Pre- and Postharvest Strategies for Pleurotus ostreatus Mushroom in a Circular Economy Approach
Abstract
:1. Introduction
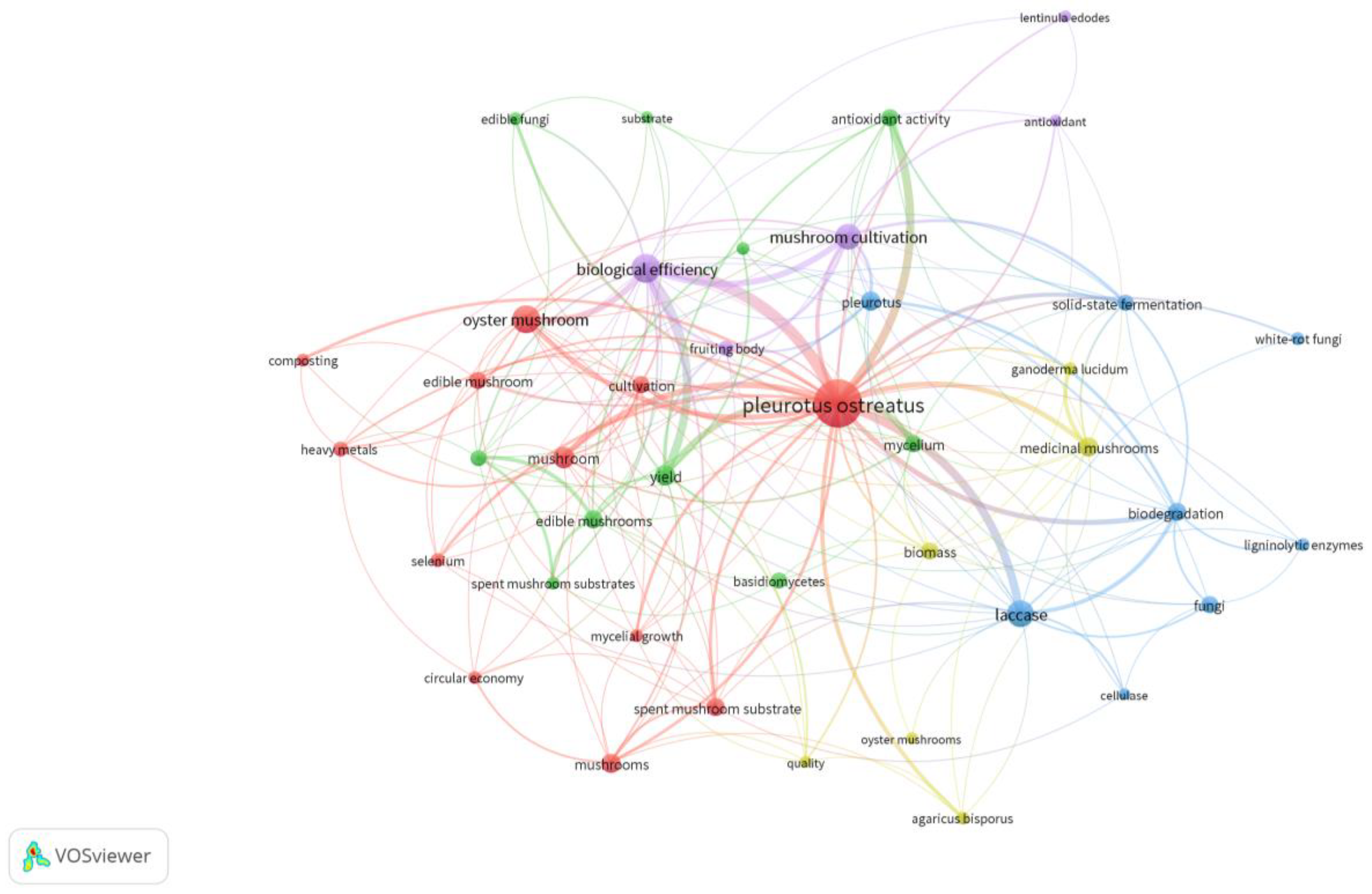
1.1. Bibliometric Study
1.2. Pleurotus ostreatus Characteristics
2. Mushroom Preharvest Activities
2.1. Spawn
2.2. Substrate Preparation
| Substrates | Reference |
|---|---|
| Sawdust, cotton seed, wheat straw, and paper waste | [53] |
| Tea residues | [54] |
| Vegetable waste and rice straw | [49] |
| Wheat straw, spent ground coffee, and cardboard | [55] |
| Defatted almond meal, chicken manure, and wheat straw | [56] |
| Olive pomace and wheat straw | [57] |
| Wheat straw and spent ground coffee | [58] |
| Wheat straw, spent ground coffee, and olive pruning residues | [9] |
| Rice straw, wheat straw, corncobs, sawdust and rice husk, and sugarcane bagasse | [10] |
| Light coarse fiber residues, Betula spp., sawdust, wheat bran | [59] |
| Coffee pulp and wheat straw | [60] |
| Spent ground coffee and sawdust | [61] |
| Alfalfa pulp | [11] |
| Spent brewery grains, wheat bran, and beech sawdust | [62] |
| Palm waste, rice bran, and wheat bran | [63] |
2.2.1. C:N Ratio
2.2.2. pH
2.2.3. Moisture
2.3. Substrate Treatment
2.4. Inoculation and Incubation
2.5. Fructification
3. Mushroom Postharvest Activities
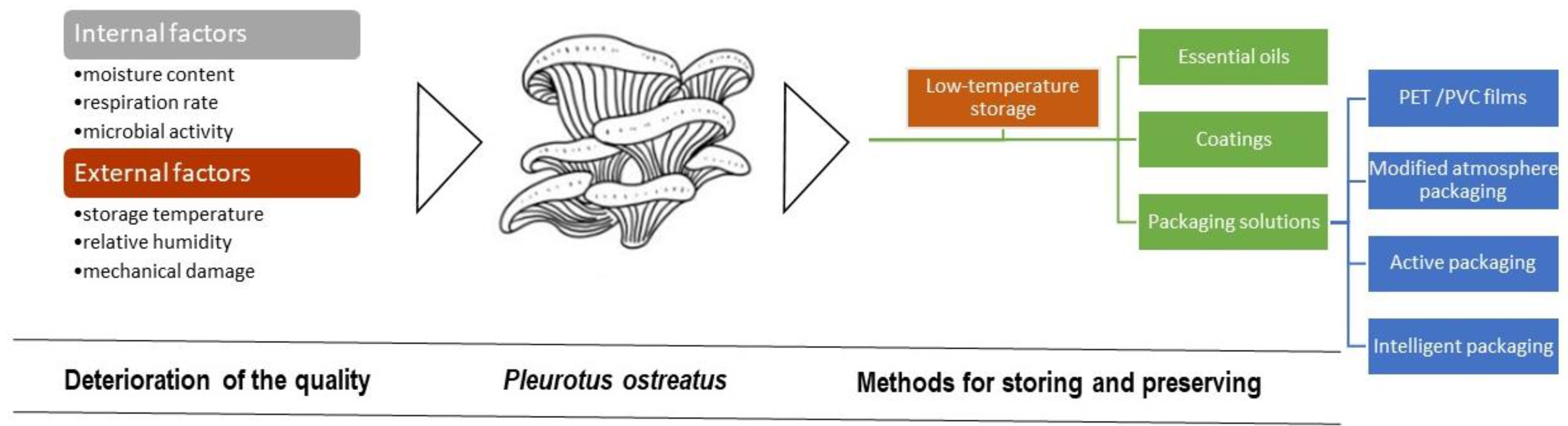
3.1. Deterioration of the Quality of Pleurotus ostreatus
3.2. Methods for Storing and Preserving Pleurotus ostreatus
3.2.1. Low-Temperature Storage
3.2.2. Essential Oil Treatment
3.2.3. Coatings
3.2.4. Packaging Solutions
4. Circular Economy Approach: Waste Reuse
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.G.; Pandey, A. Assessing the impact of industrial waste on environment and mitigation strategies: A comprehensive review. J. Hazard. Mater. 2020, 398, 123019. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, Z.; Noor, R.M.; Soon, T.K.; Ahmedy, I.; Abdullah, N.A.; Poh, Y.S. IoT Based Multidimensional Mushroom Waste Management System in Urban Area. In Proceedings of the 3rd International Conference on Sustainable Technologies for Industry 4.0 STI 2021, Dhaka, Bangladesh, 18–19 December 2021. [Google Scholar] [CrossRef]
- Comunicação da Comissão ao Parlamento Europeu, ao Conselho, ao Comité Económico e Social Europeu e ao Comité das Regiões Sobre o Regime de Acompanhamento do 8.° Programa de Ação em Matéria de Ambiente: Medir os Progressos Realizados para Alcançar os Objetivos Prioritários do Programa para 2030 e 2050. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:52022DC0357&from=EN (accessed on 14 January 2024).
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Diego, C.Z., Pardo-Giménez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Singh, V.K. Yield performance and nutritional analysis of Pleurotus citrinopileatus on different agrowastes and vegetable wastes. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Arcachon, France, 4–7 October 2011. [Google Scholar]
- Barshteyn, V.; Krupodorova, T. Utilization of agro-industrial waste by higher mushrooms: Modern view and trends. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 563–577. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; El Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Böhme, M.; Yordanova, M.H.; Sassine, Y.N. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value. PLoS ONE 2021, 16, e025579. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Halawani, R.F.; Aloufi, F.A.; Taleb, M.A.; Akter, S.; Mahmood, S. Utilization of Agro-Industrial Wastes for the Production of Quality Oyster Mushrooms. Sustainability 2022, 14, 994. [Google Scholar] [CrossRef]
- Zhou, F.; Hansen, M.; Hobley, T.J.; Jensen, P.R. Valorization of Green Biomass: Alfalfa Pulp as a Substrate for Oyster Mushroom Cultivation. Foods 2022, 11, 2519. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W.I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Technol. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Kibar, B. Influence of different drying methods and cold storage treatments on the postharvest quality and nutritional properties of P. ostreatus mushroom. Turk. J. Agric. For. 2021, 45, 565–579. [Google Scholar] [CrossRef]
- Shao, P.; Yu, J.; Chen, H.; Gao, H. Development of microcapsule bioactive paper loaded with cinnamon essential oil to improve the quality of edible fungi. Food Packag. Shelf Life 2021, 27, 100617. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Bian, S.; Ali Baig, S.; Hu, B.; Xu, X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 2017, 169, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Brennan, M.A.; Serventi, L.; Brennan, C.S. Incorporation of mushroom powder into bread dough—Effects on dough rheology and bread properties. Cereal Chem. 2018, 95, 418–427. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Narciso, J.; Guan, W.; Zhang, J.; Yuan, L.; Serventi, L.; Brennan, C. Correlations between the phenolic and fibre composition of mushrooms and the glycaemic and textural characteristics of mushroom enriched extruded products. LWT 2020, 118, 108730. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of Mushroom By-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, K.; Li, C.; Cheng, S.; Zhou, J.; Wu, Z. A Novel Biodegradable Film from Edible Mushroom (F. velutipes) By-Product: Microstructure, Mechanical, and Barrier Properties Associated with the Fiber Morphology. Innov. Food Sci. Emerg. Technol. 2018, 47, 153–160. [Google Scholar] [CrossRef]
- Sahithya, K.; Mouli, T.; Biswas, A.; Mercy, S. Remediation Potential of Mushrooms and Their Spent Substrate Against Environmental Contaminants: An Overview. Biocatal. Agric. Biotechnol. 2022, 42, 102323. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.; Zheng, F.; Long, C.; Lu, Z.; Duan, Z. Comparing Keywords Plus of WOS and Author Keywords: A Case Study of Patient Adherence Research. J. Assoc. Inf. Sci. Technol. 2016, 67, 967–972. [Google Scholar] [CrossRef]
- Kotadiya, U.; Talaviya, J.; Shah, K.; Lathiya, S. Morphological and Molecular Identification of Oyster Mushroom [Pleurotus ostreatus (Jacq.) P. Kumm]. Res. Sq. 2021, 1–9. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; van Griensven, L.; Jakovljevic, D.; Todorovic, N.; Wan-Mohtar, W.A.A.Q.I.; Vunduk, J. Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: Nature of the application. Food Sci. Hum. Wellness 2023, 12, 378–396. [Google Scholar] [CrossRef]
- Rennerova, Z.; Picó Sirvent, L.; Carvajal Roca, E.; Paśnik, J.; Logar, M.; Milošević, K.; Majtan, J.; Jesenak, M. Beta-(1,3/1,6)-D-glucan from Pleurotus ostreatus in the prevention of recurrent respiratory tract infections: An international, multicentre, open-label, prospective study. Front. Pediatr. 2022, 10, 999701. [Google Scholar] [CrossRef] [PubMed]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in Wild and Cultivated Mushrooms: Nutrition and Health. Phytochem. Rev. 2022, 21, 339–383. [Google Scholar] [CrossRef]
- Bell, V.; Silva, C.R.P.G.; Guina, J.; Fernandes, T.H. Mushrooms as future generation healthy foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef] [PubMed]
- Di Piazza, S.; Benvenuti, M.; Damonte, G.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Fungi and circular economy: Pleurotus ostreatus grown on a substrate with agricultural waste of lavender, and its promising biochemical profile. Recycling 2021, 6, 40. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Hu, D.D.; Ma, X.T.; Li, S.G.; Gu, J.G.; Hu, Q.X. Adopting Stick Spawn Reduced the Spawn Running Time and Improved Mushroom Yield and Biological Efficiency of Pleurotus eryngii. Sci. Hortic. 2014, 175, 156–159. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of Cultivation Substrate and Microbial Community on Improving Mushroom Productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Lee, M.A.; Kim, Y.G.; Lee, K.W.; Lee, B.E.; Seo, G.S. Characteristics and suitability of various cereal grains in spawn production of button mushroom. J. Mushrooms 2014, 12, 237–243. [Google Scholar] [CrossRef]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef]
- Aditya, N.; Jarial, R.S.; Jarial, K.; Bhatia, J.N. Comprehensive review on oyster mushroom species (Agaricomycetes): Morphology, nutrition, cultivation and future aspects. Heliyon 2024, 10, e26539. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Elkanah, F.A.; Afolabi, F.J.; Ogundun, O.S.; Alabi, T.F.; Oduoye, O.T. Molecular characterization of most cultivated Pleurotus species in sub-western region Nigeria with development of cost effective cultivation protocol on palm oil waste. Heliyon 2021, 7, e06215. [Google Scholar] [CrossRef]
- Ma, L.; Lin, Y.Q.; Yang, C.; Ying, Z.H.; Jiang, X.L. Production of Liquid Spawn of an Edible Mushroom, Sparassis latifolia, by Submerged Fermentation and Mycelial Growth on Pine Wood Sawdust. Sci. Hortic. 2016, 209, 22–30. [Google Scholar] [CrossRef]
- Liu, S.R.; Zhang, W.R.; Kuang, Y.B. Production of stalk spawn of an edible mushroom (Pleurotus ostreatus) in liquid culture as a suitable substitute for stick spawn in mushroom cultivation. Sci. Hortic. 2018, 240, 572–577. [Google Scholar] [CrossRef]
- Zhang, W.R.; Liu, S.R.; Kuang, Y.B.; Zheng, S.Z. Development of a Novel Spawn (Block Spawn) of an Edible Mushroom, Pleurotus ostreatus, in Liquid Culture and Its Cultivation Evaluation. Mycobiology 2019, 47, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, S.; Singh, R.; Summuna, B. Management of contaminants in mushroom spawn. Indian J. Agric. Sci. 2020, 90, 1000–1003. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, R.; Johari, N.M.K.; Annuar, M.S.M. Production of liquid spawn of an edible grey oyster mushroom, Pleurotus pulmonarius (Fr.) Quél by submerged fermentation and sporophore yield on rubber wood sawdust. Sci. Hortic. 2013, 161, 65–69. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Petre, M.; Petre, V. Environmental Biotechnology for Bioconversion of Agricultural and Forestry Wastes into Nutritive Biomass. In Environmental Biotechnology—New Approaches and Prospective Applications; Petre, M., Ed.; InTech: Seoul, Republic of Korea, 2013. [Google Scholar] [CrossRef]
- Molobele, I.I.; Masalu, R.J.; Mosha, P.R.; Mpinda, C.B. Evaluation of Enzymatic Activity during Growth of Pleurotus HK 37 on Saba comorensis Exocarp. Tanzan. J. Sci. 2022, 48, 718–726. [Google Scholar] [CrossRef]
- Masevhe, M.R.; Soundy, P.; Taylor, N.J. Alternative substrates for cultivating oyster mushrooms (Pleurotus ostreatus). S. Afr. J. Plant Soil 2016, 33, 97–103. [Google Scholar] [CrossRef]
- Singh, K. Vegetable Waste—A Potent Substrate for Cultivation of P. ostreatus. Int. J. Res. Stud. Biosci. 2016, 4, 5–9. [Google Scholar] [CrossRef]
- Philippoussis, A.N. Production of Mushrooms Using Agro-Industrial Residues as Substrates. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; nee’ Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 163–196. [Google Scholar] [CrossRef]
- Yang, W.J.; Guo, F.L.; Wan, Z.J. Yield and Size of Oyster Mushroom Grown on Rice/Wheat Straw Basal Substrate Supplemented with Cotton Seed Hull. Saudi J. Biol. Sci. 2013, 20, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Puliga, F.; Leonardi, P.; Minutella, F.; Zambonelli, A.; Francioso, O. Valorization of Hazelnut Shells as Growing Substrate for Edible and Medicinal Mushrooms. Horticulturae 2022, 8, 214. [Google Scholar] [CrossRef]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and Yield Performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (Oyster Mushroom) on Different Substrates. AMB Express 2016, 6, 87. [Google Scholar] [CrossRef]
- Yang, D.; Liang, J.; Wang, Y.; Sun, F.; Tao, H.; Xu, Q.; Zhang, L.; Zhang, Z.; Ho, C.T.; Wan, X. Tea waste: An effective and economic substrate for oyster mushroom cultivation. J. Sci. Food Agric. 2016, 96, 680–684. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Ranamukhaarachchi, S.L. Effect of different culture media, grain sources and alternate substrates on the mycelial growth of Pleurotus eryngii and Pleurotus ostreatus. Pak. J. Biol. Sci. 2020, 23, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Giménez, A.; Carrasco, J.; Roncero, J.M.; Álvarez-Ortí, M.; Zied, D.C.; Pardo-González, J.E. Recycling of the Biomass Waste Defatted Almond Meal as a Novel Nutritional Supplementation for Cultivated Edible Mushrooms. Acta Sci. Agron. 2018, 40, e39341. [Google Scholar] [CrossRef]
- Alananbeh, K.; Al-Momany, A. Production of Oyster Mushroom (Pleurotus ostreatus) on Olive Cake Agro Waste. Dirasat Agric. Sci. 2005, 32, 64–70. [Google Scholar]
- Alsanad, M.A.; Sassine, Y.N.; El Sebaaly, Z.; Abou Fayssal, S. Spent coffee grounds influence on Pleurotus ostreatus production, composition, fatty acid profile, and lignocellulose biodegradation capacity. CYTA-J. Food 2021, 19, 11–20. [Google Scholar] [CrossRef]
- Grimm, A.; Eilertsen, L.; Chen, F.; Huang, R.; Atterhem, L.; Xiong, S. Cultivation of Pleurotus ostreatus Mushroom on Substrates Made of Cellulose Fibre Rejects: Product Quality and Spent Substrate Fuel Properties. Waste Biomass Valorization 2021, 12, 4331–4340. [Google Scholar] [CrossRef]
- Salmones, D.; Mata, G.; Waliszewski, K.N. Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw: Biomass production and substrate biodegradation. Bioresour. Technol. 2005, 96, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine metabolism during cultivation of oyster mushroom (Pleurotus ostreatus) with spent coffee grounds. Appl. Microbiol. Biotechnol. 2019, 103, 5831–5841. [Google Scholar] [CrossRef] [PubMed]
- Gregori, A.; Švagelj, M.; Pahor, B.; Berovič, M.; Pohleven, F. The Use of Spent Brewery Grains for Pleurotus ostreatus Cultivation and Enzyme Production. New Biotechnol. 2008, 25, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Elkanah, F.A.; Oke, M.A.; Adebayo, E.A. Substrate composition effect on the nutritional quality of Pleurotus ostreatus (MK751847) fruiting body. Heliyon 2022, 8, e11841. [Google Scholar] [CrossRef] [PubMed]
- Yolande, M.E.; Germaine, M.J.E.; Abraham, N.T.; Germaine, Y.; Marcellin, M.L.; Aime, B.B.D.; Leroy, S.K.S. Impact of substrate methionine content on lovastatin potentiation and morphological parameters of Pleurotus ostreatus. Sci. Afr. 2023, 20, e01621. [Google Scholar] [CrossRef]
- Siwulski, M.; Budka, A.; Rzymski, P.; Gąsecka, M.; Kalač, P.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Mleczek, P.; Mleczek, M. Worldwide basket survey of multielemental composition of white button mushroom Agaricus bisporus. Chemosphere 2020, 239, 124718. [Google Scholar] [CrossRef] [PubMed]
- Golian, M.; Hegedűsová, A.; Mezeyová, I.; Chlebová, Z.; Hegedűs, O.; Urminská, D.; Vollmannová, A.; Chlebo, P. Accumulation of Selected Metal Elements in Fruiting Bodies of Oyster Mushroom. Foods 2022, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, Y.; Ren, J.; Qin, N. Yield, Nutritional Content, and Antioxidant Activity of Pleurotus ostreatus on Corncobs Supplemented with Herb Residues. Mycobiology 2018, 46, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.; Naozuka, J. Elemental Enrichment by Cultivation: Plants and Mushrooms. Quim. Nova 2020, 43, 1277–1293. [Google Scholar] [CrossRef]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J. Sci. Food Agric. 2017, 97, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Naraian, R.; Sahu, R.K.; Kumar, S.; Garg, S.K.; Singh, C.S.; Kanaujia, R.S. Influence of Different Nitrogen-Rich Supplements during Cultivation of Pleurotus florida on Corn Cob Substrate. Environmentalist 2009, 29, 1–7. [Google Scholar] [CrossRef]
- Reddy, M.S.; Kanwal, H.K. Influence of carbon, nitrogen sources, inducers, and substrates on lignocellulolytic enzyme activities of Morchella spongiola. J. Agric. Food Res. 2022, 7, 100271. [Google Scholar] [CrossRef]
- Krupodorova, T.; Barshteyn, V.Y.; Sekan, A. Review of the basic cultivation conditions influence on the growth of basidiomycetes. Curr. Res. Environ. Appl. Mycol. 2021, 11, 494–531. [Google Scholar] [CrossRef]
- Laursen, A. The Effect of Different Nitrogen Sources on Mycelial Growth of Oyster Mushroom, Pleurotus ostreatus—With a Review Concerning Cultivation of the Species; Bachelor Project in Biology; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018. [Google Scholar]
- Akcay, C.; Ceylan, F.; Arslan, R. Production of oyster mushroom (Pleurotus ostreatus) from some waste lignocellulosic materials and FTIR characterization of structural changes. Sci. Rep. 2023, 13, 12897. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Cueva, A.R.; Bernarda, M.; Hernández; Niño-Ruiz, A. Influence of C/N ratio on productivity and the protein contents of Pleurotus ostreatus grown in different residue mixtures. Rev. Fac. Cienc. Agrar. 2017, 49, 331–344. [Google Scholar]
- Hoa, H.T.; Wang, C.L. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci. Hortic. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Hossain, I.; Saifullah, M.D.; Amin, R.; Chakraborty, R. Influence of Substrate pH and Watering Frequency on the Growth of Oyster Mushroom. Int. J. Plant Biol. 2018, 6, 1097. [Google Scholar]
- Urben, A.F. Produção de Cogumelos por Meio de Tecnologia Chinesa Modificada Biotecnologia e Aplicações na Agricultura e na Saúde, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Kalita, M.K. Impact of various sterilization methods on growth and yield of oyster mushroom (Pleurotus florida). Int. J. Agric. Sci. 2015, 11, 104–107. [Google Scholar] [CrossRef]
- Oseni, T.O.; Dlamini, S.O.; Earnshaw, D.M.; Masarirambi, M.T. Effect of Substrate Pre-treatment Methods on Oyster Mushroom (Pleurotus ostreatus) Production. Int. J. Agric. Biol. 2012, 14, 251–255. [Google Scholar] [CrossRef]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkeley, CA, USA, 2000. [Google Scholar]
- Oei, P.; van Nieuwenhuijzen, B.; de Feijter, J.; de Zylva, N. Small-Scale Mushroom Cultivation: Oyster, Shiitake and Wood Ear Mushrooms, 1st ed.; Agromisa: Wageningen, The Netherlands, 2005. [Google Scholar]
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010, 85, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Savoie, J.M.; Salmones, D.; Mata, G. Hydrogen Peroxide Concentration Measured in Cultivation Substrates during Growth and Fruiting of the Mushrooms Agaricus bisporus and Pleurotus spp. J. Sci. Food Agric. 2007, 87, 1337–1344. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, M.; Jin, Q.; Fan, L.; Feng, W.; Song, T.; Fangfang, T.; Cai, W. Effects of cold stimulation on primordial initiation and yield of Pleurotus pulmonarius. Sci. Hortic. 2014, 167, 100–106. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, F.; Chen, Y.; Qi, Y.; Zhu, W.; Wang, F.; Wen, Q.; Shen, J. Effects and Mechanism of the Mycelial Culture Temperature on the Growth and Development of Pleurotus ostreatus (Jacq.) P. Kumm. Horticulturae 2023, 9, 95. [Google Scholar] [CrossRef]
- Barh, A.; Sharma, V.P.; Thakur, B.; Annepu, S.K.; Kamal, S.; Bairwa, R. Pleurotus species relationship and round the year cultivation in India. Mushroom Res. 2020, 28. [Google Scholar] [CrossRef]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef]
- Nakano, Y.; Fujii, H.; Kojima, M. Identification of blue-light photoresponse genes in oyster mushroom mycelia. Biosci. Biotechnol. Biochem. 2010, 74, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, A.; Janczewska, A.; Kobus-Cisowska, J.; Dziedziński, M.; Siwulski, M.; Czarniecka-Skubina, E.; Stuper-Szablewska, K. The effect of light conditions on the content of selected active ingredients in anatomical parts of the oyster mushroom (Pleurotus ostreatus L.). PLoS ONE 2022, 17, e0262279. [Google Scholar] [CrossRef] [PubMed]
- Gallotti, F.; Lavelli, V. The Effect of UV Irradiation on Vitamin D2 Content and Antioxidant and Antiglycation Activities of Mushrooms. Foods 2020, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, X.; Song, Z.; Kong, W.; Kang, Y.; Kong, W.; Ng, T.B. Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during postharvest storage. Food Chem. 2021, 352, 129357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhang, M.; Shan, L.; You, Y.; Salokhe, V.M. Extension of the shelf-life of fresh oyster mushrooms (Pleurotus ostreatus) by modified atmosphere packaging with chemical treatments. Afr. J. Biotechnol. 2011, 10, 9509–9517. [Google Scholar] [CrossRef]
- Dawadi, E.; Magar, P.B.; Bhandari, S.; Subedi, S.; Shrestha, S.; Shrestha, J. Nutritional and Post-harvest Quality Preservation of Mushrooms: A Review. Heliyon 2022, 8, e12093. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Qi, Z.; Deng, Z.; Han, A.; Long, H.; Wang, J.; Yao, W.; et al. Advances in Postharvest Storage and Preservation Strategies for Pleurotus eryngii. Foods 2023, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Ko, J.A.; Lee, J.S.; Park, H.J.; Hanna, M.A. Effect of Modified Atmosphere Packaging on the Shelf-life of Coated, Whole, and Sliced Mushrooms. LWT-Food Sci. Technol. 2006, 39, 365–372. [Google Scholar] [CrossRef]
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh Mushroom Preservation Techniques. Foods 2021, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Phillippoussis, A. Cultivated Mushrooms: Preservation and Processing. In Handbook of Vegetable Preservation and Processing, 2nd ed.; Hui, Y.H., Özgül Evranuz, E., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 516–547. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Gao, X.; Chen, J.; Zheng, Y.; Gao, Y.; Qiu, L. The molecular mechanism for the ethylene regulation of postharvest button mushrooms maturation and senescence. Postharvest Biol. Technol. 2019, 156, 110930. [Google Scholar] [CrossRef]
- Lin, X.; Sun, D.W. Research Advances in Browning of Button Mushroom (Agaricus bisporus): Affecting Factors and Controlling Methods. Trends Food Sci. Technol. 2019, 90, 63–75. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.V.; Geyer, M.; Linke, M.; Pant, A.; Saengerlaub, S.; Caleb, O.J. Application of humidity-regulating tray for packaging of mushrooms. Postharvest Biol. Technol. 2015, 108, 102–110. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Navarro-Martínez, A.; Barón, M.; Navarro-Segura, L.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Martínez-Hernández, G.B. Packaging of Fresh Sliced Mushrooms with Essential Oils Vapours: A New Technology for Maintaining Quality and Extending Shelf Life. Foods 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Schill, S.; Stessl, B.; Meier, N.; Tichy, A.; Wagner, M.; Ludewig, M. Microbiological Safety and Sensory Quality of Cultivated Mushrooms (Pleurotus eryngii, Pleurotus ostreatus, and Lentinula edodes) at Retail Level and Post-Retail Storage. Foods 2021, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Sajben, E.; Manczinger, L.; Nagy, A.; Kredics, L.; Vágvölgyi, C. Characterization of pseudomonads isolated from decaying sporocarps of oyster mushroom. Microbiol. Res. 2011, 166, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Azu Okorley, B.; Leo Sossah, F.; Dai, D.; Xu, S.; Liu, Z.; Song, B.; Sheng, H.; Fu, Y.; Li, Y. Resistance Sources to Brown Blotch Disease (Pseudomonas tolaasii) in a Diverse Collection of Pleurotus Mushroom Strains. Pathogens 2019, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.A.; de Oliveira, M.S. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Palacios, I.; Moro, C.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; García-Lafuente, A.; Villares, A. Use of modified atmosphere packaging to preserve mushroom quality during storage. Recent Pat. Food Nutr. Agric. 2011, 3, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Villaescusa, R.; Gil, M. Quality improvement of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Azevedo, S.; Cunha, L.M.; Oliveira, J.C.; Mahajan, P.V.; Fonseca, S.C. Modelling the influence of time, temperature and relative humidity conditions on the mass loss rate of fresh oyster mushrooms. J. Food Eng. 2017, 212, 108–112. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Han, A.; Deng, Z.; Qi, Z.; Long, H.; Wang, J.; Yao, W.; et al. Advances in the Role and Mechanisms of Essential Oils and Plant Extracts as Natural Preservatives to Extend the Postharvest Shelf Life of Edible Mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Dama, C.L.; Kumar, S.; Mishra, B.K.; Shukla, K.B.; Mathur, S.; Doshi, A. Antioxidative Enzymatic Profile of Mushrooms Stored at Low Temperature. J. Food Sci. Technol. 2010, 47, 650–655. [Google Scholar] [CrossRef]
- bin Harun, A. Post Harvest Control for Maintenance of Quality Mushrooms. Food and Fertilizer Technology Center for the Asian and Pacific Region. 2017. Available online: https://ap.fftc.org.tw/article/1249 (accessed on 6 December 2023).
- Gui, H.; Zhao, M.; Zhang, S.; Yin, R.; Hu, C.; Fan, M.; Li, L. Active Antioxidant Packaging from Essential Oils Incorporated Polylactic Acid/Poly(butylene adipate-co-terephthalate)/Thermoplastic Starch for Preserving Straw Mushroom. Foods 2022, 11, 2252. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, F.; Ruiz, F. An optimal control approach to steam distillation of essential oils from aromatic plants. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- de Souza, E.L.; Lundgren, G.A.; de Oliveira, K.Á.R.; Berger, L.R.R.; Magnani, M. An Analysis of the Published Literature on the Effects of Edible Coatings Formed by Polysaccharides and Essential Oils on Postharvest Microbial Control and Overall Quality of Fruit. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1947–1967. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 228506. [Google Scholar] [CrossRef] [PubMed]
- Radünz, M.; Dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; da Rosa Zavareze, E. Antimicrobial potential of spray drying encapsulated thyme (Thymus vulgaris) essential oil on the conservation of hamburger-like meat products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef] [PubMed]
- Chagas, E.; Majolo, C.; Monteiro, P.; Oliveira, M.; Gama, P.; Bizzo, H.; Chaves, F. Composition of essential oils of Mentha species and their antimicrobial activity against Aeromonas spp. J. Essent. Oil Res. 2020, 32, 209–215. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Deng, Y.; Liu, T.; Lu, D. Effect of starch film containing thyme essential oil microcapsules on the physicochemical activity of mango. LWT 2020, 131, 109700. [Google Scholar] [CrossRef]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Fiorentino, D.; Frabboni, L.; Benvenuti, S.; Orlandini, G.; Pellati, F.; Gallone, A. Lavender and peppermint essential oils as effective mushroom tyrosinase inhibitors: A basic study. Flavour Fragr. J. 2011, 26, 441–446. [Google Scholar] [CrossRef]
- Aly, A.A.; Mohammed, M.K.; Maraei, R.W.; Abdalla, A.E.; Abouel-Yazeed, A.M. Improving the nutritional quality and bio-ingredients of stored white mushrooms using gamma irradiation and essential oils fumigation. Radiochim. Acta 2023, 111, 387–399. [Google Scholar] [CrossRef]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with Essential Oils Improves Sensory Quality and Enhances Antioxidant Ability of Shiitake Mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, S.; Uzun, Y.; Yilmaz, N.; Ercisli, S.; Eren, E.; Ekiert, H.; Elansary, H.O.; Szopa, A. Maintaining the Quality and Storage Life of Button Mushrooms (Agaricus bisporus) with Gum, Agar, Sodium Alginate, Egg White Protein, and Lecithin Coating. J. Fungi 2021, 7, 614. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Villalobos-Carvajal, R.; Reyes-Parra, J.; Jara-Quijada, E.; Ruiz, C.; Andrades, P.; Gacitúa, J.; Beldarraín-Iznaga, T. Preservation of mushrooms (Agaricus bisporus) by an alginate-based-coating containing a cinnamaldehyde essential oil nanoemulsion. Food Packag. Shelf Life 2021, 28, 100662. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Pérez-Santaescolástica, C.; Munekata, P.E.S.; Feng, X.; Liu, Y.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Active edible coatings and films with Mediterranean herbs to improve food shelf-life. Crit. Rev. Food Sci. Nutr. 2022, 62, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Feng, L.; Zheng, X. Effect of Chitosan Coating Enriched with Thyme Oil on Postharvest Quality and Shelf Life of Shiitake Mushroom (Lentinus edodes). J. Agric. Food Chem. 2012, 60, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of Chitosan and Guar Gum-Based Composite Edible Coating on Quality of Mushroom (Lentinus edodes) during Postharvest Storage. Sci. Hortic. 2019, 253, 382–389. [Google Scholar] [CrossRef]
- Shenbagam, A.; Kumar, N.; Rahul, K.; Upadhyay, A.; Gniewosz, M.; Kieliszek, M. Characterization of Aloe Vera Gel-Based Edible Coating with Orange Peel Essential Oil and Its Preservation Effects on Button Mushroom (Agaricus bisporus). Food Bioprocess Technol. 2023, 16, 2877–2897. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Joshi, K.; Warby, J.; Valverde, J.; Tiwari, B.; Cullen, P.J.; Frias, J.M. Impact of cold chain and product variability on quality attributes of modified atmosphere packed mushrooms (Agaricus bisporus) throughout distribution. J. Food Eng. 2018, 232, 44–55. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, 135, 3–11. Available online: https://eur-lex.europa.eu/eli/reg/2009/450/oj (accessed on 1 May 2024).
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Thermally buffered corrugated packaging for preserving the postharvest freshness of mushrooms (Agaricus bisporus). J. Food Eng. 2018, 216, 11–19. [Google Scholar] [CrossRef]
- Chang, C.-K.; Cheng, K.-C.; Hou, C.-Y.; Wu, Y.-S.; Hsieh, C.-W. Development of Active Packaging to Extend the Shelf Life of Agaricus bisporus by Using Plasma Technology. Polymers 2021, 13, 2120. [Google Scholar] [CrossRef] [PubMed]
- Shonte, T.T.; Mulla, M.F.; Foley, L.; Pathania, S. Mechanisms of Action and Preservation Effects of Packaging Systems for Mushrooms: Novel Approaches to Preserve Irish Edible Mushrooms. Coatings 2024, 14, 172. [Google Scholar] [CrossRef]
- Wrona, M.; Bentayeb, K.; Nerín, C. A novel active packaging for extending the shelf-life of fresh mushrooms (Agaricus bisporus). Food Control 2015, 54, 200–207. [Google Scholar] [CrossRef]
- Han Lyn, F.; Maryam Adilah, Z.A.; Nor-Khaizura, M.A.R.; Jamilah, B.; Nur Hanani, Z.A. Application of modified atmosphere and active packaging for oyster mushroom (Pleurotus ostreatus). Food Packag. Shelf Life 2020, 23, 100451. [Google Scholar] [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent Packaging for Real-Time Monitoring of Food-Quality: Current and Future Developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.-L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Li, H.; Zhong, Y. Novel colorimetric films based on polyvinyl alcohol/sodium carboxymethyl cellulose doped with anthocyanins and betacyanins to monitor pork freshness. Food Chem. 2023, 404, 134426. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Mu, H.; Gao, H.; Chen, H.; Wu, W.; Fang, X.; Liu, R.; Niu, B. Preparation of PVA/PLA-based intelligent packaging to indicate the quality of shiitake mushrooms. J. Agric. Food Res. 2023, 12, 100589. [Google Scholar] [CrossRef]
- Liu, L.; Wu, W.; Zheng, L.; Yu, J.; Sun, P.; Shao, P. Intelligent packaging films incorporated with anthocyanins-loaded ovalbumin-carboxymethyl cellulose nanocomplexes for food freshness monitoring. Food Chem. 2022, 387, 132908. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chávez, A.M.; Alberti, M.M.; Albertó, E. Evaluation of ligninolytic activity in spent mushroom substrate from four cultivated mushrooms. J. Bioresour. Bioprod. 2022, 7, 288–294. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Chang, B.-V.; Fan, S.-N.; Tsai, Y.-C.; Chung, Y.-L.; Tu, P.-X.; Yang, C.-W. Removal of emerging contaminants using spent mushroom compost. Sci. Total Environ. 2018, 634, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.; Alba-Elías, F. Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus. Water 2019, 11, 454. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Andrades, M.S.; Villalba Eguren, G.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Organic Amendment for the Recovery of Vineyard Soils: Effects of a Single Application on Soil Properties over Two Years. Processes 2022, 10, 317. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, M.; Li, R.; Zhang, X.; Wang, G.; Liu, X.; Qu, J.; Jin, Y. Spent mushroom substrate combined with alkaline amendment passivates cadmium and improves soil property. Environ. Sci. Pollut. Res. Int. 2020, 27, 16317–16325. [Google Scholar] [CrossRef] [PubMed]
- García-Delgado, C.; Jiménez-Ayuso, N.; Frutos, I.; Gárate, A.; Eymar, E. Cadmium and lead bioavailability and their effects on polycyclic aromatic hydrocarbons biodegradation by spent mushroom substrate. Environ. Sci. Pollut. Res. 2013, 20, 8690–8699. [Google Scholar] [CrossRef] [PubMed]
- Zied, D.C.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of spent mushroom substrate in new mushroom crops to promote the transition towards a circular economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Hu, W.; Di, Q.; Liang, T.; Liu, J.; Zhang, J. Effects of spent mushroom substrate biochar on growth of oyster mushroom (Pleurotus ostreatus). Environ. Technol. Innov. 2022, 28, 102729. [Google Scholar] [CrossRef]
- Rajavat, A.S.; Rai, S.; Pandiyan, K.; Kushwaha, P.; Choudhary, P.; Kumar, M.; Chakdar, H.; Singh, A.; Karthikeyan, N.; Bagul, S.Y.; et al. Sustainable use of the spent mushroom substrate of Pleurotus florida for production of lignocellulolytic enzymes. J. Basic Microbiol. 2020, 60, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Grujić, M.; Dojnov, B.; Potočnik, I.; Duduk, B.; Vujčić, Z. Spent mushroom compost as substrate for the production of industrially important hydrolytic enzymes by fungi Trichoderma spp. and Aspergillus niger in solid state fermentation. Int. Biodeterior. Biodegrad. 2015, 104, 290–298. [Google Scholar] [CrossRef]
- He, J.; Qiu, Y.; Ji, X.; Liu, X.; Qiu, Z.; Xu, J.; Xia, J. A novel strategy for producing cellulase from Trichoderma reesei with ultrasound-assisted fermentation using spent mushroom substrate. Process Biochem. 2021, 104, 110–116. [Google Scholar] [CrossRef]
- Branà, M.; Sergio, L.; Haidukowski, M.; Logrieco, A.; Altomare, C. Degradation of Aflatoxin B1 by a Sustainable Enzymatic Extract from Spent Mushroom Substrate of Pleurotus eryngii. Toxins 2020, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.; Almeida, M.; Paié-Ribeiro, J.; Barros, A.N.; Rodrigues, M. Unlocking the Potential of Spent Mushroom Substrate (SMS) for Enhanced Agricultural Sustainability: From Environmental Benefits to Poultry Nutrition. Life 2023, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.H.; Kim, Y.I.; Ahmadi, F.; Oh, Y.K.; Park, J.M.; Kwak, W.S. Effect of microbial inoculant or molasses on fermentative quality and aerobic stability of sawdust-based spent mushroom substrate. Bioresour. Technol. 2016, 216, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Kim, M.; Reddy, K.E.; Oh, Y.; Jung, Y.; Yeo, J.; Choi, H. Rumen fermentation and digestibility of spent mushroom (Pleurotus ostreatus) substrate inoculated with Lactobacillus brevis for Hanwoo steers. Rev. Colomb. Cienc. Pecu. 2017, 30, 267–277. [Google Scholar] [CrossRef]
- Nakatsuka, H.; Oda, M.; Hayashi, Y.; Tamura, K. Effects of fresh spent mushroom substrate of Pleurotus ostreatus on soil micromorphology in Brazil. Geoderma 2016, 269, 54–60. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Zhou, X.; Baig, S.A.; Hu, B.; Xu, X. Composition variability of spent mushroom substrates during continuous cultivation, composting process and their effects on mineral nitrogen transformation in soil. Geoderma 2017, 307, 30–37. [Google Scholar] [CrossRef]
- Lopes, A.D.; de Melo Santana Gomes, S.; Schwengber, R.P.; Carpi, M.C.G.; Dias-Arieira, C.R. Control of Meloidogyne javanica with Pleurotus djamor spent mushroom substrate. Chem. Biol. Technol. Agric. 2023, 10, 13. [Google Scholar] [CrossRef]
- Huang, Z.; Guan, H.; Zheng, H.; Wang, M.; Xu, P.; Dong, S.; Yang, Y.; Xiao, J. Novel liquid organic fertilizer: A potential way to effectively recycle spent mushroom substrate. J. Clean. Prod. 2022, 376, 134368. [Google Scholar] [CrossRef]
- Van Tam, N.; Wang, C.-H. Use of Spent Mushroom Substrate and Manure Compost for Honeydew Melon Seedlings. J. Plant Growth Regul. 2015, 34, 417–424. [Google Scholar] [CrossRef]
- Najafi, B.; Faizollahzadeh Ardabili, S.; Shamshirband, S.; Chau, K. Spent mushroom compost (SMC) as a source for biogas production in Iran. Eng. Appl. Comput. Fluid Mech. 2019, 13, 967–982. [Google Scholar] [CrossRef]
- Grover, R.; Goel, A.; Wati, L.; Raj, K. Ethanol production from spent oyster mushroom substrate. Pollut. Res. 2015, 34, 121–124. Available online: https://www.researchgate.net/publication/283131084 (accessed on 29 April 2024).
- de Almeida Moreira, B.R.; da Silva Viana, R.; Magalhães, A.C.; Caraschi, J.C.; Zied, D.C.; Dias, E.S.; Rinker, D.L. Production of Pleurotus ostreatus var. florida on briquettes and recycling its spent substrate as briquettes for fuel grade biosolids. J. Clean. Prod. 2020, 274, 123919. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Halim-Lim, S.A.; Kamarudin, N.Z.; Rukayadi, Y.; Abd Rahim, M.H.; Jamaludin, A.A.; Ilham, Z. Fruiting-body-base flour from an Oyster mushroom waste in the development of antioxidative chicken patty. J. Food Sci. 2020, 85, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Wan-Mohtar, W.A.A.Q.I.; Mahmud, N.; Supramani, S.; Ahmad, R.; Zain, N.A.M.; Hassan, N.A.M.; Peryasamy, J.; Halim-Lim, S.A. Fruiting-body-base flour from an oyster mushroom—A waste source of antioxidative flour for developing potential functional cookies and steamed-bun. AIMS Agric. Food 2018, 3, 481–492. [Google Scholar] [CrossRef]
- Wang, L.; Brennan, M.A.; Guan, W.; Liu, J.; Zhao, H.; Brennan, C.S. Edible mushrooms dietary fibre and antioxidants: Effects on glycaemic load manipulation and their correlations pre-and post-simulated in vitro digestion. Food Chem. 2021, 351, 129320. [Google Scholar] [CrossRef] [PubMed]
- Harada-Padermo, S.D.S.; Dias-Faceto, L.S.; Selani, M.M.; Alvim, I.D.; Floh, E.I.S.; Macedo, A.F.; Bogusz, S.; Dias, C.T.D.S.; Conti-Silva, A.C.; Vieira, T.M.F.S. Umami Ingredient: Flavor enhancer from shiitake (Lentinula edodes) byproducts. Food Res. Int. 2020, 137, 109540. [Google Scholar] [CrossRef] [PubMed]
- Van Ba, H.; Seo, H.-W.; Cho, S.-H.; Kim, Y.-S.; Kim, J.-H.; Ham, J.-S.; Park, B.Y.; Pil-Nam, S. Antioxidant and anti-foodborne bacteria activities of shiitake by-product extract in fermented sausages. Food Control 2016, 70, 201–209. [Google Scholar] [CrossRef]
- Van Ba, H.; Seo, H.-W.; Cho, S.-H.; Kim, Y.-S.; Kim, J.-H.; Ham, J.-S.; Park, B.Y.; Pil-Nam, S. Effects of extraction methods of shiitake by-products on their antioxidant and antimicrobial activities in fermented sausages during storage. Food Control 2017, 79, 109–118. [Google Scholar] [CrossRef]
- Banerjee, D.K.; Das, A.K.; Banerjee, R.; Pateiro, M.; Nanda, P.K.; Gadekar, Y.P.; Biswas, S.; McClements, D.J.; Lorenzo, J.M. Application of Enoki Mushroom (Flammulina Velutipes) Stem Wastes as Functional Ingredients in Goat Meat Nuggets. Foods 2020, 9, 432. [Google Scholar] [CrossRef]
- Gonzalez, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez-Jasso, R.M.; Contreras, J.C.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef] [PubMed]



| Taxonomic Rank | Classification |
|---|---|
| Kingdom | Fungi |
| Phylum | Basidiomycota |
| Class | Agaricomycetes |
| Order | Agaricales |
| Family | Pleurotaceae |
| Genus | Pleurotus |
| Species | Pleurotus ostreatus |
| Parameters | Incubation | Primordia Formation | Basidioma Development |
|---|---|---|---|
| Temperature (°C) | 24 | 10–15 | 10–21 |
| RH (%) | 85–95 | 95–100 | 85–90 |
| [CO2] (ppm) | 5000–20,000 | <1000 | <1000 |
| Time (days) | 12–21 | 3–5 | 4–7 |
| Luminosity (Lux) | - | 1000–1500 | 1000–1500 |
| Essential Oil | Mushroom Species | Research Outcome | References |
|---|---|---|---|
| Clove and peppermint EOs | Volvariella volvacea | The active antioxidant packaging composed of EOs was effective mainly with peppermint oil. | [116] |
| Eugenol, bergamot, and grapefruit EOs | Agaricus bisporus | Vaporized EOs within the MAP reduce the quality loss of sliced mushrooms during postharvest storage. | [105] |
| Clove, cinnamaldehyde, and thyme EOs | Lentinula edodes | EO fumigation maintained sensory quality during storage and increased AOx. | [130] |
| Cinnamon EO | Agaricus bisporus | Bioactive food packaging was suitable for extending the shelf-life of high-moisture-content products. | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Pre- and Postharvest Strategies for Pleurotus ostreatus Mushroom in a Circular Economy Approach. Foods 2024, 13, 1464. https://doi.org/10.3390/foods13101464
Silva M, Ramos AC, Lidon FJ, Reboredo FH, Gonçalves EM. Pre- and Postharvest Strategies for Pleurotus ostreatus Mushroom in a Circular Economy Approach. Foods. 2024; 13(10):1464. https://doi.org/10.3390/foods13101464
Chicago/Turabian StyleSilva, Mafalda, Ana Cristina Ramos, Fernando J. Lidon, Fernando H. Reboredo, and Elsa M. Gonçalves. 2024. "Pre- and Postharvest Strategies for Pleurotus ostreatus Mushroom in a Circular Economy Approach" Foods 13, no. 10: 1464. https://doi.org/10.3390/foods13101464






