Liquid Chromatography–Mass Spectrometry-Based Metabolomics Reveals Dynamic Metabolite Changes during Early Postmortem Aging of Donkey Meat
Abstract
:1. Introduction
2. Materials and Methods
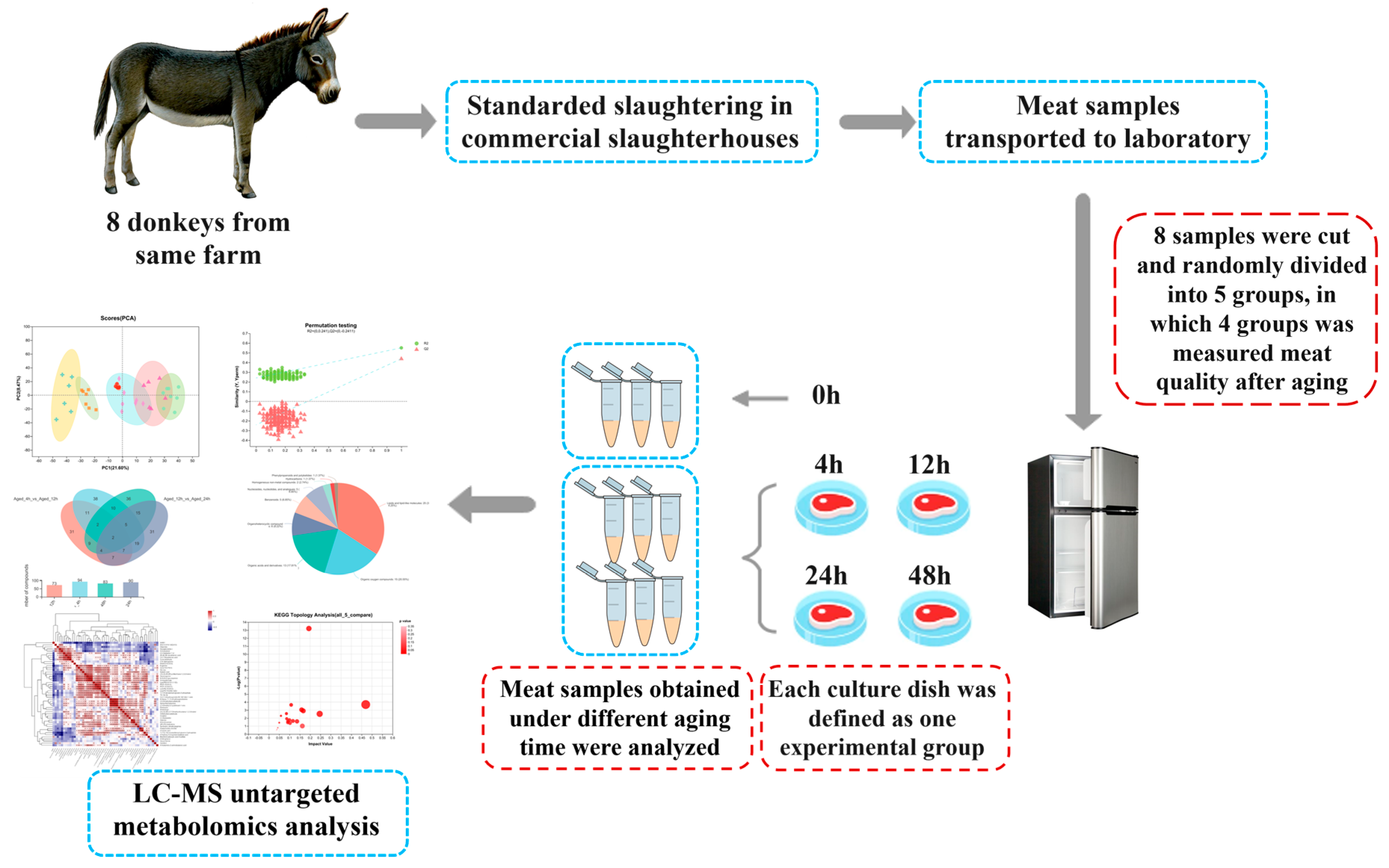
2.1. Samples
2.2. Meat Quality Analysis
2.2.1. pH Measurement
2.2.2. Cooking Loss
2.2.3. Shear Force
2.3. LC–MS Untargeted Metabolomics Determination
2.3.1. Sample Preparation
2.3.2. UHPLC–MS Untargeted Metabolomics Analysis
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Meat Quality
3.2. Metabolomics Analysis
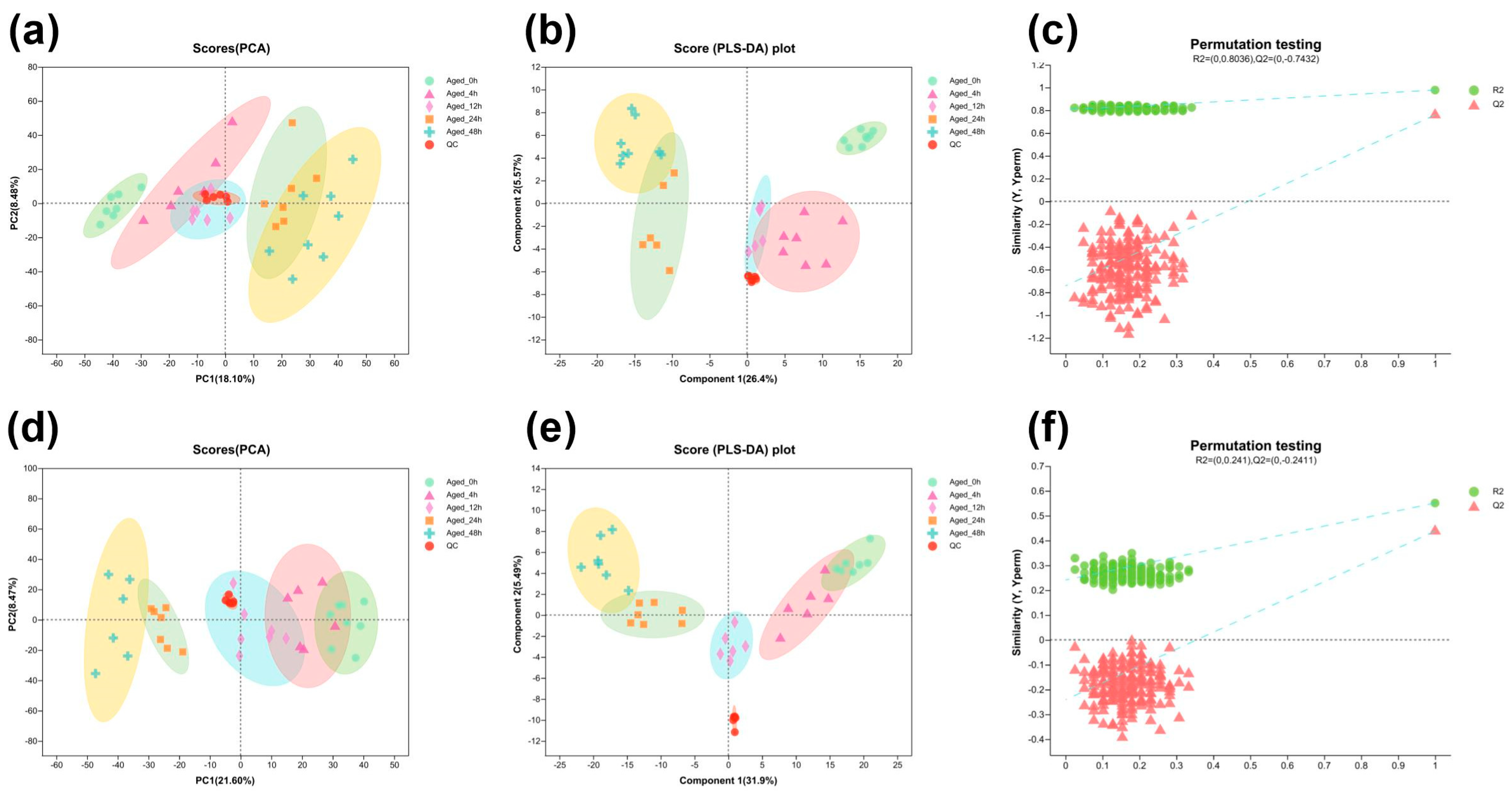
3.2.1. Multivariate Analysis
3.2.2. Qualitative and Quantitative Analysis of Different Aging Time
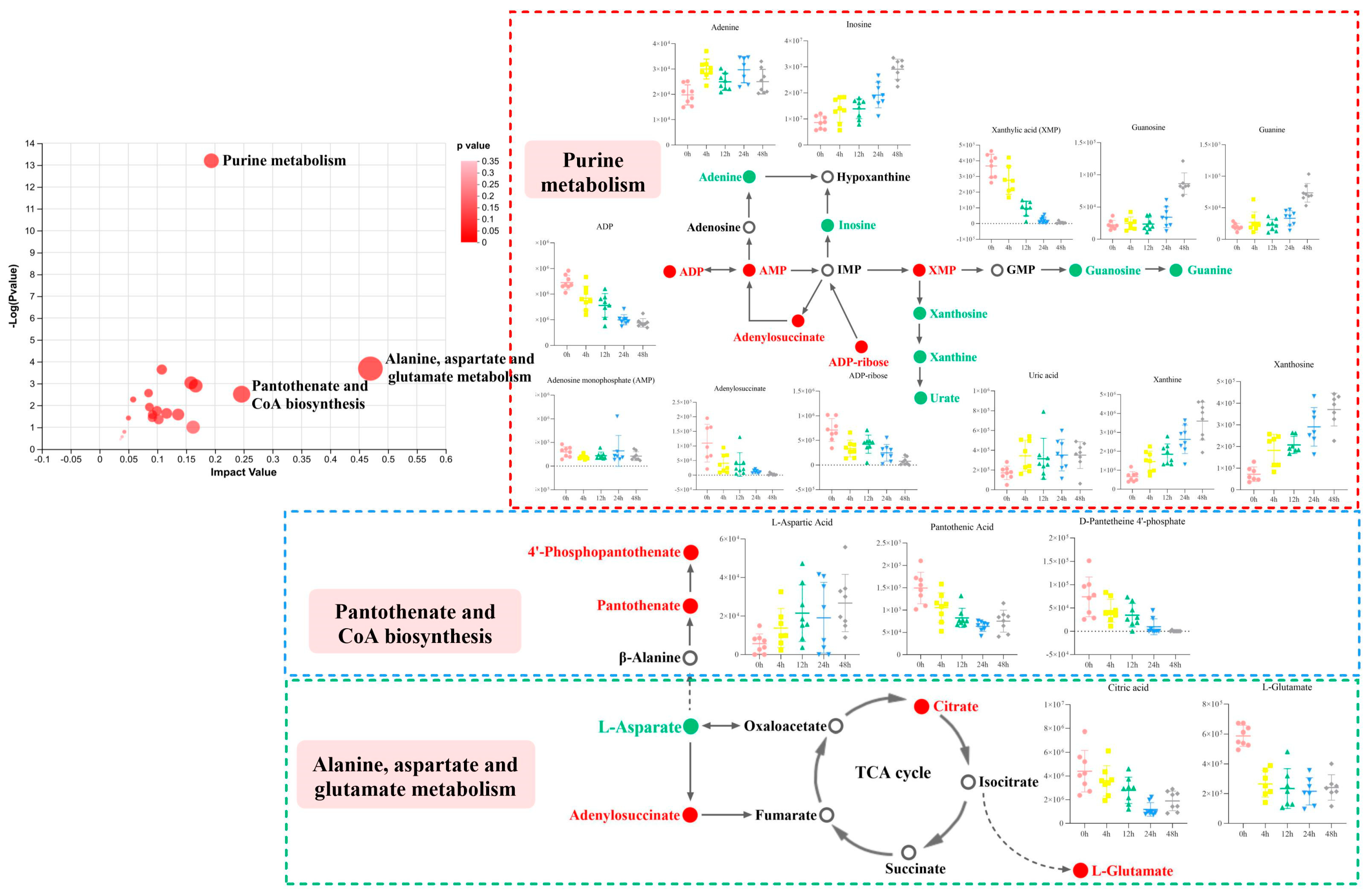
3.2.3. Enrichment of the Differential Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, W.; Qu, H.; Ma, Q.; Zhu, M.; Li, M.; Zhan, Y.; Liu, Z.; Xu, J.; Yao, H.; Li, Z. RNA-seq analysis identifies differentially expressed gene in different types of donkey skeletal muscles. Anim. Biotechnol. 2023, 34, 1786–1795. [Google Scholar] [CrossRef]
- Marino, R.; Albenzio, M.; Della Malva, A.; Muscio, A.; Sevi, A. Nutritional properties and consumer evaluation of donkey bresaola and salami: Comparison with conventional products. Meat Sci. 2015, 101, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Xu, J.; Qu, H.; Ma, Q.; Zhu, M.; Li, M.; Zhan, Y.; Wang, T.; Gao, J.; Yao, H. Differential proteomic analysis to identify potential biomarkers associated with quality traits of Dezhou donkey meat using a data-independent acquisition (DIA) strategy. LWT 2022, 166, 113792. [Google Scholar] [CrossRef]
- Piao, M.Y.; Jo, C.; Kim, H.J.; Lee, H.J.; Kim, H.J.; Ko, J.-Y.; Baik, M. Comparison of carcass and sensory traits and free amino acid contents among quality grades in loin and rump of Korean cattle steer. Asian-Australas. J. Anim. Sci. 2015, 28, 1629. [Google Scholar] [CrossRef]
- Kodani, Y.; Miyakawa, T.; Komatsu, T.; Tanokura, M. NMR-based metabolomics for simultaneously evaluating multiple determinants of primary beef quality in Japanese Black cattle. Sci. Rep. 2017, 7, 1297. [Google Scholar] [CrossRef] [PubMed]
- Melody, J.; Lonergan, S.M.; Rowe, L.; Huiatt, T.W.; Mayes, M.S.; Huff-Lonergan, E. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 2004, 82, 1195–1205. [Google Scholar] [CrossRef]
- Scheffler, T.; Gerrard, D. Mechanisms controlling pork quality development: The biochemistry controlling postmortem energy metabolism. Meat Sci. 2007, 77, 7–16. [Google Scholar] [CrossRef]
- Gašperlin, L.; Žlender, B.; Abram, V. Colour of beef heated to different temperatures as related to meat ageing. Meat Sci. 2001, 59, 23–30. [Google Scholar] [CrossRef]
- Jayasooriya, S.; Torley, P.; D’arcy, B.; Bhandari, B. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007, 75, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Boakye, K.; Mittal, G. Changes in colour of beef M. longissimus dorsi muscle during ageing. Meat Sci. 1996, 42, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Tian, X.; Shao, L.; Xu, L.; Dai, R.; Li, X. Label-free proteomic strategy to compare the proteome differences between longissimus lumborum and psoas major muscles during early postmortem periods. Food Chem. 2018, 269, 427–435. [Google Scholar] [CrossRef]
- Luo, B.; Groenke, K.; Takors, R.; Wandrey, C.; Oldiges, M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography–mass spectrometry. J. Chromatogr. A 2007, 1147, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Lv, H.; Xu, Z.; Zhao, S.; Huang, T.; Manyande, A.; Xiong, S. The mechanism for improving the flesh quality of grass carp (Ctenopharyngodon idella) following the micro-flowing water treatment using a UPLC-QTOF/MS based metabolomics method. Food Chem. 2020, 327, 126777. [Google Scholar] [CrossRef] [PubMed]
- Dannenberger, D.; Nuernberg, G.; Nuernberg, K.; Will, K.; Schauer, N.; Schmicke, M. Effects of diets supplemented with n–3 or n–6 PUFA on pig muscle lipid metabolites measured by non-targeted LC–MS lipidomic profiling. J. Food Compos. Anal. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, T.; Li, Y.; Liu, Y.; Liu, J. An untargeted LC-MS metabolomics approach to the metabolic profiles of bottom cultured scallops (Mizuhopecten yessoensis) subjected to mechanical shock in early post-harvest handling. Aquaculture 2021, 533, 736061. [Google Scholar] [CrossRef]
- Xiao, Z.; Ge, C.; Zhou, G.; Zhang, W.; Liao, G. 1H NMR-based metabolic characterization of Chinese Wuding chicken meat. Food Chem. 2019, 274, 574–582. [Google Scholar] [CrossRef]
- Bi, H.; Cai, D.; Zhang, R.; Zhu, Y.; Zhang, D.; Qiao, L.; Liu, Y. Mass spectrometry-based metabolomics approach to reveal differential compounds in pufferfish soups: Flavor, nutrition, and safety. Food Chem. 2019, 301, 125261. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, R.; Shi, L.; Zhang, F.; Chang, J.; Chu, X. Effects of spices on the formation of biogenic amines during the fermentation of dry fermented mutton sausage. Food Chem. 2020, 321, 126723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.H.; Seo, J.K.; Setyabrata, D.; Kim, Y.H.B. Effects of aging/freezing sequence and freezing rate on meat quality and oxidative stability of pork loins. Meat Sci. 2018, 139, 162–170. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.; Wu, G.; Young, O. The development of meat tenderness is likely to be compartmentalised by ultimate pH. Meat Sci. 2014, 96, 646–651. [Google Scholar] [CrossRef]
- Polidori, P.; Santini, G.; Klimanova, Y.; Zhang, J.-J.; Vincenzetti, S. Effects of ageing on donkey meat chemical composition, fatty acid profile and volatile compounds. Foods 2022, 11, 821. [Google Scholar] [CrossRef]
- Immonen, K.; Puolanne, E. Variation of residual glycogen-glucose concentration at ultimate pH values below 5.75. Meat Sci. 2000, 55, 279–283. [Google Scholar] [CrossRef]
- White, A.; O’sullivan, A.; Troy, D.; O’Neill, E. Manipulation of the pre-rigor glycolytic behaviour of bovine M. longissimus dorsi in order to identify causes of inconsistencies in tenderness. Meat Sci. 2006, 73, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Thompson, J. The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Sci. 2001, 58, 167–174. [Google Scholar] [CrossRef]
- Takahashi, G.; Wang, S.-M.; Lochner, J.; Marsh, B. Effects of 2-Hz and 60-Hz electrical stimulation on the microstructure of beef. Meat Sci. 1987, 19, 65–76. [Google Scholar] [CrossRef]
- Dransfield, E. Modelling post-mortem tenderisation—III: Role of calpain I in conditioning. Meat Sci. 1992, 31, 85–94. [Google Scholar] [CrossRef]
- Simmons, N.; Singh, K.; Dobbie, P.; Devine, C. The effect of pre-rigor holding temperature on calpain and calpastatin activity and meat tenderness. In Proceedings of the 42nd International Congress of Meat Science and Technology, Lillehammer, Norway, 1–6 September 1996; pp. 414–415. [Google Scholar]
- Ribeiro, F.A.; Lau, S.K.; Furbeck, R.A.; Herrera, N.J.; Henriott, M.L.; Bland, N.A.; Fernando, S.C.; Subbiah, J.; Sullivan, G.A.; Calkins, C.R. Ultimate pH effects on dry-aged beef quality. Meat Sci. 2021, 172, 108365. [Google Scholar] [CrossRef]
- Kristensen, L.; Purslow, P.P. The effect of ageing on the water-holding capacity of pork: Role of cytoskeletal proteins. Meat Sci. 2001, 58, 17–23. [Google Scholar] [CrossRef]
- Rowe, L.; Huff-Lonergan, E.; Lonergan, S. Desmin degradation influences water-holding capacity and tenderness of fresh pork. J. Anim. Sci. 2001, 79, 443. [Google Scholar]
- Yu, Q.; Tian, X.; Shao, L.; Li, X.; Dai, R. Mitochondria changes and metabolome differences of bovine longissimus lumborum and psoas major during 24 h postmortem. Meat Sci. 2020, 166, 108112. [Google Scholar] [CrossRef]
- Yu, Q.; Tian, X.; Shao, L.; Li, X.; Dai, R. Targeted metabolomics to reveal muscle-specific energy metabolism between bovine longissimus lumborum and psoas major during early postmortem periods. Meat Sci. 2019, 156, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; You, L.; Luo, R. Proteomics and metabolomics combined study on endopathic changes of water-soluble precursors in Tan lamb during postmortem aging. Food Sci. Nutr. 2022, 10, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Cornet, M.; Bousset, J. Free amino acids and dipeptides in porcine muscles: Differences between ‘red’ and ‘white’ muscles. Meat Sci. 1999, 51, 215–219. [Google Scholar] [CrossRef]
- Sewell, D.A.; Harris, R.; Marlin, D.; Dunnett, M. Estimation of the carnosine content of different fibre types in the middle gluteal muscle of the thoroughbred horse. J. Physiol. 1992, 455, 447–453. [Google Scholar] [CrossRef]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochem. C/C Biokhimiia 2000, 65, 757–765. [Google Scholar]
- Pösö, A.R.; Puolanne, E. Carbohydrate metabolism in meat animals. Meat Sci. 2005, 70, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Antonelo, D.; Gómez, J.F.; Cônsolo, N.R.; Beline, M.; Colnago, L.A.; Schilling, W.; Zhang, X.; Suman, S.P.; Gerrard, D.E.; Balieiro, J.C. Metabolites and metabolic pathways correlated with beef tenderness. Meat Muscle Biol. 2020, 4, 1–19. [Google Scholar] [CrossRef]
- Muroya, S.; Oe, M.; Ojima, K.; Watanabe, A. Metabolomic approach to key metabolites characterizing postmortem aged loin muscle of Japanese Black (Wagyu) cattle. Asian-Australas. J. Anim. Sci. 2019, 32, 1172. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Scheffler, T.L.; Gerrard, D.E. The conversion of muscle to meat. In Lawrie’s Meat Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 159–194. [Google Scholar]
- Martinez, H.A.; Arnold, A.N.; Brooks, J.C.; Carr, C.C.; Gehring, K.B.; Griffin, D.B.; Hale, D.S.; Mafi, G.G.; Johnson, D.D.; Lorenzen, C.L. National beef tenderness survey—2015: Palatability and shear force assessments of retail and foodservice beef. Meat Muscle Biol. 2017, 1, 138–148. [Google Scholar] [CrossRef]

| Group | Metabolite | Formula | VIP | FC | p_Value |
|---|---|---|---|---|---|
| 0–4 h | (R)-S-Lactoylglutathione | C13H21N3O8S | 2.4988 | 1.07 | 0.0134 |
| 1D-Myo-inositol 3,4-bisphosphate | C6H14O12P2 | 2.4565 | 1.09 | 0.0472 | |
| 5-Hydroxyindoleacetic acid | C10H9NO3 | 1.5848 | 0.97 | 0.0084 | |
| 5-Hydroxy-L-tryptophan | C11H12N2O3 | 1.7972 | 1.05 | 0.0207 | |
| Acetylcarnitine | C9H17NO4 | 1.3735 | 1.02 | 0.0178 | |
| Adenine | C5H5N5 | 2.0209 | 0.96 | 0.0001 | |
| Adenosine monophosphate | C10H14N5O7P | 1.5615 | 1.03 | 0.0220 | |
| Adenylosuccinate | C14H18N5O11P | 2.8708 | 1.12 | 0.0120 | |
| ADP | C10H15N5O10P2 | 1.4256 | 1.02 | 0.0055 | |
| ADP-ribose | C15H23N5O14P2 | 2.4531 | 1.06 | 0.0035 | |
| Alpha-D-Glucose 1,6-bisphosphate | C6H14O12P2 | 2.3833 | 1.08 | 0.0095 | |
| Arbutin | C12H16O7 | 1.1971 | 0.98 | 0.0493 | |
| Biocytin | C16H28N4O4S | 2.2409 | 0.89 | 0.0230 | |
| Creatine | C4H9N3O2 | 1.8926 | 0.98 | 0.0000 | |
| D-Fructose 2,6-bisphosphate | C6H14O12P2 | 2.5075 | 1.11 | 0.0416 | |
| DG (8:0/15:0/0:0) | C26H50O5 | 1.0996 | 1.01 | 0.0208 | |
| D-myo-Inositol 1,4-bisphosphate | C6H14O12P2 | 2.6324 | 1.07 | 0.0182 | |
| D-Xylose | C5H10O5 | 1.3285 | 0.97 | 0.0410 | |
| Fructose 1,6-bisphosphate | C6H14O12P2 | 2.4915 | 1.09 | 0.0182 | |
| Glutathione, oxidized | C20H32N6O12S2 | 1.7348 | 1.02 | 0.0020 | |
| Inosine | C10H12N4O5 | 1.6979 | 0.97 | 0.0304 | |
| L-Aspartic Acid | C4H7NO4 | 2.4854 | 0.90 | 0.0412 | |
| L-Glutamate | C5H9NO4 | 2.3490 | 1.06 | 0.0014 | |
| LysoSM (d18:1) | C23H50N2O5P+ | 1.7929 | 1.03 | 0.0048 | |
| N-Acetyl-alpha-D-glucosamine 1-phosphate | C8H16NO9P | 2.3395 | 0.93 | 0.0029 | |
| Pantothenic Acid | C9H17NO5 | 1.3555 | 1.03 | 0.0350 | |
| Phosphocreatine | C4H10N3O5P | 2.0266 | 0.89 | 0.0471 | |
| Phosphoric acid | H3O4P | 1.2767 | 0.98 | 0.0320 | |
| Pyrophosphate | H4O7P2 | 1.3779 | 0.98 | 0.0223 | |
| S-Lactoylglutathione | C13H21N3O8S | 2.3409 | 1.07 | 0.0234 | |
| Uric acid | C5H4N4O3 | 2.2319 | 0.94 | 0.0137 | |
| Xanthine | C5H4N4O2 | 2.6059 | 0.94 | 0.0012 | |
| Xanthosine | C10H12N4O6 | 2.5790 | 0.93 | 0.0095 | |
| 4–12 h | Adenine | C5H5N5 | 1.2586 | 1.02 | 0.0189 |
| Alpha-D-Glucose 1,6-bisphosphate | C6H14O12P2 | 2.9650 | 1.10 | 0.0191 | |
| Arbutin | C12H16O7 | 1.3174 | 1.02 | 0.0071 | |
| Calcitriol | C27H44O3 | 1.9349 | 0.97 | 0.0093 | |
| D-Fructose 2,6-bisphosphate | C6H14O12P2 | 2.9761 | 1.18 | 0.0456 | |
| DG (8:0/15:0/0:0) | C26H50O5 | 1.3920 | 0.99 | 0.0071 | |
| Fructose 1,6-bisphosphate | C6H14O12P2 | 4.4890 | 1.21 | 0.0016 | |
| Glutathione, oxidized | C20H32N6O12S2 | 1.3512 | 1.02 | 0.0495 | |
| Glyceric acid | C3H6O4 | 1.9644 | 0.95 | 0.0171 | |
| Oxidized glutathione | C20H32N6O12S2 | 2.2871 | 1.04 | 0.0113 | |
| S-Adenosylhomocysteine | C14H20N6O5S | 1.3135 | 0.98 | 0.0311 | |
| 12–24 h | (R)-S-Lactoylglutathione | C13H21N3O8S | 3.8322 | 1.20 | 0.0258 |
| 1D-Myo-inositol 3,4-bisphosphate | C6H14O12P2 | 6.5078 | 3.32 | 0.0027 | |
| 9,10,13-TriHOME | C18H34O5 | 1.2677 | 0.97 | 0.0194 | |
| Acetylcarnitine | C9H17NO4 | 1.9120 | 1.03 | 0.0167 | |
| Adenine | C5H5N5 | 1.3744 | 0.97 | 0.0194 | |
| Adenosine diphosphate ribose | C15H23N5O14P2 | 5.4947 | 0.70 | 0.0001 | |
| ADP | C10H15N5O10P2 | 1.4856 | 1.03 | 0.0101 | |
| Calcitriol | C27H44O3 | 1.6821 | 0.97 | 0.0280 | |
| Cinnavalininate | C14H8N2O6 | 1.0077 | 0.99 | 0.0009 | |
| Citric acid | C6H8O7 | 2.5784 | 1.06 | 0.0018 | |
| Creatine | C4H9N3O2 | 1.2022 | 0.99 | 0.0001 | |
| D-Erythrose 4-phosphate | C4H9O7P | 1.9235 | 1.04 | 0.0132 | |
| D-Fructose 2,6-bisphosphate | C6H14O12P2 | 2.8330 | 1.21 | 0.0139 | |
| D-Mannose 6-phosphate | C6H13O9P | 1.8994 | 1.04 | 0.0175 | |
| D-myo-Inositol 1,4-bisphosphate | C6H14O12P2 | 3.8493 | 1.20 | 0.0070 | |
| D-Myoinositol 4-phosphate | C6H13O9P | 1.7607 | 1.03 | 0.0063 | |
| D-Pantetheine 4’-phosphate | C11H23N2O7PS | 3.2471 | 1.17 | 0.0191 | |
| D-Xylose | C5H10O5 | 1.6564 | 0.95 | 0.0045 | |
| Glyceric acid | C3H6O4 | 2.8808 | 0.91 | 0.0003 | |
| Inosine | C10H12N4O5 | 1.1417 | 0.98 | 0.0384 | |
| Isocitrate | C6H8O7 | 2.6414 | 1.08 | 0.0003 | |
| Pyrophosphate | H4O7P2 | 1.2376 | 0.97 | 0.0359 | |
| S-Adenosylhomocysteine | C14H20N6O5S | 1.5438 | 0.98 | 0.0044 | |
| S-Lactoylglutathione | C13H21N3O8S | 3.1306 | 1.19 | 0.0220 | |
| Uridine diphosphate-N-acetylglucosamine | C17H27N3O17P2 | 1.7501 | 1.03 | 0.0016 | |
| Urocanic acid | C6H6N2O2 | 1.0355 | 0.99 | 0.0028 | |
| Xanthine | C5H4N4O2 | 1.3893 | 0.97 | 0.0339 | |
| Xanthylic acid | C10H13N4O9P | 3.7871 | 1.21 | 0.0023 | |
| 24–48 h | Guanosine | C10H13N5O5 | 3.5774 | 0.91 | 0.0003 |
| Guanine | C5H5N5O | 3.3936 | 0.92 | 0.0002 | |
| 3-Methylindole | C9H9N | 1.3661 | 0.98 | 0.0229 | |
| LysoSM(d18:1) | C23H50N2O5P+ | 2.5364 | 1.04 | 0.0108 | |
| DG (14:0/16:1(9Z)/0:0) | C33H62O5 | 1.2358 | 1.01 | 0.0144 | |
| S-Adenosylhomocysteine | C14H20N6O5S | 1.3707 | 0.99 | 0.0085 | |
| (R)-S-Lactoylglutathione | C13H21N3O8S | 3.5401 | 1.16 | 0.0353 | |
| ADP-ribose | C15H23N5O14P2 | 3.3305 | 1.12 | 0.0313 | |
| D-myo-Inositol 1,4-bisphosphate | C6H14O12P2 | 3.3973 | 1.14 | 0.0240 | |
| Inosine | C10H12N4O5 | 2.0781 | 0.97 | 0.0015 | |
| Theobromine | C7H8N4O2 | 1.4483 | 0.98 | 0.0014 | |
| 1,4-beta-D-Glucan | C18H32O18 | 2.3819 | 0.96 | 0.0028 | |
| Glyceric acid | C3H6O4 | 3.2365 | 0.93 | 0.0002 | |
| Biocytin | C16H28N4O4S | 2.4494 | 0.92 | 0.0439 | |
| KAPA | C9H17NO3 | 1.9517 | 0.97 | 0.0190 | |
| Palmitoyl-L-carnitine | C23H45NO4 | 2.3837 | 1.04 | 0.0491 | |
| 9,10,13-TriHOME | C18H34O5 | 3.0082 | 0.91 | 0.0104 | |
| Adenylosuccinate | C14H18N5O11P | 3.9232 | 1.21 | 0.0035 | |
| Adenosine diphosphate ribose | C15H23N5O14P2 | 3.3072 | 0.90 | 0.0084 | |
| D-Xylose | C5H10O5 | 2.7604 | 0.93 | 0.0000 | |
| Xanthylic acid | C10H13N4O9P | 3.4229 | 1.18 | 0.0171 | |
| Malic acid | C4H6O5 | 1.9394 | 1.03 | 0.0069 |
| Pathway Name | Total | Hit | Impact Value | p Value |
|---|---|---|---|---|
| Alanine, aspartate and glutamate metabolism | 28 | 4 | 0.469565 | 0.002409 |
| Pantothenate and CoA biosynthesis | 27 | 3 | 0.246255 | 0.011784 |
| Purine metabolism | 81 | 13 | 0.193812 | 0.000000 |
| Citrate cycle (TCA cycle) | 20 | 3 | 0.166651 | 0.006359 |
| Aminoacyl-tRNA biosynthesis | 52 | 2 | 0.162162 | 0.138972 |
| Caffeine metabolism | 18 | 3 | 0.158590 | 0.005423 |
| Biotin metabolism | 23 | 2 | 0.136285 | 0.055364 |
| Amino sugar and nucleotide sugar metabolism | 107 | 4 | 0.116323 | 0.054167 |
| Glyoxylate and dicarboxylate metabolism | 54 | 5 | 0.107896 | 0.001992 |
| Glycolysis/Gluconeogenesis | 31 | 2 | 0.102451 | 0.073197 |
| Fructose and mannose metabolism | 52 | 3 | 0.099593 | 0.043905 |
| Arginine biosynthesis | 23 | 2 | 0.091707 | 0.055364 |
| Pyruvate metabolism | 28 | 3 | 0.091410 | 0.067980 |
| Inositol phosphate metabolism | 45 | 3 | 0.086398 | 0.035429 |
| Tryptophan metabolism | 56 | 4 | 0.084945 | 0.012193 |
| Histidine metabolism | 33 | 3 | 0.058318 | 0.018566 |
| Arginine and proline metabolism | 72 | 3 | 0.050089 | 0.067010 |
| Neomycin, kanamycin and gentamicin biosynthesis | 76 | 2 | 0.042962 | 0.211768 |
| Glycerolipid metabolism | 32 | 1 | 0.039099 | 0.301516 |
| Pentose and glucuronate interconversions | 56 | 1 | 0.036312 | 0.354392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, W.; Wang, L.; Li, T.; Wang, T.; Wang, X.; Yan, M.; Zhu, M.; Gao, J.; Wang, C.; Ma, Q.; et al. Liquid Chromatography–Mass Spectrometry-Based Metabolomics Reveals Dynamic Metabolite Changes during Early Postmortem Aging of Donkey Meat. Foods 2024, 13, 1466. https://doi.org/10.3390/foods13101466
Chai W, Wang L, Li T, Wang T, Wang X, Yan M, Zhu M, Gao J, Wang C, Ma Q, et al. Liquid Chromatography–Mass Spectrometry-Based Metabolomics Reveals Dynamic Metabolite Changes during Early Postmortem Aging of Donkey Meat. Foods. 2024; 13(10):1466. https://doi.org/10.3390/foods13101466
Chicago/Turabian StyleChai, Wenqiong, Liyuan Wang, Tong Li, Tianqi Wang, Xinrui Wang, Miao Yan, Mingxia Zhu, Jingrong Gao, Changfa Wang, Qiugang Ma, and et al. 2024. "Liquid Chromatography–Mass Spectrometry-Based Metabolomics Reveals Dynamic Metabolite Changes during Early Postmortem Aging of Donkey Meat" Foods 13, no. 10: 1466. https://doi.org/10.3390/foods13101466






