7.14 ± 0.056 | 0.07 ± 0.15 | 2.64 ± 0.002 | 0.08 ± 0.001 | 6.54 ± 0.088 |
| 8766 | 11.43 ± 0.061 | 1.28 ± 0.334 | 7.41 ± 0.022 | 0.15 ± 0.008 | 2.61 ± 0.025 | 0.2 ± 0.057 | 7.03 ± 0.028 |
| 14093 | 11.47 ± 0.004 | 1.39 ± 0.105 | 7.11 ± 0.03 | 0.11 ± 0.003 | 2.56 ± 0.035 | 0.07 ± 0.062 | 6.68 ± 0.055 |
| 9500 | 11.75 ± 0.033 | 1.35 ± 0.136 | 7.24 ± 0.006 | 0.15 ± 0.024 | 2.49 ± 0.039 | 0.18 ± 0.017 | 7.21 ± 0.116 |
| 9501 | 11.58 ± 0.014 | 1.40 ± 0.130 | 6.76 ± 0.013 | 0.11 ± 0.004 | 2.05 ± 0.012 | 0.39 ± 0.007 | 7.48 ± 0.128 |
| 9507 | 11.74 ± 0.019 | 1.48 ± 0.160 | 7.25 ± 0.017 | 0.16 ± 0.011 | 2.54 ± 0.016 | 0.18 ± 0.059 | 6.97 ± 0.09 |
| 9508 | 11.96 ± 0.028 | 1.67 ± 0.073 | 7.5 ± 0.039 | 0.13 ± 0.002 | 2.67 ± 0.016 | 0.12 ± 0.057 | 7.19 ± 0.046 |
| 9530 | 11.97 ± 0.004 | 1.24 ± 0.055 | 7.97 ± 0.022 | 0.10 ± 0.018 | 2.83 ± 0.055 | 0.33 ± 0.022 | 7.75 ± 0.033 |
| CM | 8.14 ± 0.012 | 39.51 ± 0.014 | 7.48 ± 0.023 | 0.14 ± 0.002 | 2.47 ± 0.047 | 0 | 5.88 ± 0.043 |
| ITEM | TPC (µgGAE/mL) | TPC % Reduction | TEAC (μmolTE/mL) | TEAC % Reduction |
|---|---|---|---|---|
| 6993 | 600.81 ± 1.10 dc | −32% | 4.49 ± 0.06 cd | −21% |
| 6920 | 701.70 ± 0.60 b | −20% | 4.56 ± 0.16 cd | −19% |
| 8766 | 611.59 ± 37.60 cde | −30% | 4.38 ± 0.02 cd | −22% |
| 14093 | 630.71 ± 0.76 bcde | −28% | 4.44 ± 0.07 cd | −21% |
| 9500 | 699.74 ± 1.41 bc | −20% | 4.62 ± 0.02 bc | −18% |
| 9501 | 551.04 ± 20.00 ef | −37% | 4.22 ± 0.14 d | −25% |
| 9507 | 712.13 ± 2.49 b | −19% | 4.51 ± 0.02 cd | −20% |
| 9508 | 801.64 ± 53.60 a | −9% | 4.97 ± 0.20 b | −12% |
| 9530 | 639.39 ± 26.60 bcd | −27% | 4.43 ± 0.04 cd | −22% |
| CM | 502.04 ± 0.13 f | −43% | 3.12 ± 0.02 f | −44% |
| Must | 876.57 ± 5.15 a | // | 5.66 ± 0.04 a | // |
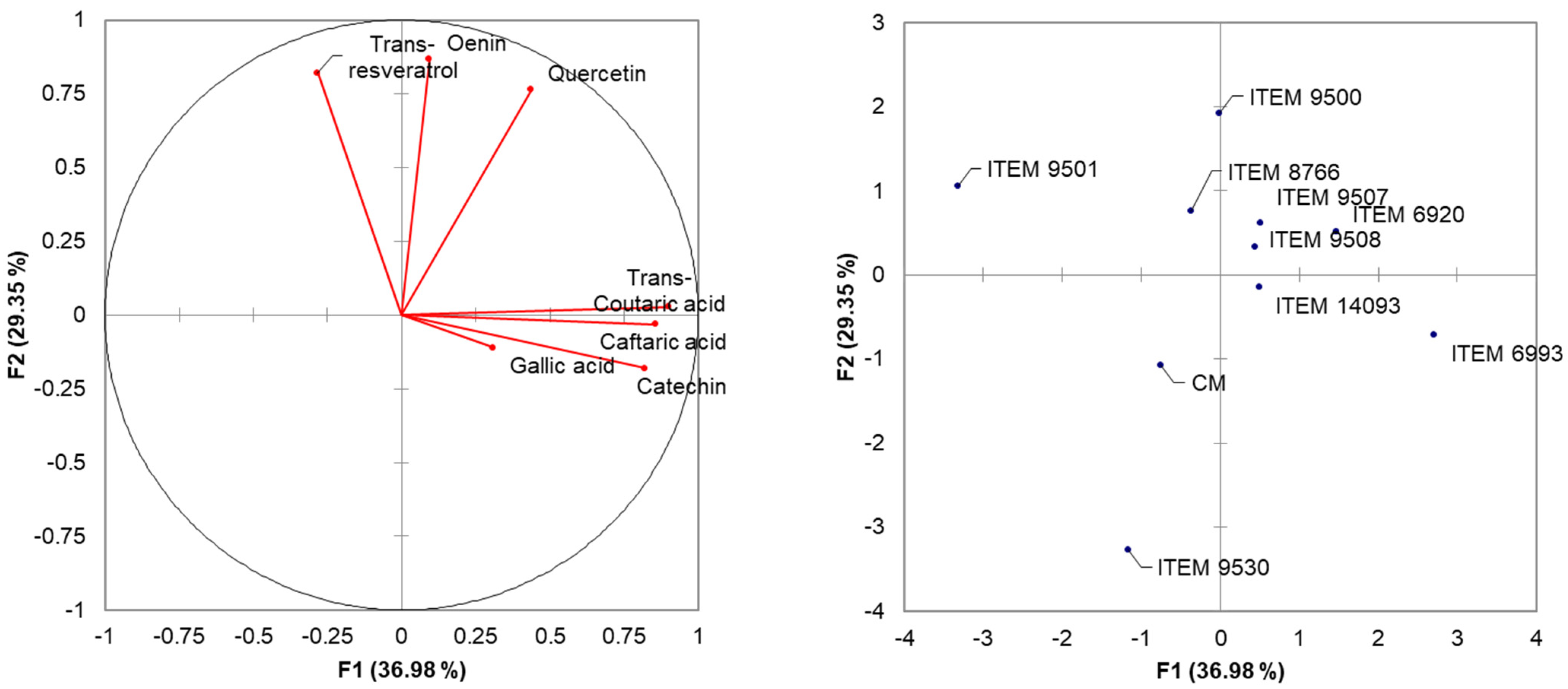
| Strain | Quercetin | Gallic Acid | Catechin | Oenin | Trans-Resveratrol | Trans-Coutaric Acid | Caftaric Acid |
| mg/L | |||||||
| 6993 | 2.857 ± 0.091 b | 32.872 ± 0.486 b | 58.207 ± 2.593 b | 115.251 ± 1.096 b | 1.412 ± 0.118 a | 14.097 ± 0.113 b | 52.723 ± 0.900 b |
| 6920 | 2.433 ± 0.152 b | 30.381 ± 0.549 a | 49.160 ± 4.002 a | 120.966 ± 0.643 a | 2.766 ± 0.097 b | 16.039 ± 0.214 b | 56.158 ± 0.751 b |
| 8766 | 2.369 ± 0.391 b | 25.187 ± 0.590 b | 44.896 ± 0.375 a | 134.179 ± 1.729 b | 1.710 ± 0.069 a | 9.447 ± 0.157 b | 43.842 ± 0.614 b |
| 14093 | 2.456 ± 0.295 b | 30.530 ± 0.344 a | 50.545 ± 3.924 a | 121.750 ± 0.924 a | 1.721 ± 0.016 a | 8.806 ± 0.059 b | 42.246 ± 0.198 a |
| 9500 | 3.375 ± 0.441 b | 27.015 ± 0.427 b | 48.366 ± 0.335 a | 131.042 ± 2.551 b | 3.085 ± 0.067 b | 8.341 ± 0.052 b | 42.143 ± 0.332 a |
| 9501 | 2.132 ± 0.391 b | 29.516 ± 0.673 a | 42.259 ± 3.506 a | 121.123 ± 3.725 a | 3.528 ± 0.088 b | 0.563 ± 0.000 b | 3.130 ± 0.113 b |
| 9507 | 2.184 ± 0.115 b | 24.349 ± 0.179 b | 44.707 ± 0.086 a | 127.983 ± 1.007 a | 2.139 ± 0.446 b | 14.093 ± 0.046 b | 60.821 ± 1.713 b |
| 9508 | 2.902 ± 0.189 b | 26.484 ± 0.875 b | 47.336 ± 0.815 a | 125.542 ± 3.056 a | 1.442 ± 0.028 a | 8.730 ± 0.138 b | 54.666 ± 1.254 b |
| 9530 | 0.671 ± 0.101 a | 26.277 ± 0.834 b | 46.072 ± 0.704 a | 92.511 ± 0.632 b | 0.515 ± 0.007 b | 7.157 ± 0.154 a | 37.062 ± 0.796 b |
| CM | 0.677 ± 0.059 a | 29.559 ± 0.009 a | 47.319 ± 3.573 a | 124.157 ± 0.709 a | 1.565 ± 0.079 a | 6.832 ± 0.017 a | 40.754 ± 0.010 a |
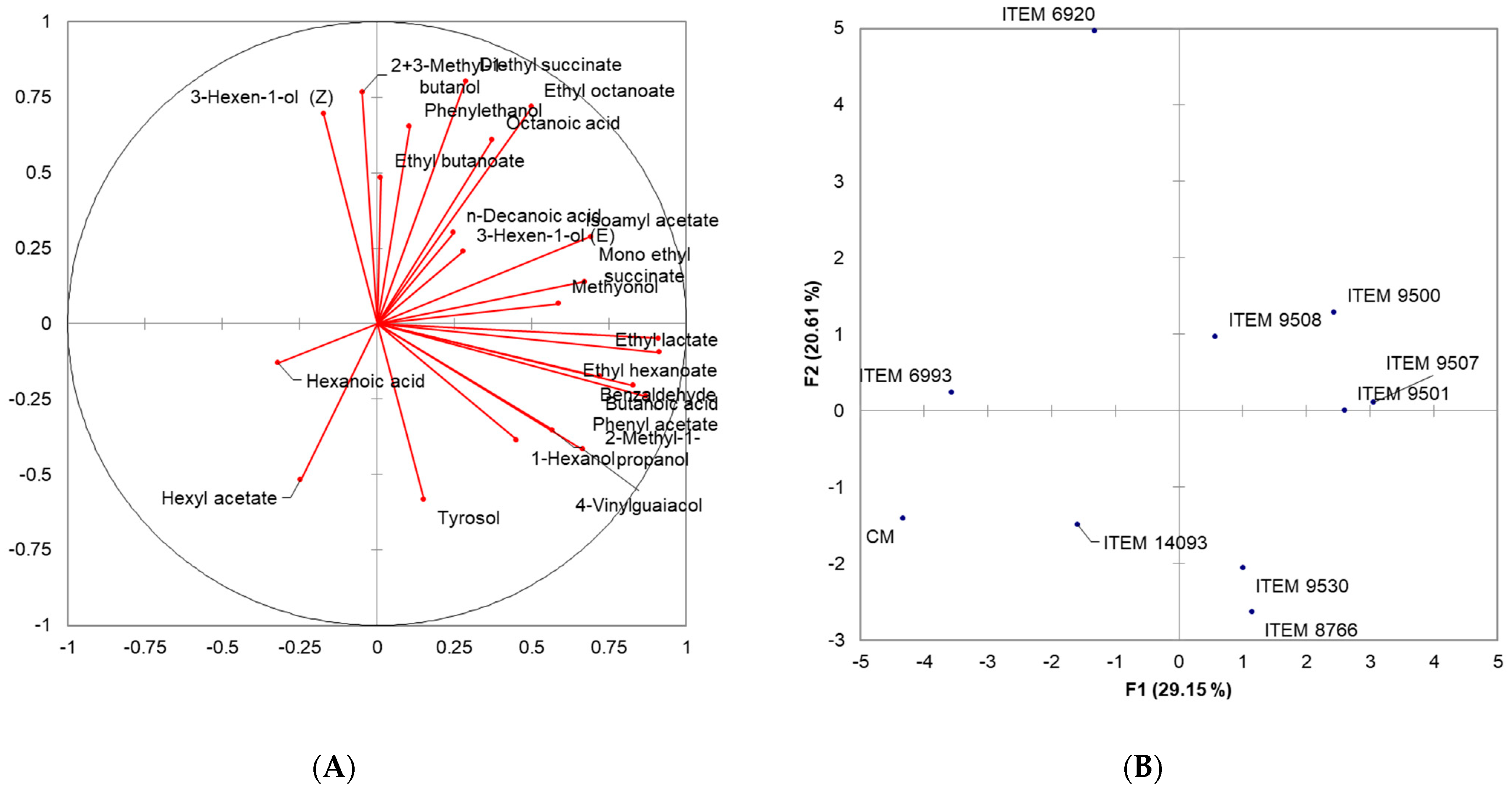
| Volatiles (mg/L) | ITEM 6993 | ±SD | ITEM 6920 | ±SD | ITEM 8766 | ±SD | ITEM 14093 | ±SD | ITEM 9500 | ±SD | ITEM 9501 | ±SD | ITEM 9507 | ±SD | ITEM 9508 | ±SD | ITEM 9530 | ±SD | CM | ±SD | Statistical Significance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esters | |||||||||||||||||||||
| Ethyl butanoate | 0.110 | 0.050 | 0.715 | 0.210 | 0.142 | 0.060 | 0.150 | 0.06 | 0.760 | 0.180 | 0.450 | 0.170 | 0.650 | 0.170 | 0.611 | 0.017 | 0.204 | 0.042 | 0.940 | 0.140 | *** |
| Isoamyl acetate | 0.722 | 0.210 | 1.442 | 0.620 | 1.197 | 0.340 | 0.170 | 0.05 | 1.820 | 0.640 | 1.080 | 0.023 | 2.050 | 0.820 | 0.610 | 0.032 | 1.450 | 0.260 | 0.514 | 0.095 | * |
| Ethyl hexanoate | 0.149 | 0.060 | 0.158 | 0.050 | 0.492 | 0.080 | 0.127 | 0.04 | 0.514 | 0.120 | 0.430 | 0.110 | 0.610 | 0.210 | 0.450 | 0.120 | 0.340 | 0.070 | 0.110 | 0.040 | ** |
| Hexyl acetate | nd | nd | 0.093 | 0.014 | 0.076 | 0.012 | 0.095 | 0.014 | 0.056 | 0.014 | 0.066 | 0.014 | 0.053 | 0.011 | 0.087 | 0.015 | 0.250 | 0.074 | *** | ||
| Ethyl lactate | nd | 0.092 | 0.014 | 0.235 | 0.092 | 0.217 | 0.051 | 0.460 | 0.080 | 0.560 | 0.080 | 0.411 | 0.130 | 0.405 | 0.210 | 0.326 | 0.066 | 0.042 | 0.011 | ** | |
| Ethyl octanoate | 0.079 | 0.020 | 0.570 | 0.070 | 0.214 | 0.014 | 0.156 | 0.07 | 0.440 | 0.130 | 0.380 | 0.110 | 0.278 | 0.080 | 0.316 | 0.080 | 0.320 | 0.080 | 0.147 | 0.040 | ** |
| Diethyl succinate | 0.123 | 0.060 | 0.750 | 0.130 | 0.341 | 0.032 | 0.188 | 0.012 | 0.470 | 0.170 | 0.270 | 0.070 | 0.310 | 0.070 | 0.310 | 0.040 | 0.167 | 0.030 | 0.122 | 0.040 | *** |
| Phenyl acetate | 0.353 | 0.140 | 0.207 | 0.080 | 1.269 | 0.510 | 1.160 | 0.33 | 1.650 | 0.910 | 1.770 | 0.640 | 2.110 | 0.940 | 0.850 | 0.170 | 0.950 | 0.210 | 0.281 | 0.104 | * |
| Mono ethyl succinate | 0.654 | 0.210 | 1.096 | 0.340 | 1.390 | 0.660 | 0.860 | 0.24 | 0.94 | 0.18 | 1.04 | 0.15 | 0.930 | 0.360 | 0.910 | 0.310 | 0.870 | 0.140 | 0.142 | 0.080 | ns |
| Total Esters | 2.19 | 5.03 | 5.37 | 3.10 | 7.15 | 6.04 | 7.42 | 4.52 | 4.71 | 2.55 | |||||||||||
| Alcohols | |||||||||||||||||||||
| 2-Methyl-1-propanol | 0.20 | 0.06 | 0.76 | 0.12 | 2.54 | 0.57 | 0.95 | 0.2 | 1.25 | 0.04 | 0.92 | 0.17 | 1.62 | 0.35 | 0.47 | 0.06 | 0.88 | 0.20 | 0.55 | 0.14 | *** |
| 3-Methyl-1-butanol | 27.15 | 2.35 | 28.72 | 5.04 | 16.12 | 4.05 | 18.93 | 5.1 | 24.76 | 5.11 | 16.90 | 4.32 | 21.88 | 4.15 | 31.16 | 4.28 | 11.87 | 3.17 | 14.22 | 2.07 | ** |
| 1-Hexanol | 1.06 | 0.04 | 0.54 | 0.08 | 0.82 | 0.12 | 0.95 | 0.18 | 1.37 | 0.34 | 1.78 | 0.65 | 1.96 | 0.62 | 1.26 | 0.33 | 2.87 | 0.64 | 1.25 | 0.04 | ** |
| 3-Hexen-1-ol (E) | 0.17 | 0.02 | 0.33 | 0.03 | 0.18 | 0.04 | 0.26 | 0.08 | 0.27 | 0.07 | 0.44 | 0.10 | 0.22 | 0.07 | 0.31 | 0.01 | 0.35 | 0.08 | 0.28 | 0.11 | ns |
| 3-Hexen-1-ol (Z) | 0.17 | 0.05 | 0.55 | 0.08 | 0.19 | 0.06 | 0.25 | 0.06 | 0.18 | 0.06 | 0.34 | 0.07 | 0.21 | 0.04 | 0.17 | 0.05 | 0.12 | 0.04 | 0.27 | 0.15 | ** |
| Methyonol | 0.29 | 0.07 | 0.36 | 0.10 | 0.60 | 0.17 | 0.17 | 0.03 | 0.55 | 0.10 | 0.37 | 0.08 | 0.35 | 0.03 | 0.22 | 0.04 | 0.28 | 0.06 | 0.150 | 0.04 | ** |
| Phenylethanol | 19.23 | 3.520 | 18.607 | 4.070 | 11.156 | 3.070 | 9.450 | 1.51 | 19.400 | 3.610 | 8.550 | 2.170 | 20.140 | 5.140 | 17.980 | 3.910 | 10.500 | 2.180 | 10.240 | 1.82 | * |
| Total Alcohols | 48.27 | 49.87 | 31.61 | 30.96 | 47.78 | 29.30 | 46.38 | 51.57 | 26.86 | 26.96 | |||||||||||
| Aldehydes | |||||||||||||||||||||
| Benzaldehyde | 0.10 | 0.0300 | 0.330 | 0.0700 | 0.420 | 0.1100 | 0.92 | 0.24 | 0.56 | 0.14 | 1.10 | 0.03 | 1.15 | 0.07 | 0.85 | 0.18 | 0.870 | 0.1700 | 0.25 | 0.06 | *** |
| Volatiles phenol | |||||||||||||||||||||
| 4-Vinylguaiacol | 1.480 | 0.550 | nd | 4.45 | 0.95 | 1.76 | 0.61 | 2.55 | 0.61 | 2.87 | 0.51 | 3.98 | 0.72 | 5.38 | 0.94 | 4.10 | 0.92 | 0.65 | 0.17 | *** | |
| Tyrosol | 0.995 | 0.370 | nd | 5.53 | 1.04 | nd | 0.36 | 0.08 | 0.77 | 0.18 | 0.95 | 0.18 | nd | 1.76 | 0.34 | 1.040 | 0.320 | *** | |||
| Total Volatile Phenols | 2.47 | 9.97 | 1.76 | 2.91 | 3.64 | 4.93 | 5.38 | 5.86 | 1.69 | ||||||||||||
| Volatile acids | |||||||||||||||||||||
| Butanoic acid | nd | nd | 0.26 | 0.06 | 0.41 | 0.07 | 0.55 | 0.11 | 0.63 | 0.25 | 0.41 | 0.07 | 0.35 | 0.08 | 0.36 | 0.07 | nd | *** | |||
| Hexanoic acid | 1.63 | 0.27 | 0.83 | 0.18 | 1.18 | 0.04 | 0.87 | 0.18 | 0.77 | 0.21 | 0.93 | 0.18 | 0.88 | 0.13 | 1.34 | 0.33 | 0.95 | 0.16 | 0.880 | 0.1700 | * |
| Octanoic acid | 1.74 | 0.14 | 1.71 | 0.42 | 0.63 | 0.17 | 0.94 | 0.24 | 1.45 | 0.43 | 1.76 | 0.34 | 1.88 | 1.04 | 1.33 | 0.17 | 1.36 | 0.35 | 0.620 | 0.2300 | ns |
| n-Decanoic acid | 0.43 | 0.08 | 0.52 | 0.17 | 0.34 | 0.05 | nd | 0.56 | 0.07 | 0.87 | 0.21 | 0.55 | 0.14 | 1.62 | 0.54 | 0.41 | 0.11 | 0.550 | 0.1600 | ** | |
| Total Volatile Acids | 3.79 | 3.05 | 2.41 | 2.22 | 3.33 | 4.19 | 3.72 | 4.64 | 3.08 | 2.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, G.; Taurino, M.; Gerardi, C.; Tufariello, M.; Lenucci, M.; Grieco, F. Yeast Starter Culture Identification to Produce of Red Wines with Enhanced Antioxidant Content. Foods 2024, 13, 312. https://doi.org/10.3390/foods13020312
Romano G, Taurino M, Gerardi C, Tufariello M, Lenucci M, Grieco F. Yeast Starter Culture Identification to Produce of Red Wines with Enhanced Antioxidant Content. Foods. 2024; 13(2):312. https://doi.org/10.3390/foods13020312
Chicago/Turabian StyleRomano, Giuseppe, Marco Taurino, Carmela Gerardi, Maria Tufariello, Marcello Lenucci, and Francesco Grieco. 2024. "Yeast Starter Culture Identification to Produce of Red Wines with Enhanced Antioxidant Content" Foods 13, no. 2: 312. https://doi.org/10.3390/foods13020312