Characteristics of Citrate-Esterified Starch and Enzymatically Debranched Starch and Their Effects on Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chickpea Starch
2.3. Preparation of Chickpea RS
2.3.1. Preparation of Enzymatically Debranched Starch (EDS)
2.3.2. Preparation of Citrate-Esterified Starch (CCS)
2.4. Determination of Starch Digestibility
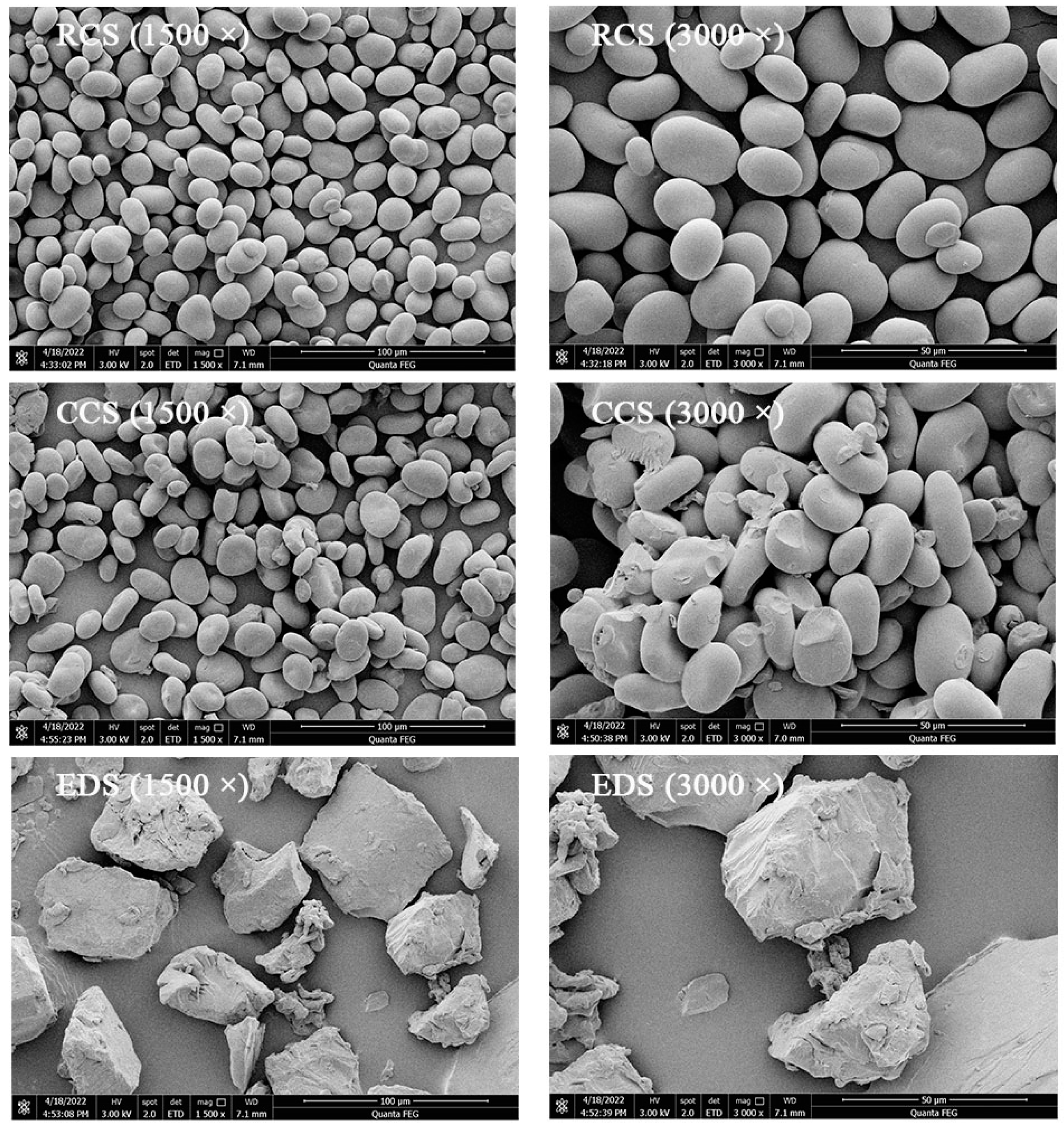
2.5. Scanning Electron Microscopy (SEM)
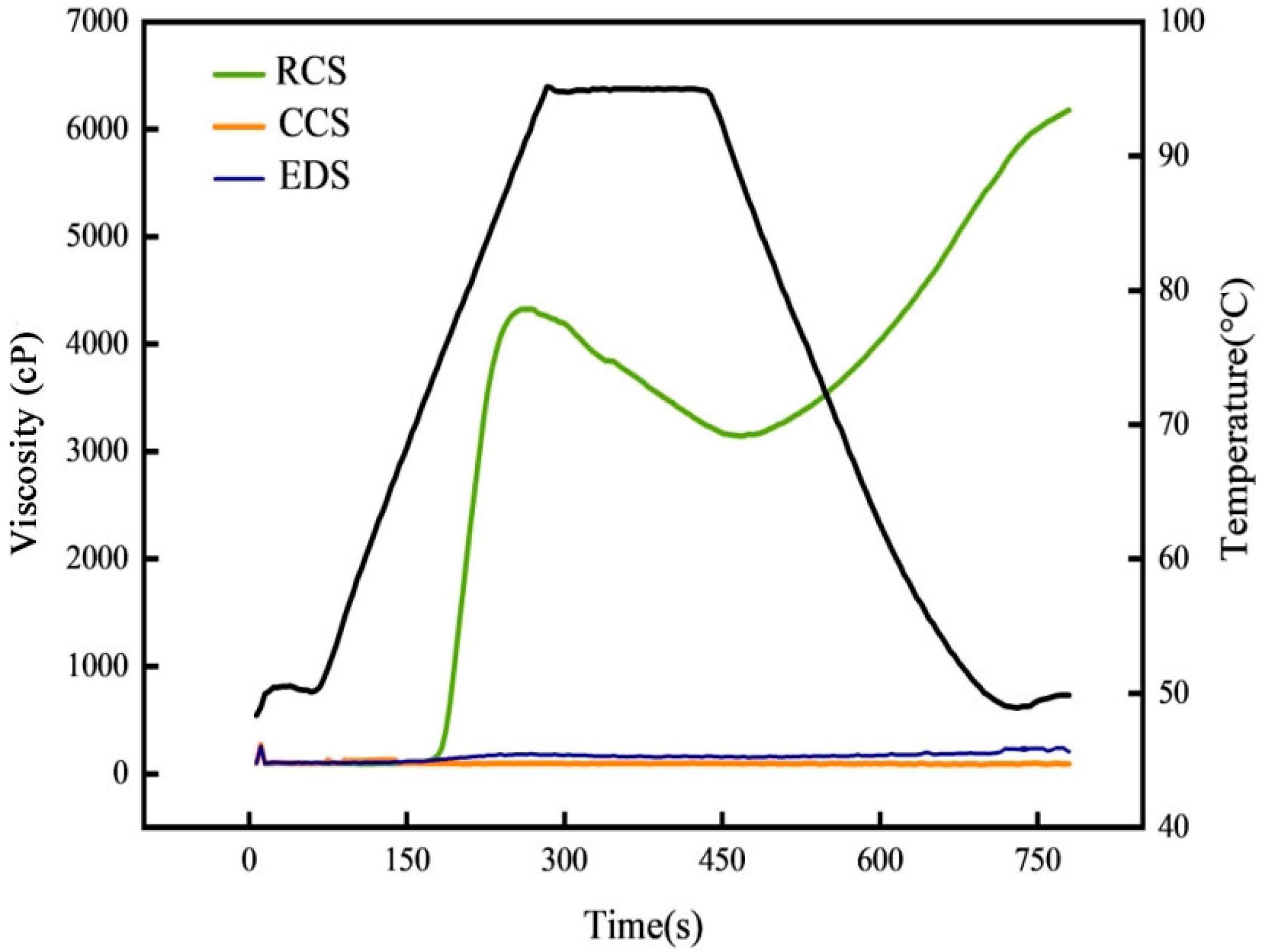
2.6. Viscosity
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
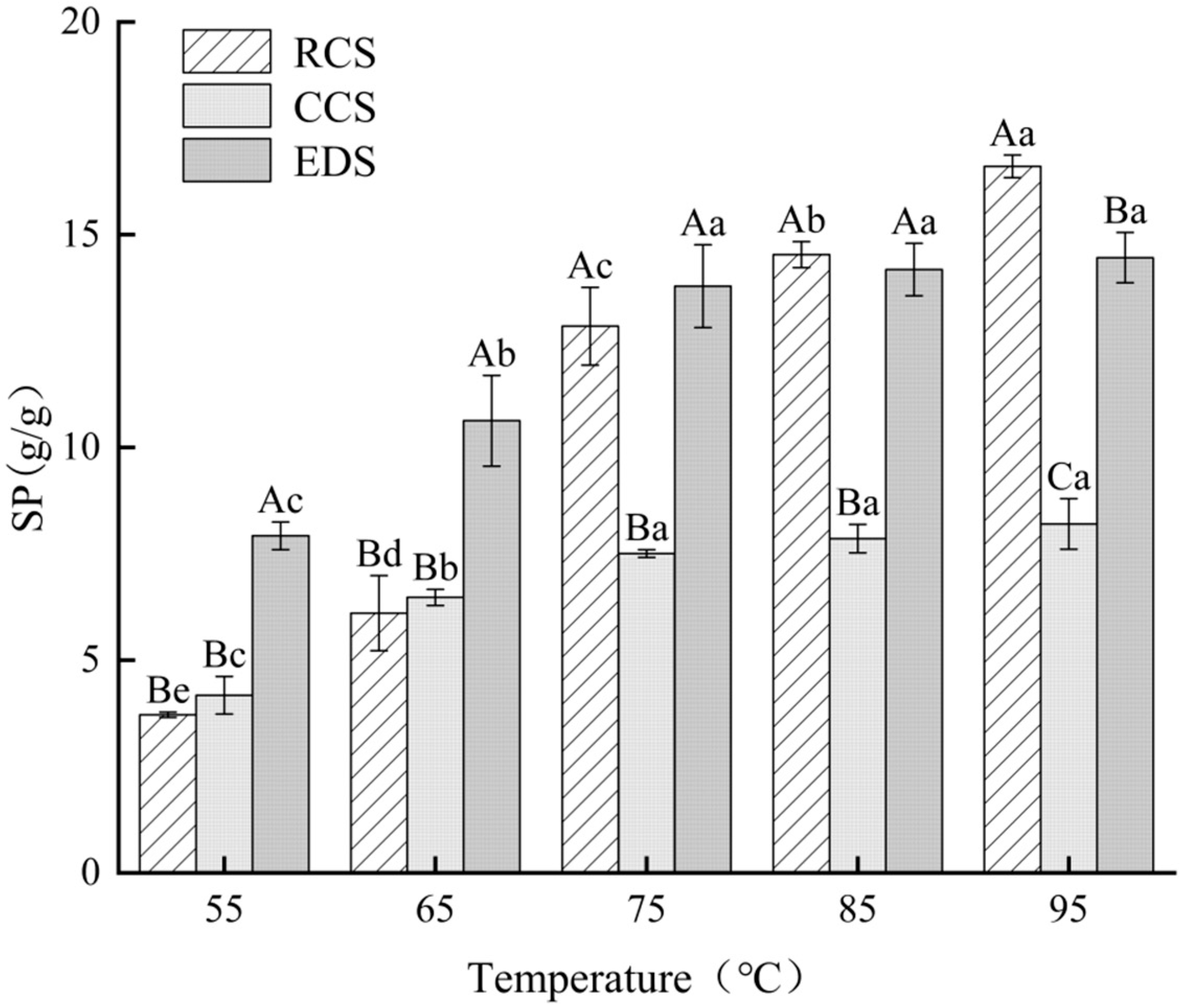
2.8. Expansion Force
2.9. Animals and Treatments
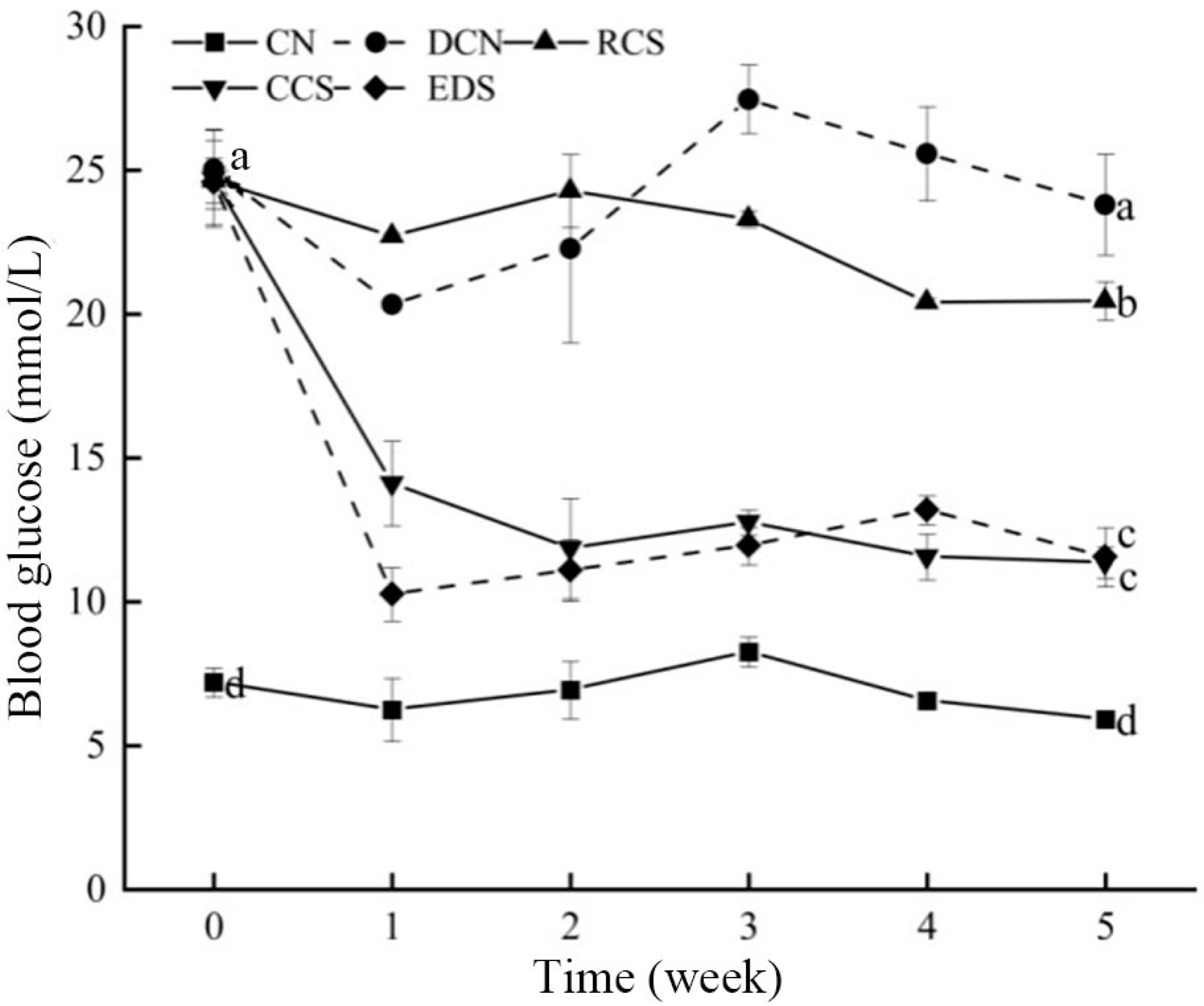
2.10. Analysis of Blood Glucose
2.11. Evaluation of Serum Lipid Metabolism and Inflammatory Factors
2.12. Morphology of Liver Slices
2.13. Analysis of Intestinal Flora
2.14. Statistical Analysis
3. Results and Discussion
3.1. Digestive Properties of Modified Chickpea Starch
3.2. Microstructure of Modified Starch
3.3. Viscosity Properties of Modified Starch
3.4. Short-Range Orderliness of Modified Starch
3.5. Expansion Force Analysis
3.6. Effect of Modified Chickpea Starch on Blood Glucose in Mice
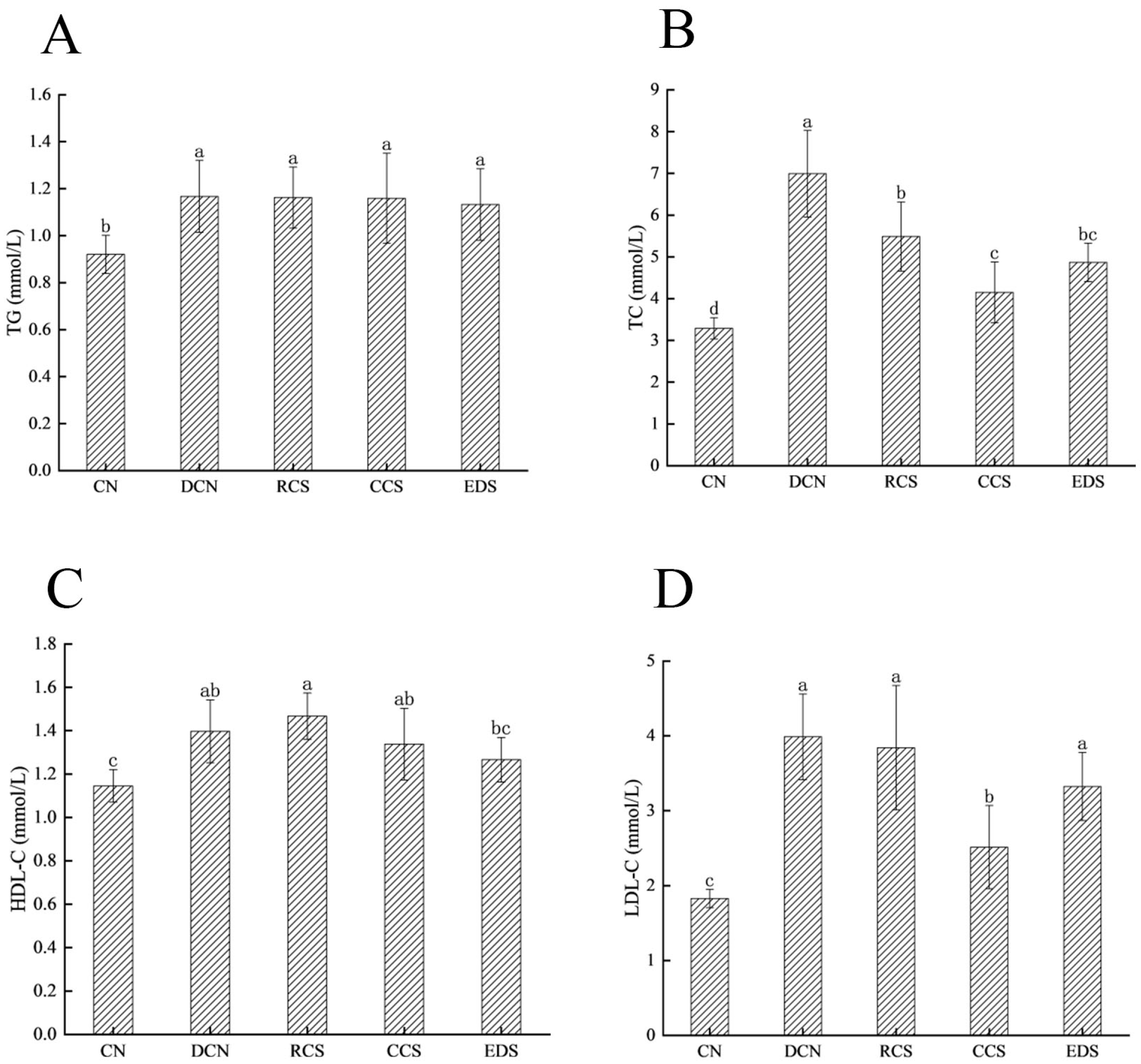
3.7. Effect of Modified Chickpea Starch on Lipid Metabolism in Mice
3.8. Effects of Different Modified Chickpea Starches on Inflammatory Factors in Mice
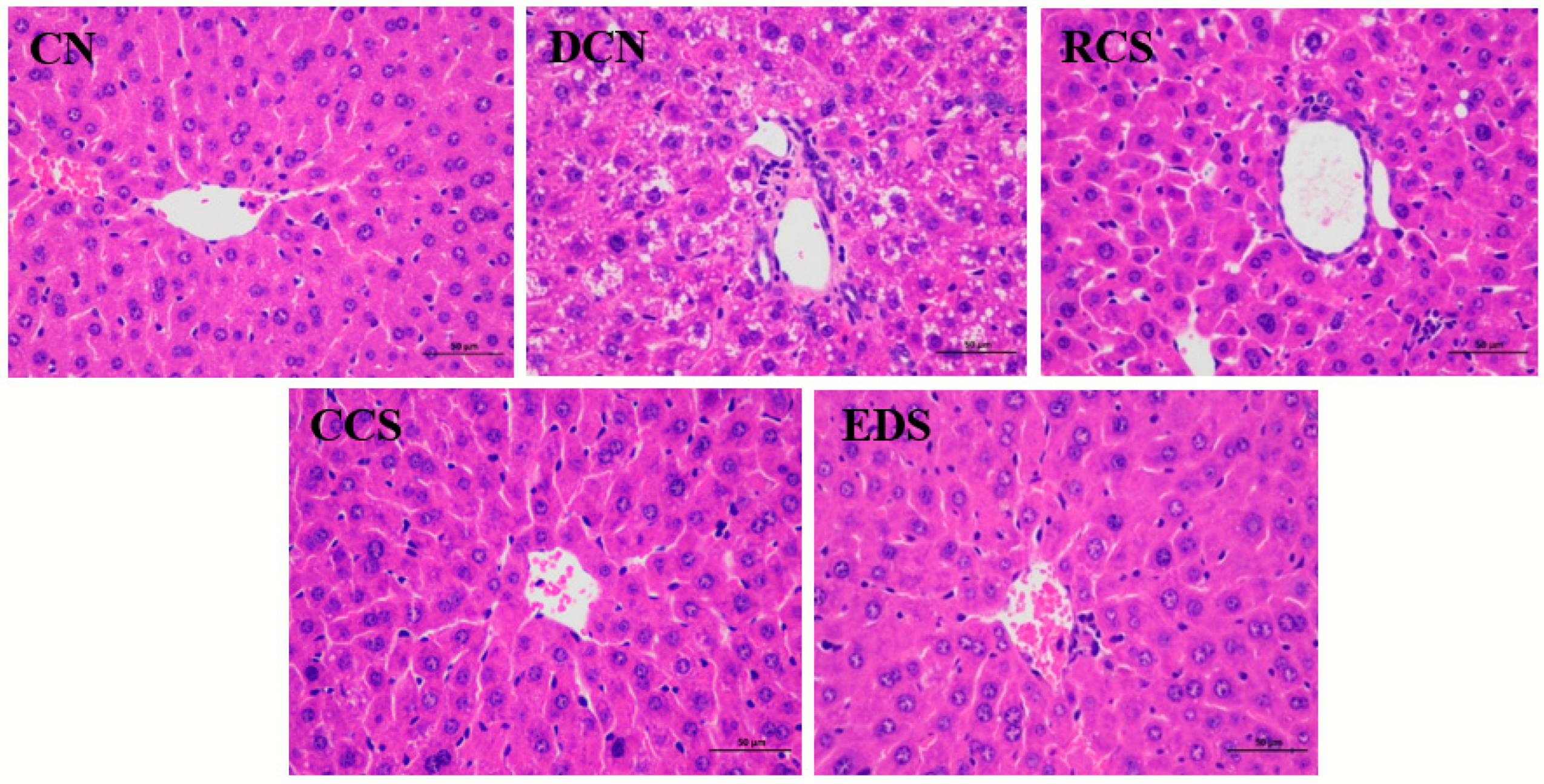
3.9. Effect of Modified Chickpea Starch on Microstructure of Mouse Liver Tissue
3.10. Bioinformatic Analysis of Mouse Intestinal Flora
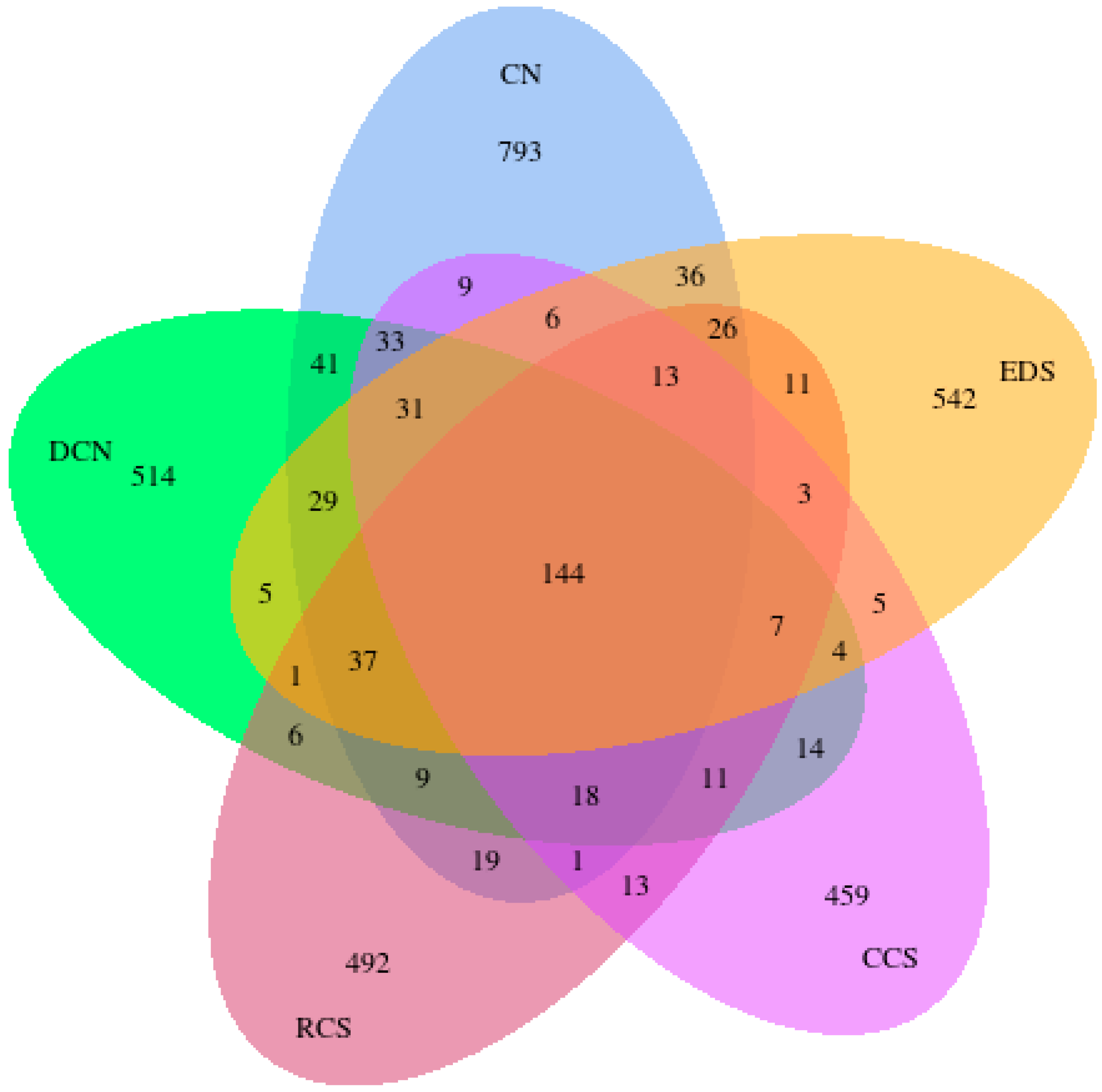
3.10.1. Venn Diagram
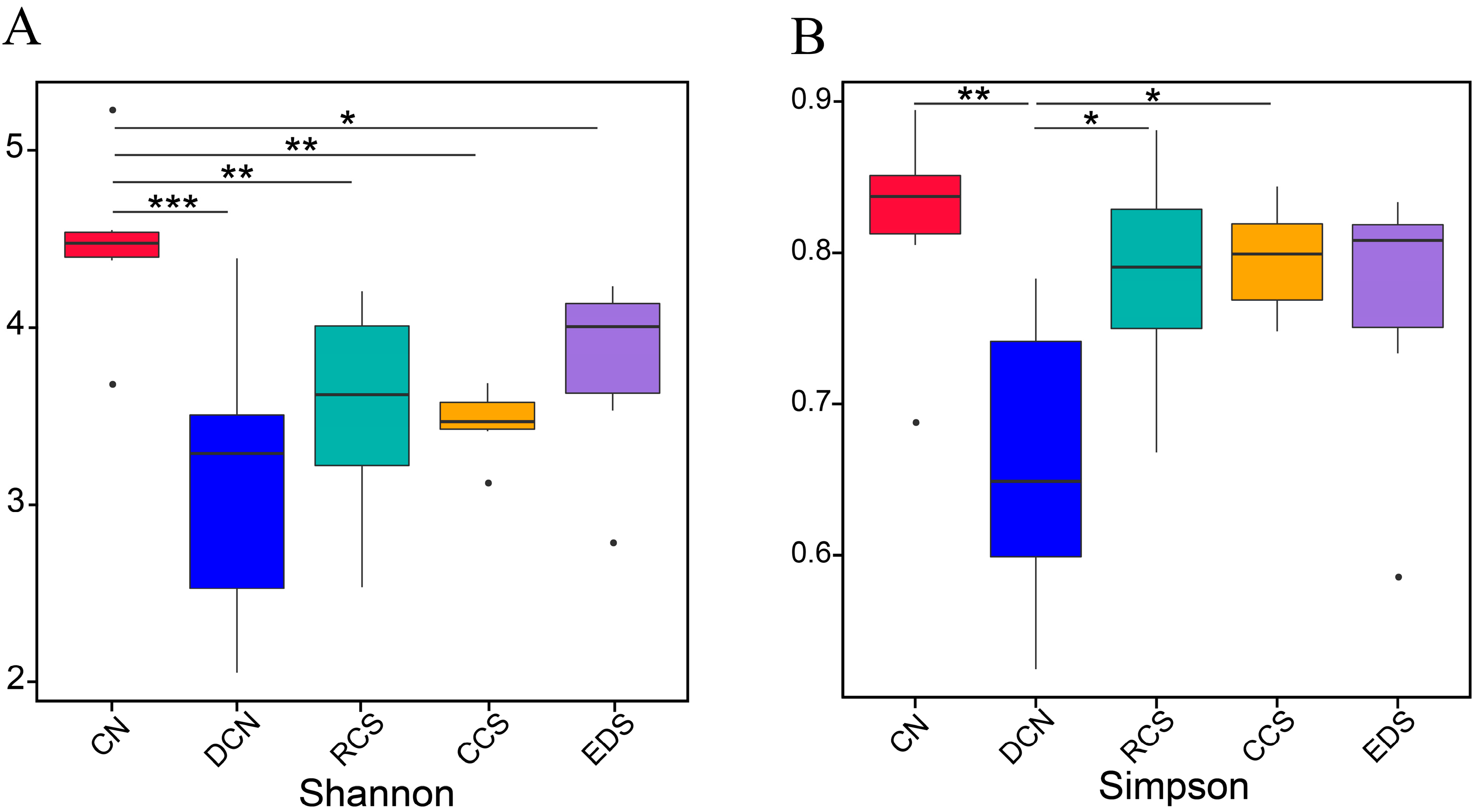
3.10.2. Analysis of Differences between Groups
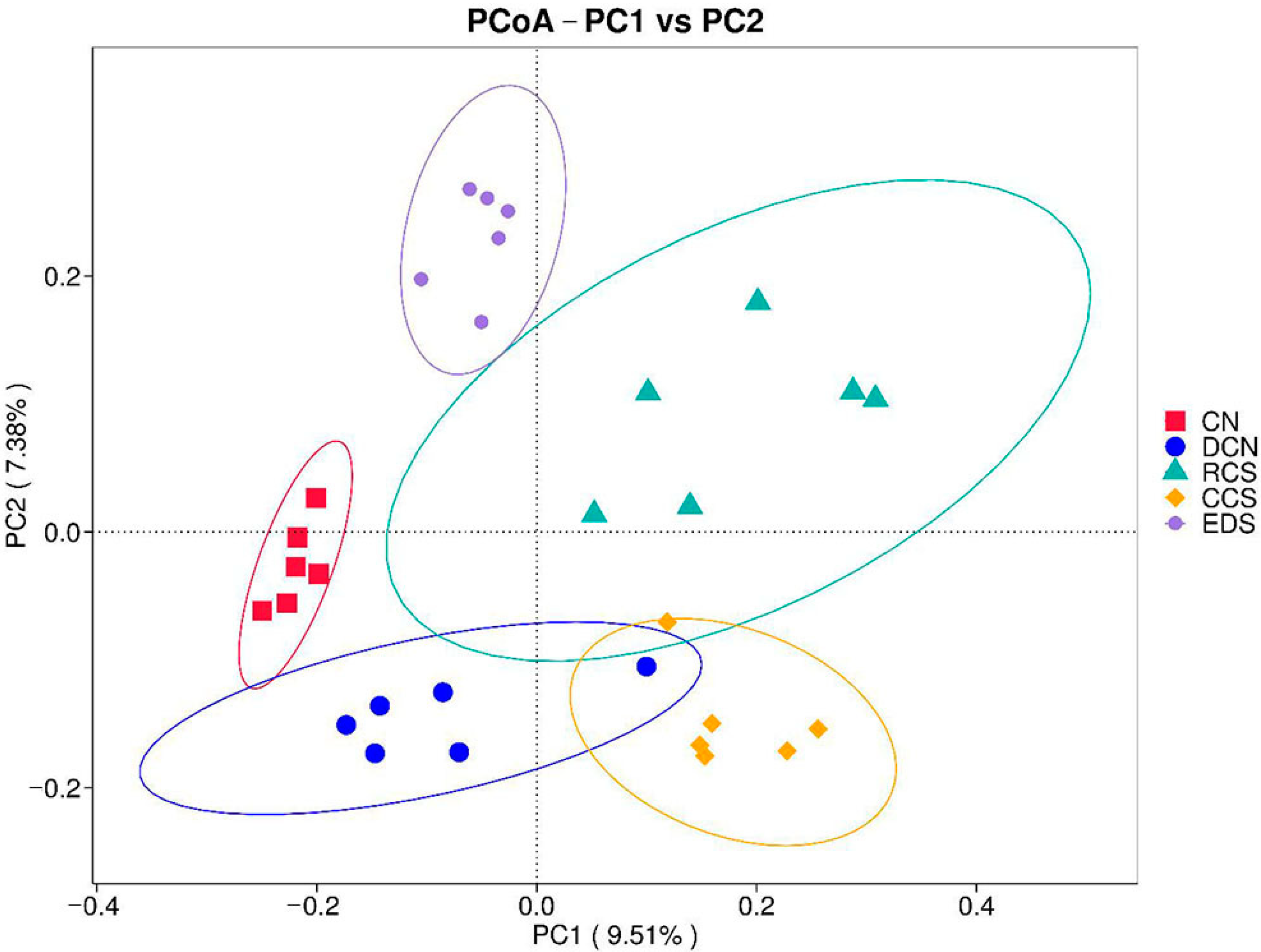
3.10.3. Principal Coordinate Analysis (PCoA)
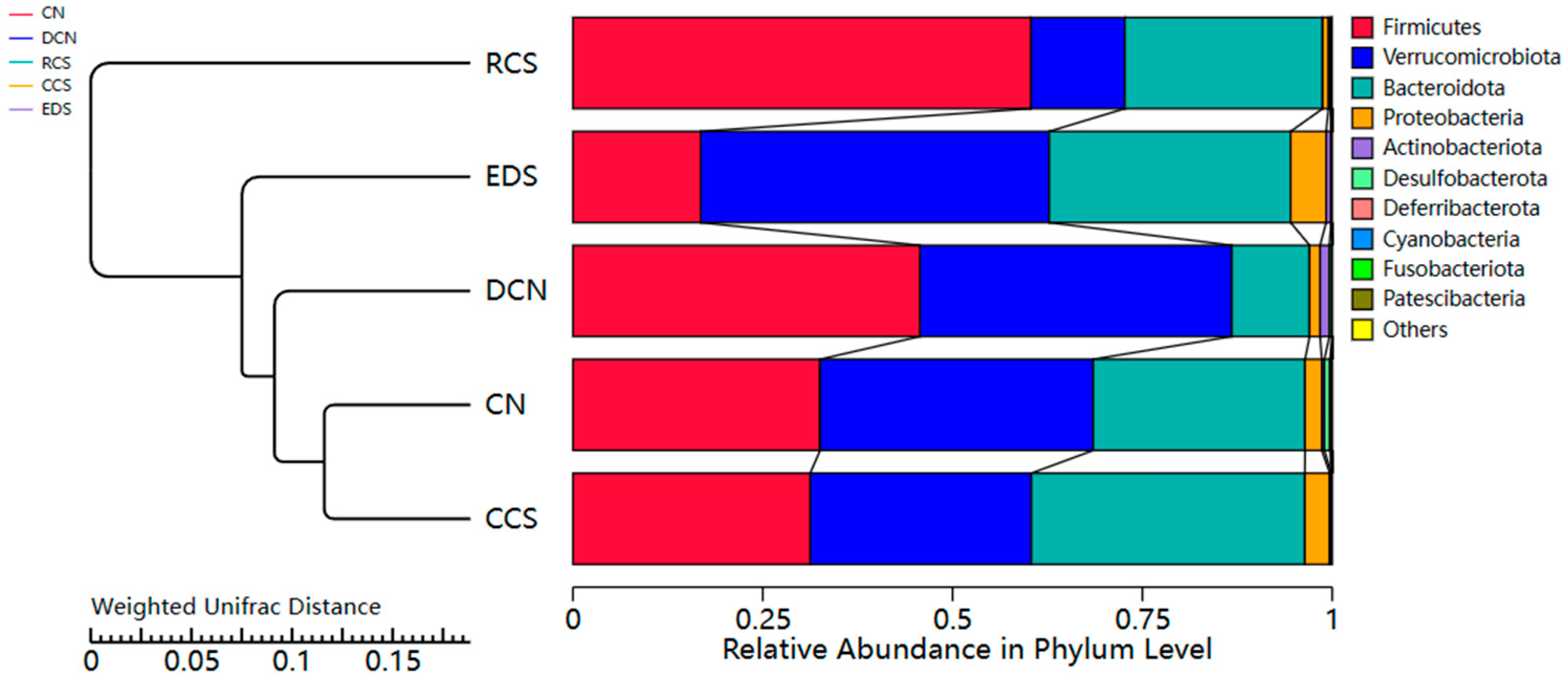
3.10.4. Unweighted Pair-Group Method with Arithmetic Mean Analysis (UPGMA)
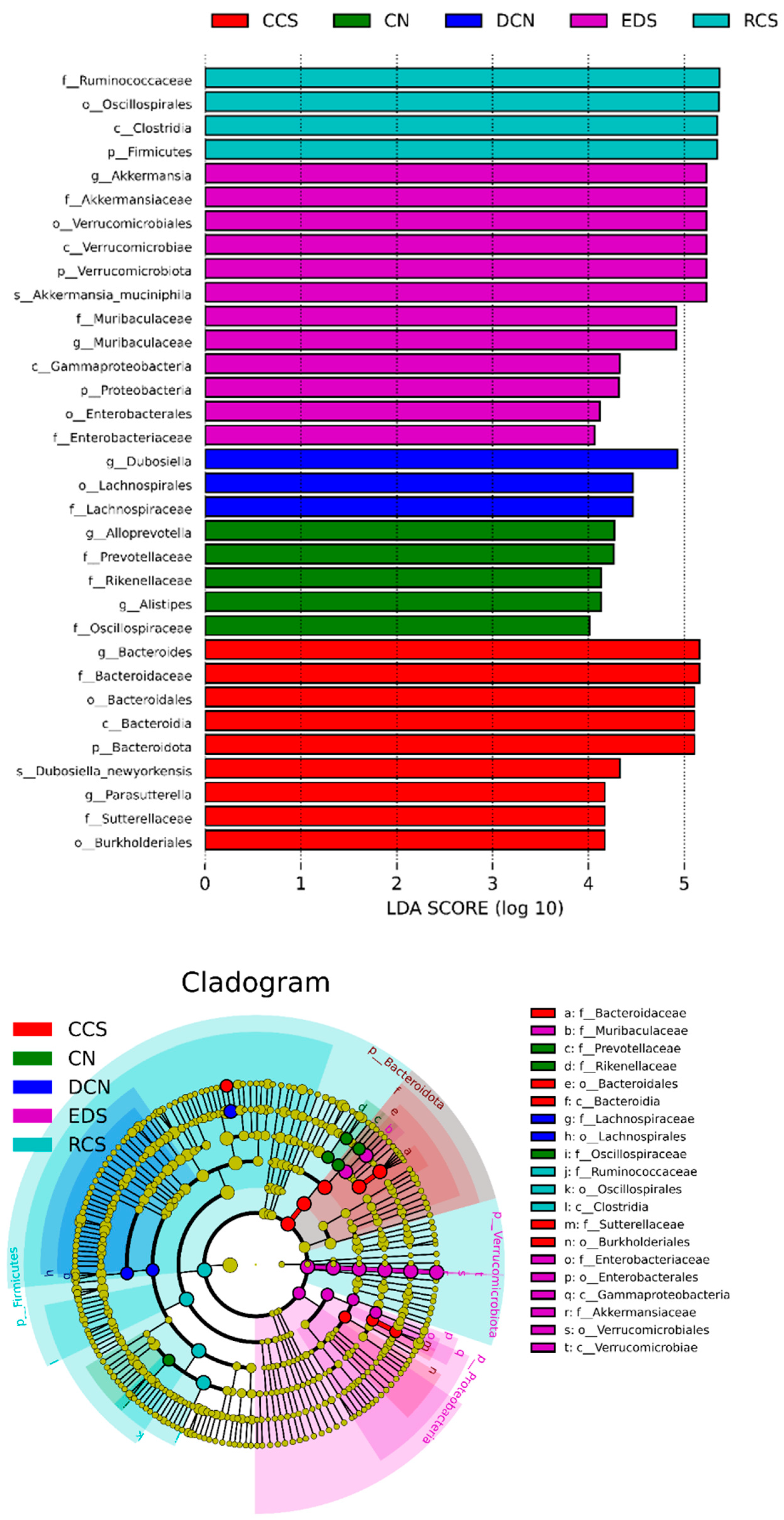
3.10.5. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
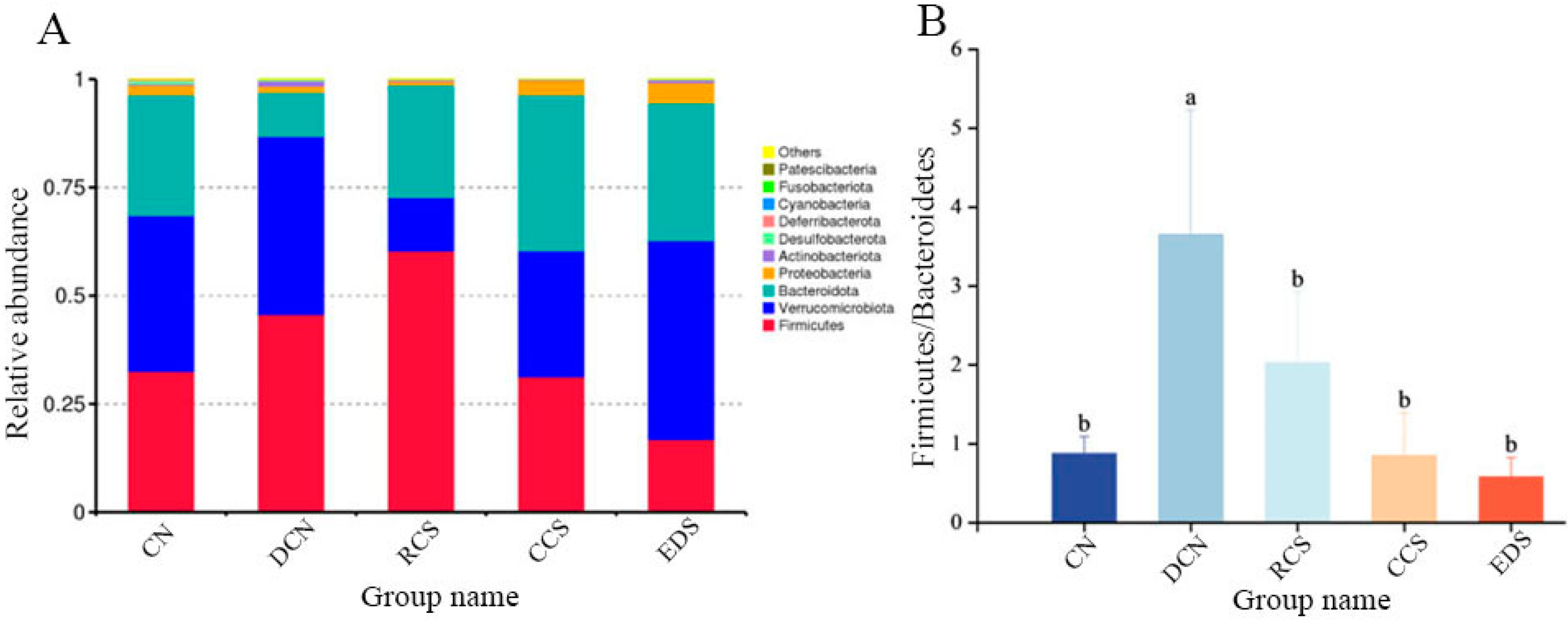
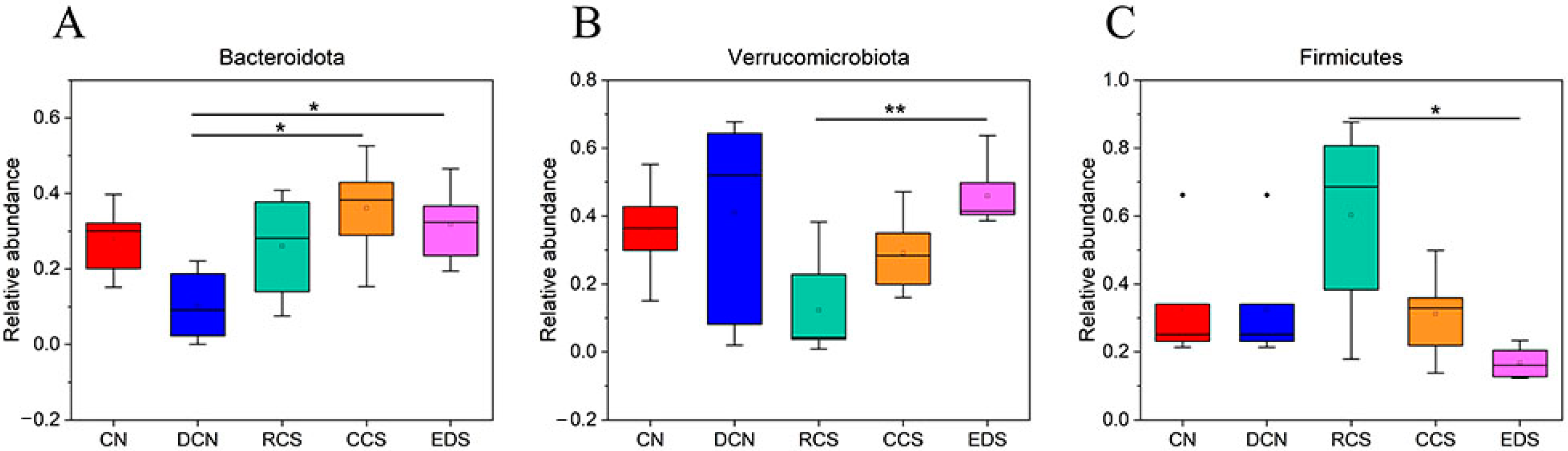
3.10.6. Phylum-Level Species Analysis
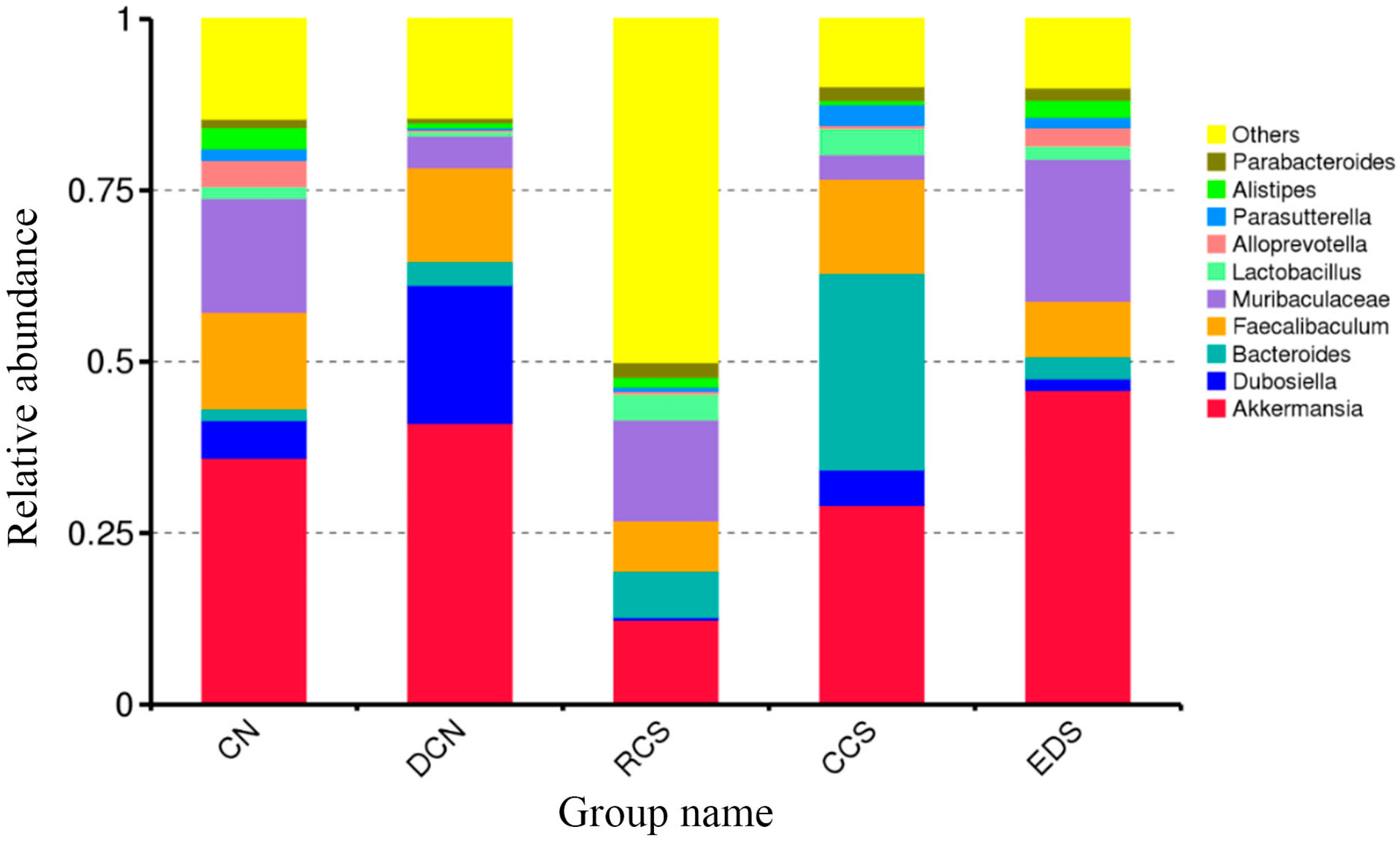
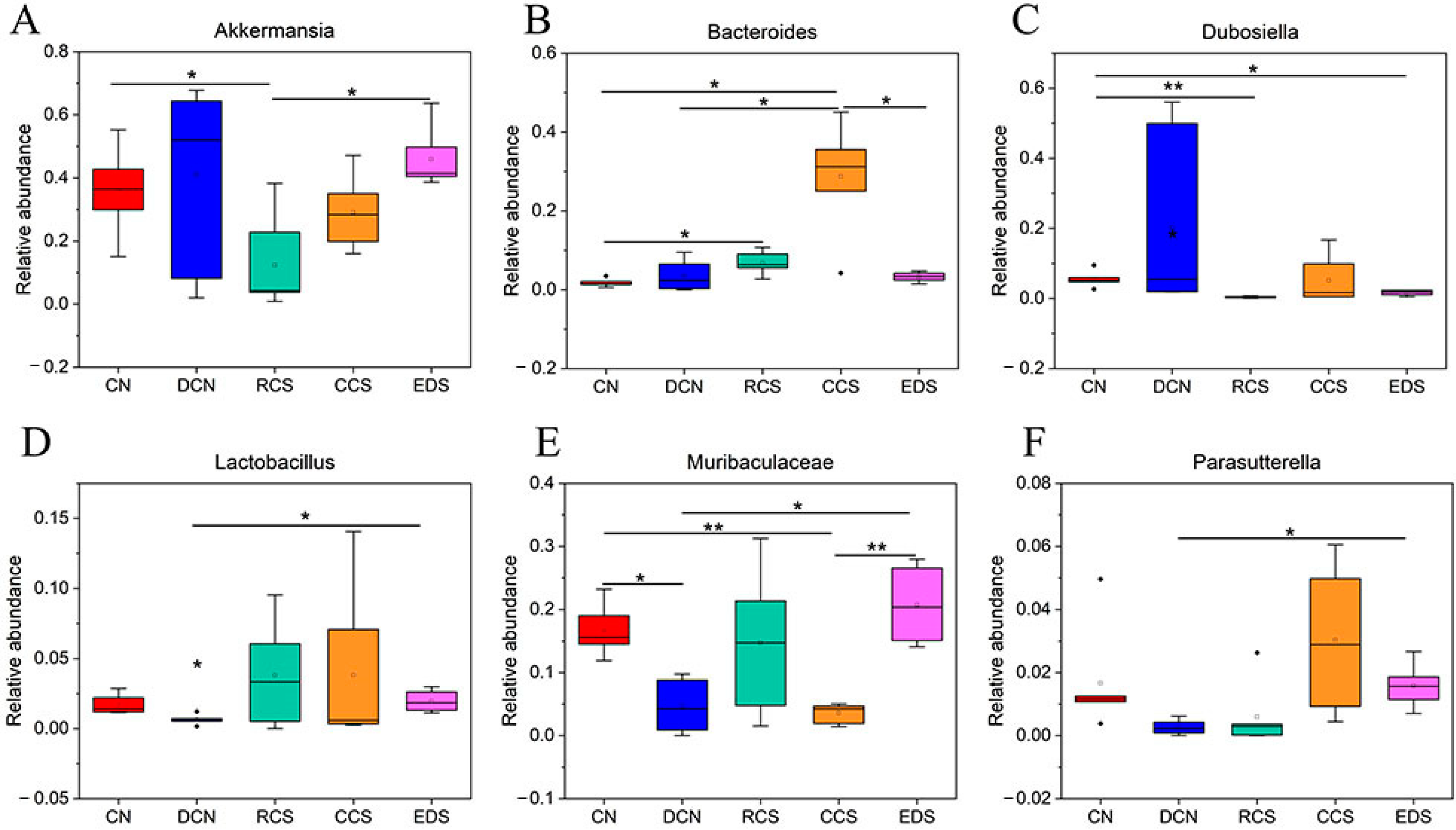
3.10.7. A Species Structure Analysis at the Genus Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ba, J.; Chen, Y.; Liu, D. Fatigue in adults with type 2 diabetes: A systematic review and meta-analysis. West J. Nurs. Res. 2021, 43, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Lv, C.; Zhou, R.; Wang, Y.; Zhou, X.; Qin, S. The Molecular mechanism underlying the therapeutic effect of dihydromyricetin on type 2 diabetes mellitus based on network pharmacology, molecular docking, and transcriptomics. Foods 2024, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, Z.; Yang, F.; Wang, Y.; Pang, X.; Sun, J.; Li, X.; Lu, Y. Broccoli improves lipid metabolism and intestinal flora in mice with Type 2 Diabetes induced by HFD and STZ diet. Foods 2024, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.R.; Boren, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, N.C.D.R.F. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar]
- Singh, R.; Sharma, P.; Varshney, R.K.; Sharma, S.K.; Singh, N.K. Chickpea improvement: Role of wild species and genetic markers. Biotechnol. Genet. Eng. Rev. 2008, 25, 267–314. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Prasad, K. Technological, processing and nutritional aspects of chickpea (Cicer arietinum)—A review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- De Pasquale, I.; Verni, M.; Verardo, V.; Gómez-Caravaca, A.M.; Rizzello, C.G. Nutritional and functional advantages of the use of fermented black chickpea flour for semolina-pasta fortification. Foods 2021, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant starch and its nanoparticles: Recent advances in their green synthesis and application as functional food ingredients and bioactive delivery systems. Trends Food Sci. Technol. 2022, 119, 90–100. [Google Scholar] [CrossRef]
- Jiali, L.; Wu, Z.; Liu, L.; Yang, J.; Wang, L.; Li, Z.; Liu, L. The research advance of resistant starch: Structural characteristics, modification method, immunomodulatory function, and its delivery systems application. Crit. Rev. Food Sci. Nutr. 2023, 6, 1–18. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Shah, A.; Wani, I.A.; Masoodi, A.F. Preparation, health benefits and applications of resistant starch—A review. Starch-Starke 2016, 68, 287–301. [Google Scholar] [CrossRef]
- Li, W.; Yao, S. Extraction process of chickpea resistant starch and its effect on reducing blood lipids. Modern Food 2018, 7, 188–192. [Google Scholar]
- Song, X.; Dong, H.; Zang, Z.; Wu, W.; Zhu, W.; Zhang, H.; Guan, Y. Kudzu resistant starch: An effective regulator of type 2 diabetes mellitus. Oxid. Med. Cell. Longev. 2021, 2021, 4448048. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, F.; Ren, X.; Wang, Y.; Blanchard, C. Resistant starch manipulated hyperglycemia/hyperlipidemia and related genes expression in diabetic rats. Int. J. Biol. Macromol. 2015, 75, 316–321. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S.K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Tacer-Caba, Z.; Nilufer-Erdil, D. Pullulanase treatments to increase resistant starch content of black chickpea (Cicer arietinum L.) starch and the effects on starch properties. Int. J. Biol. Macromol. 2018, 111, 505–513. [Google Scholar] [CrossRef]
- Zhao, M.; Cui, W.; Hu, X.; Ma, Z. Anti-hyperlipidemic and ameliorative effects of chickpea starch and resistant starch in mice with high fat diet induced obesity are associated with their multi-scale structural characteristics. Food Funct. 2022, 13, 5135–5152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, M.; Cao, T.; Xu, W. The Effect of acetylation on the physicochemical properties of chickpea starch. Foods 2023, 12, 2462. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q. Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch-Staerke 2004, 56, 364–370. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- GB/T 24852-2010; Determination of the Pasting Properties of Rice-Rapid Visco Analyzer Method. China National Standardization Administration: Beijing, China, 2010.
- Li, D.; Zhang, S.; Yang, C.; Li, Q.; Wang, S.; Xu, X.; Hao, J.; Li, C. A novel PTP1B inhibitor-phosphate of polymannuronic acid ameliorates insulin resistance by regulating IRS-1/Akt signaling. Int. J. Mol. Sci. 2021, 22, 12693. [Google Scholar] [CrossRef] [PubMed]
- Hugerth, L.W.; Andersson, A.F. Analysing microbial community composition through amplicon sequencing: From sampling to hypothesis testing. Front. Microbiol. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, Q.; Liang, C. Preparation and properties of pea resistant starch by different methods. Food Ferment. Ind. 2011, 37, 119–123. [Google Scholar]
- Navaf, M.; Sunooj, K.V.; Aaliya, B.; Sudheesh, C.; Akhila, P.P.; Sabu, S.; Sasidharan, A.; George, J. Talipot palm (Corypha umbraculifera L.) a nonconventional source of starch: Effect of citric acid on structural, rheological, thermal properties and in vitro digestibility. Int. J. Biol. Macromol. 2021, 182, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, Y.; Yu, S.; Gao, Q. Preparation and properties of RS III from waxy maize starch with pullulanase. Food Hydrocoll. 2013, 33, 19–25. [Google Scholar] [CrossRef]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef]
- Srikaeo, K.; Hao, P.T.; Lerdluksamee, C. Effects of heating temperatures and acid concentrations on physicochemical properties and starch digestibility of citric acid esterified tapioca starches. Starch-Starke 2019, 71, 1800065. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z. Preparation of products rich in resistant starch from maize starch by an enzymatic method. Carbohydr. Polym. 2011, 86, 1610–1614. [Google Scholar] [CrossRef]
- Sudheesh, C.; Sunooj, K.V.; Anjali, K.U.; Aaliya, B.; Navaf, M.; Kumar, S.; Sajeevkumar, V.A.; George, J. Effect of lysine incorporation, annealing and heat moisture treatment alone and in combination on the physico-chemical, retrogradation, rheological properties and in vitro digestibility of kithul (Caryota urens L.) starch. Int. J. Food Sci. Technol. 2020, 55, 2391–2398. [Google Scholar] [CrossRef]
- Shukri, R.; Shi, Y.C. Structure and pasting properties of alkaline-treated phosphorylated cross-linked waxy maize starches. Food Chem. 2017, 214, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Surendra Babu, A.; Parimalavalli, R.; Rudra, S.G. Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches. Int. J. Biol. Macromol. 2015, 80, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.H.M.; Hashim, R.; Sulaiman, N.S.; Mohamed, M.; Sulaiman, O. Citric acid-modified starch as an environmentally friendly binder for wood composite making. Bioresources 2020, 15, 4234–4248. [Google Scholar] [CrossRef]
- Li, M.N.; Xie, Y.; Chen, H.Q.; Zhang, B. Effects of heat-moisture treatment after citric acid esterification on structural properties and digestibility of wheat starch, A- and B-type starch granules. Food Chem. 2019, 272, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, K.Y.; Lee, H.G. Effect of different pH conditions on the in vitro digestibility and physicochemical properties of citric acid-treated potato starch. Int. J. Biol. Macromol. 2018, 107, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.Q.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Surendra Babu, A.; Parimalavalli, R. Effect of pullulanase debranching and storage temperatures on structural characteristics and digestibility of sweet potato starch. J. Saudi Soc. Agric. Sci. 2018, 17, 208–216. [Google Scholar] [CrossRef]
- Tang, J.; Liang, Q.; Ren, X.; Raza, H.; Ma, H. Insights into ultrasound-induced starch-lipid complexes to understand physicochemical and nutritional interventions. Int. J. Biol. Macromol. 2022, 222, 950–960. [Google Scholar] [CrossRef]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Li, Y.; He, S.; Li, C.; Shen, K.; Yang, M.; Tao, W.; Yang, Y.; Shi, L.; Yao, Y. Evidence of association between single-nucleotide polymorphisms in lipid metabolism-related genes and type 2 diabetes mellitus in a Chinese population. Int. J. Med. Sci. 2021, 18, 356–363. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, N.; Zhang, M.; Pan, R.; Dang, Y.; Niu, Y. The association between blood glucose levels and lipids or lipid ratios in type 2 diabetes patients: A cross-sectional study. Front. Endocrinol. 2022, 13, 969080. [Google Scholar] [CrossRef]
- Yuan, H.C.; Meng, Y.; Bai, H.; Shen, D.Q.; Wan, B.C.; Chen, L.Y. Meta-analysis indicates that resistant starch lowers serum total cholesterol and low-density cholesterol. Nutr. Res. 2018, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Díaz-Rubio, M.E.; Dardevet, D.; Martin, J.F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.L.; Mazur, A.; Comte, B. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Pietrani, N.T.; Bosco, A.A.; Campos, F.M.F.; Sandrim, V.C.; Gomes, K.B. IL-6, TNF-α, and IL-10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch. Endocrinol. Metab. 2017, 61, 438–446. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone disease, obesity and the firmicutes/bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, T.; Li, W.; Zhao, Y.; Long, H.; Liu, R.; Sui, W.; Zhang, M. Lactobacillus casei LC89 exerts antidiabetic effects through regulating hepatic glucagon response and gut microbiota in type 2 diabetic mice. Food Funct. 2021, 12, 8288–8299. [Google Scholar] [CrossRef] [PubMed]


| Basic Feed (g/kg) | Diabetes Control Group (g/kg) | Chickpea Starch Group (g/kg) | Citrate-Esterified Starch Group (CCS Group, g/kg) | Enzymatically Debranched Starch Group (EDS Group, g/kg) | |
|---|---|---|---|---|---|
| Chickpea starch powder | 0 | 0 | 234.5 | 0 | 0 |
| Citrate-esterified starch (CCS) | 0 | 0 | 0 | 234.5 | 0 |
| Enzymatically debranched starch (EDS) | 0 | 0 | 0 | 0 | 234.5 |
| Corn starch | 397.5 | 234.5 | 0 | 0 | 0 |
| Casein | 200 | 118 | 118 | 118 | 118 |
| Dextrin | 132 | 77.88 | 77.88 | 77.88 | 77.88 |
| Soybean oil | 70 | 41.3 | 41.3 | 41.3 | 41.3 |
| Sucrose | 100 | 59 | 59 | 59 | 59 |
| Microcrystalline cellulose | 50 | 29.5 | 29.5 | 29.5 | 29.5 |
| Cysteine | 3 | 1.77 | 1.77 | 1.77 | 1.77 |
| TBHQ | 0.014 | 0.083 | 0.083 | 0.083 | 0.083 |
| CMC | 2.5 | 1.48 | 1.48 | 1.48 | 1.48 |
| Mixed dimension | 10 | 5.9 | 5.9 | 5.9 | 5.9 |
| Mixed ore | 35 | 20.65 | 20.65 | 20.65 | 20.65 |
| Egg yolk | 0 | 30 | 30 | 30 | 30 |
| Lard | 0 | 180 | 180 | 180 | 180 |
| Sucrose | 0 | 200 | 200 | 200 | 200 |
| Starch | RDS (%) | SDS (%) | RS (%) | 1047/1022 cm−1 |
|---|---|---|---|---|
| RCS | 83.31 ± 1.23 a | 9.12 ± 1.71 a | 7.57 ± 0.58 c | 0.69 ± 0.006 b |
| EDS | 58.06 ± 4.22 b | 2.53 ± 1.18 b | 38.87 ± 3.05 b | 0.73 ± 0.021 a |
| CCSb | 25.16 ± 1.95 c | 0.92 ± 0.26 b | 74.18 ± 1.74 a | 0.59 ± 0.015 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Ding, C.; Xie, Y.; Meng, J.; Fan, X.; Fan, D.; Wan, H.; Jiang, Z. Characteristics of Citrate-Esterified Starch and Enzymatically Debranched Starch and Their Effects on Diabetic Mice. Foods 2024, 13, 1486. https://doi.org/10.3390/foods13101486
Wang N, Ding C, Xie Y, Meng J, Fan X, Fan D, Wan H, Jiang Z. Characteristics of Citrate-Esterified Starch and Enzymatically Debranched Starch and Their Effects on Diabetic Mice. Foods. 2024; 13(10):1486. https://doi.org/10.3390/foods13101486
Chicago/Turabian StyleWang, Nannan, Changhe Ding, Yingying Xie, Jun Meng, Xing Fan, Duoduo Fan, Haowei Wan, and Zhengqiang Jiang. 2024. "Characteristics of Citrate-Esterified Starch and Enzymatically Debranched Starch and Their Effects on Diabetic Mice" Foods 13, no. 10: 1486. https://doi.org/10.3390/foods13101486




