Evaluation of Antimicrobial and Preservative Effects of Cinnamaldehyde and Clove Oil in Catfish (Ictalurus punctatus) Fillets Stored at 4 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antimicrobials Formulation
2.2. Bacterial Strains, Growth, Inoculum Preparation and Zone of Inhibition
2.3. Bacterial Death Curve
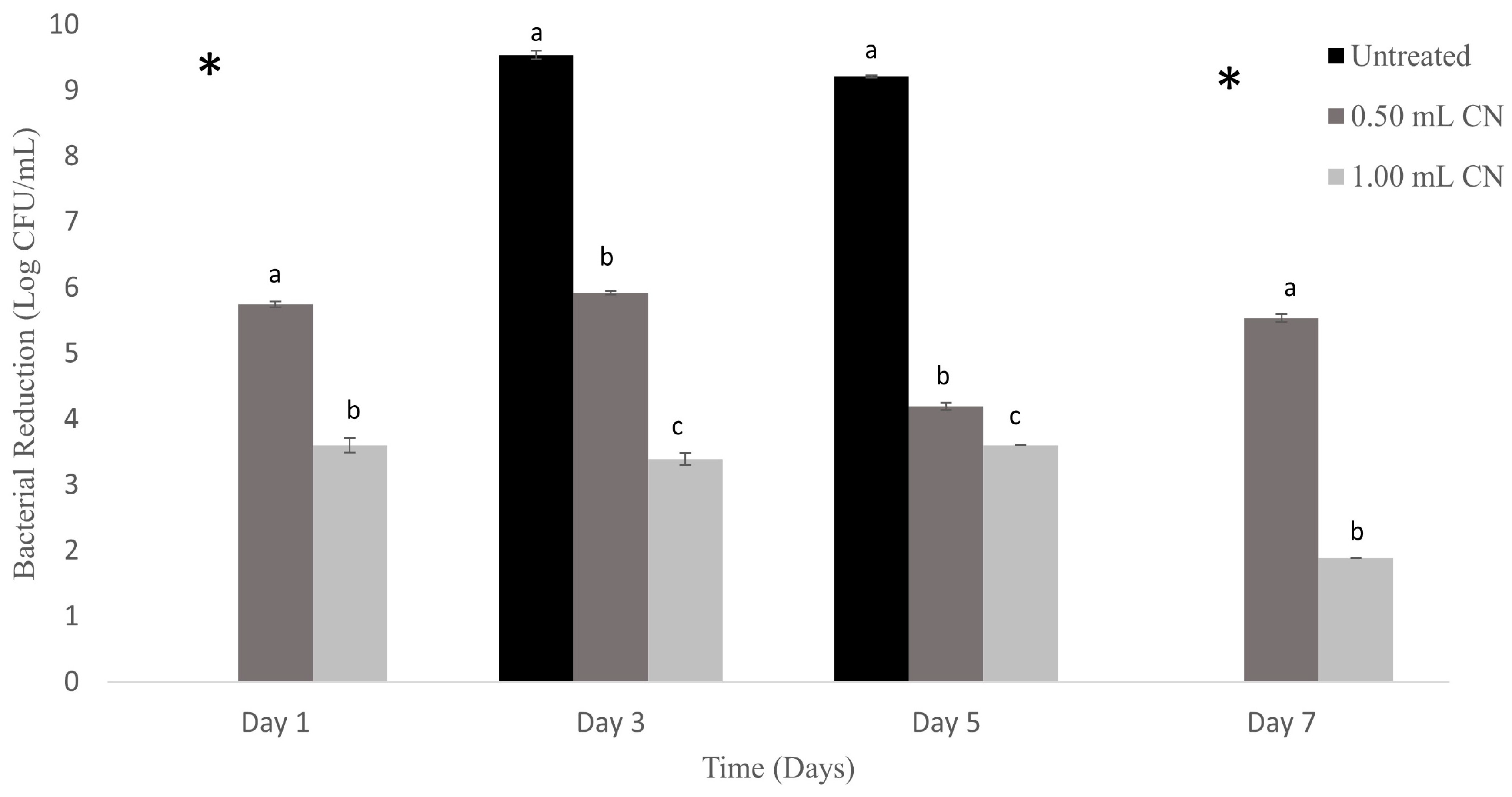
2.4. Absorbent Food Pad Inoculation
2.5. Statistical Analysis
3. Results
3.1. Zone of Inhibition
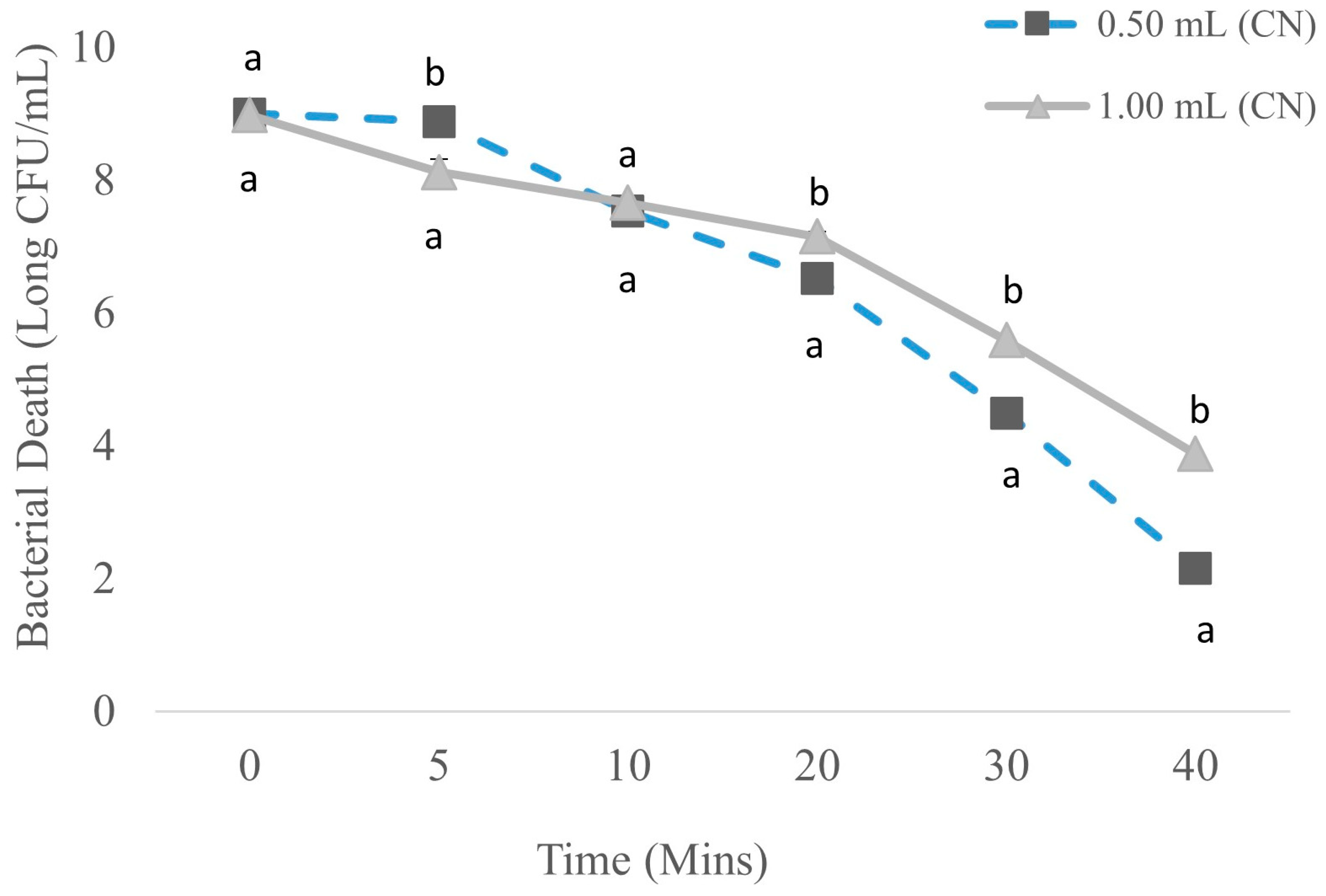
3.2. Bacterial Death Curve
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, P.; Sharma, M.; Kewat, A. Spoilage in Fish and Shellfish Products. Limnology 2022, 2, 59. [Google Scholar]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Pradesh, U. Significance of fish nutrients for human health. Int. J. Fish. Aquat. Res. 2020, 5, 47–49. [Google Scholar]
- Venegas-Calerón, M.; Napier, J.A. New alternative sources of omega-3 fish oil. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2023; pp. 343–398. [Google Scholar]
- Krittanawong, C.; Isath, A.; Hahn, J.; Wang, Z.; Narasimhan, B.; Kaplin, S.L.; Jneid, H.; Virani, S.S.; Tang, W.W. Fish consumption and cardiovascular health: A systematic review. Am. J. Med. 2021, 134, 713–720. [Google Scholar] [PubMed]
- Qiu, Q.; Dewey-Mattia, D.; Subramhanya, S.; Cui, Z.; Griffin, P.M.; Lance, S.; Lanier, W.; Wise, M.E.; Crowe, S.J. Food recalls associated with foodborne disease outbreaks, United States, 2006–2016. Epidemiol. Infect. 2021, 149, e190. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Parisi, A.; Conversano, M.C.; Iannacci, A.; D’Emilio, F.; Mercurio, V.; Normanno, G. Food-borne bacteria associated with seafoods: A brief review. J. Food Qual. Hazards Control 2020. [Google Scholar]
- Garcia, S.M.; Rosenberg, A.A. Food security and marine capture fisheries: Characteristics, trends, drivers and future perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2869–2880. [Google Scholar] [CrossRef] [PubMed]
- Guillen, J.; Natale, F.; Carvalho, N.; Casey, J.; Hofherr, J.; Druon, J.-N.; Fiore, G.; Gibin, M.; Zanzi, A.; Martinsohn, J.T. Global seafood consumption footprint. Ambio 2019, 48, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Bennett, N.J.; Le Billon, P.; Green, S.J.; Cisneros-Montemayor, A.M.; Amongin, S.; Gray, N.J.; Sumaila, U.R. Oil, fisheries and coastal communities: A review of impacts on the environment, livelihoods, space and governance. Energy Res. Soc. Sci. 2021, 75, 102009. [Google Scholar] [CrossRef]
- Gephart, J.A.; Golden, C.D.; Asche, F.; Belton, B.; Brugere, C.; Froehlich, H.E.; Fry, J.P.; Halpern, B.S.; Hicks, C.C.; Jones, R.C.; et al. Scenarios for global aquaculture and its role in human nutrition. Rev. Fish. Sci. Aquac. 2020, 29, 122–138. [Google Scholar] [CrossRef]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2021, 123, 107697. [Google Scholar] [CrossRef]
- Ekonomou, S.; Parlapani, F.; Kyritsi, M.; Hadjichristodoulou, C.; Boziaris, I. Preservation status and microbial communities of vacuum-packed hot smoked rainbow trout fillets. Food Microbiol. 2022, 103, 103959. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Hong, H.; Zhang, L.; Luo, Y. Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 252–288. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh fish degradation and advances in preservation using physical emerging technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kołakowska, A.; Burt, J.R. Postharvest biochemical and microbial changes. In Seafood; CRC Press: Boca Raton, FL, USA, 2020; pp. 55–75. [Google Scholar]
- Kumar, K. Food Processing and Preservation; Scientific Publisher: Hackensack, NJ, USA, 2020; p. 13. [Google Scholar]
- Wu, T.; Wang, M.; Wang, P.; Tian, H.; Zhan, P. Advances in the formation and control methods of undesirable flavors in fish. Foods 2022, 11, 2504. [Google Scholar] [CrossRef]
- Ebirim, R.I. Spoilage and Sensory Observation of Cinnamaldehyde and Clove Oil Application to Control Microbial Populations in Catfish (Ictalurus punctatus) and Trout (Oncorhynchus mykiss) Fillet Packaging. Master’s Thesis, Delaware State University ProQuest Dissertations Publishing, Delaware State University, Dover, DE, USA, 2020. [Google Scholar]
- Huang, Y.; Zhang, H.; Lv, B.; Tang, C.; Du, J.; Jin, H. Sulfur dioxide: Endogenous generation, biological effects, detection, and therapeutic potential. Antioxid. Redox Signal. 2022, 36, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.D. Human health effects of exposure to nitrate, nitrite, and nitrogen dioxide. In Just Enough Nitrogen: Perspectives on How to Get There for Regions with too Much and too Little Nitrogen; The Institute of Food Technologist: Chicago, IL, USA, 2020; pp. 283–294. [Google Scholar]
- Singh, V.P. Recent approaches in food bio-preservation-a review. Open Vet. J. 2018, 8, 104–111. [Google Scholar] [CrossRef]
- Ranathunga, N.S.; Wijayasekara, K.N.; Abeyrathne, E.D.N.S. Application of bio-preservation to enhance food safety: A review. Food Sci. Preserv. 2023, 30, 179–189. [Google Scholar] [CrossRef]
- Hussein, A.R. Foods bio-preservation: A review. Int. J. Res. Appl. Sci. Biotechnol. 2022, 9, 212–217. [Google Scholar]
- Kirchner, M.T.; Bläser, D.; Boese, R.; Thakur, T.S.; Desiraju, G.R. Weak C—H⋯ O hydrogen bonds in anisaldehyde, salicylaldehyde and cinnamaldehyde. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2011, 67, o387–o390. [Google Scholar] [CrossRef]
- Ashakirin, S.N.; Tripathy, M.; Patil, U.K.; Majeed, A.B.A. Chemistry and bioactivity of cinnamaldehyde: A natural molecule of medicinal importance. Int. J. Pharm. Sci. Res. 2017, 8, 2333–2340. [Google Scholar]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Gooderham, N.J.; Cohen, S.M.; Eisenbrand, G.; Fukushima, S.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS assessment of natural flavor complexes: Clove, cinnamon leaf and West Indian bay leaf-derived flavoring ingredients. Food Chem. Toxicol. 2020, 145, 111585. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Pereira, J.A.; Silva, P.; Perestrelo, R.; Câmara, J.S. Food fingerprints–A valuable tool to monitor food authenticity and safety. Food Chem. 2019, 278, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Suppakul, P. Cinnamaldehyde and eugenol: Use in antimicrobial packaging. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 479–490. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Long, W., III; Sarker, M.I.; Liu, C.K. Cinnamaldehyde/Lactic Acid Spray Wash Treatment for Meat Safety and Byproduct Quality Assurance. Am. J. Food Sci. Technol. 2018, 6, 280–289. [Google Scholar]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [PubMed]
- Zamuner, C.F.; Dilarri, G.; Bonci, L.C.; Saldanha, L.L.; Behlau, F.; Marin, T.G.; Sass, D.C.; Bacci, M.; Ferreira, H. A cinnamaldehyde-based formulation as an alternative to sodium hypochlorite for post-harvest decontamination of citrus fruit. Trop. Plant Pathol. 2020, 45, 701–709. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and antimicrobial activity of biodegradable active packaging enriched with clove and thyme essential oil for food packaging application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Gengatharan, A.; Rahim, M.H.A. The application of clove extracts as a potential functional component in active food packaging materials and model food systems: A mini-review. Appl. Food Res. 2023, 3, 100283. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Hossen Jenia, S. Study on Nutritional Composition, Bioactive Compounds, Antioxidant and Antimicrobial Activity of the Clove (Syzygium Aromaticum). Master’s Thesis, Chattogram Veterinary and Animal Sciences University, Chattogram, Bangladesh, 2019. [Google Scholar]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. Lipids Essent. Oils Antimicrob. Agents 2011, 203–238. [Google Scholar]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Diogo, R.; Oliveira, C.; Chardon, M. The origin and transformation of the palatine-maxillary system of catfish (Teleostei: Siluriformes): An example of macroevolution. Neth. J. Zool. 2000, 50, 373–388. [Google Scholar] [CrossRef]
- Gormaz, J.G.; Fry, J.P.; Erazo, M.; Love, D.C. Public health perspectives on aquaculture. Curr. Environ. Health Rep. 2014, 1, 227–238. [Google Scholar] [CrossRef]
- Triantafyllidis, A.; Krieg, F.; Cottin, C.; Abatzopoulos, T.J.; Triantaphyllidis, C.; Guyomard, R. Genetic structure and phylogeography of European catfish (Silurus glanis) populations. Mol. Ecol. 2002, 11, 1039–1055. [Google Scholar] [CrossRef]
- Day, J.J.; Wilkinson, M. On the origin of the Synodontis catfish species flock from Lake Tanganyika. Biol. Lett. 2006, 2, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Dauda, A.B.; Natrah, I.; Karim, M.; Kamarudin, M.S.; Bichi, A.U.H. African catfish aquaculture in Malaysia and Nigeria: Status, trends and prospects. Fish. Aquac. J. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Castrica, M.; Miraglia, D.; Menchetti, L.; Branciari, R.; Ranucci, D.; Balzaretti, C.M. Antibacterial effect of an active absorbent pad on fresh beef meat during the shelf-life: Preliminary results. Appl. Sci. 2020, 10, 7904. [Google Scholar] [CrossRef]
- Pettersen, M.K.; Nilsen-Nygaard, J.; Hansen, A.; Carlehög, M.; Liland, K.H. Effect of liquid absorbent pads and packaging parameters on drip loss and quality of chicken breast fillets. Foods 2021, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Hayden, M.; Qiao, M.; Huang, T.-S.; Ren, X.; Weese, J. Absorbent pads containing N-halamine compound for potential antimicrobial use for chicken breast and ground chicken. J. Agric. Food Chem. 2018, 66, 1941–1948. [Google Scholar] [CrossRef]
- Gouvêa, D.M.; Mendonça, R.C.S.; Lopez, M.E.S.; Batalha, L.S. Absorbent food pads containing bacteriophages for potential antimicrobial use in refrigerated food products. LWT-Food Sci. Technol. 2016, 67, 159–166. [Google Scholar] [CrossRef]
- Kilinc, B.; Altas, S. Effect of absorbent pads containing black seed or rosemary oils on the shelf life of sardine [Sardina pilchardus (Walbaum, 1792)] fillets. J. Appl. Ichthyol. 2016, 32, 552–558. [Google Scholar] [CrossRef]
- Oral, N.; Vatansever, L.; Sezer, Ç.; Aydın, B.; Güven, A.; Gülmez, M.; Başer, K.H.C.; Kürkçüoğlu, M. Effect of absorbent pads containing oregano essential oil on the shelf life extension of overwrap packed chicken drumsticks stored at four degrees Celsius. Poult. Sci. 2009, 88, 1459–1465. [Google Scholar] [CrossRef]
- Ren, T.; Qiao, M.; Huang, T.-S.; Weese, J.; Ren, X. Efficacy of N-halamine compound on reduction of microorganisms in absorbent food pads of raw beef. Food Control 2018, 84, 255–262. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of cinnamaldehyde and eugenol and their activity after incorporation into cellulose-based packaging films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; El-Boshra, I.M.; El-Khalifa, E.A. Nutritive value of clove (Syzygium aromaticum) and detection of antimicrobial effect of its bud oil. Res. J. Microbiol. 2007, 2, 266–271. [Google Scholar]


| Treatment Conc. (mL/mL) | ATCC Aeromonas hydrophila and CN | |||
| Trial 1 (cm) | Trial 2 (cm) | Trial 3 (cm) | Average | |
| 0.125 | 1.00 | 1.00 | 1.00 | 1.00 ± 0.00 a |
| 0.25 | 1.40 | 1.50 | 1.30 | 1.40 ± 0.10 c |
| 0.50 | 1.70 | 1.80 | 1.60 | 1.70 ± 0.01 d |
| 0.75 | 1.90 | 1.90 | 1.80 | 1.85 ± 0.07 de |
| 1.00 | 2.00 | 2.30 | 2.00 | 2.10 ± 0.17 e |
| ATCC Aeromonas hydrophila and CO | ||||
| Trial 1 (cm) | Trial 2 (cm) | Trial 3 (cm) | Average | |
| 0.125 | 1.00 | 1.10 | 1.00 | 1.03 ± 0.05 a |
| 0.25 | 1.10 | 1.30 | 1.30 | 1.23 ± 0.11 b |
| 0.50 | 1.50 | 1.50 | 1.50 | 1.50 ± 0.00 c |
| 0.75 | 1.70 | 2.00 | 2.00 | 1.90 ± 0.17 e |
| 1.00 | 2.00 | 2.00 | 1.90 | 1.96 ± 0.05 e |
| Treatment Conc. (mL/mL) | NTCC 10735, Shewanella baltica and CN | |||
| Trial 1 (cm) | Trial 2 (cm) | Trial 3 (cm) | Average | |
| 0.125 | 1.00 | 1.10 | 1.00 | 1.03 ± 0.06 a |
| 0.25 | 1.20 | 1.10 | 1.00 | 1.10 ± 0.10 a |
| 0.50 | 2.00 | 1.90 | 1.80 | 1.90 ± 0.01 b |
| 0.75 | 2.20 | 2.00 | 2.00 | 2.07 ± 0.12 b |
| 1.00 | 2.30 | 2.20 | 2.00 | 2.17 ± 0.15 b |
| NTCC 10735 Shewanella baltica and CO | ||||
| Trial 1 (cm) | Trial 2 (cm) | Trial 3 (cm) | Average | |
| 0.125 | 0.80 | 0.80 | 0.80 | 0.80 ± 0.00 a |
| 0.25 | 1.80 | 1.70 | 1.75 | 1.75 ± 0.07 b |
| 0.50 | 2.50 | 2.70 | 2.60 | 2.60 ± 0.14 c |
| 0.75 | 2.80 | 2.60 | 2.70 | 2.70 ± 0.14 c |
| 1.00 | 2.90 | 3.00 | 2.95 | 2.90 ± 0.07 c |
| Treatment Conc. (mL/mL) | Catfish and CN | |||
| Trial 1 (cm) | Trial 2 (cm) | Trial 3 (cm) | Average | |
| 0.125 | 0.80 | 0.80 | 0.80 | 0.80 ± 0.00 a |
| 0.25 | 0.90 | 0.90 | 0.90 | 0.90 ± 0.00 ab |
| 0.50 | 1.00 | 1.00 | 1.00 | 1.00 ± 0.00 ab |
| 0.75 | 1.00 | 1.00 | 1.10 | 1.03 ± 0.05 bc |
| 1.00 | 1.10 | 1.20 | 1.00 | 1.10 ± 0.10 bc |
| Catfish and CO | ||||
| Trial 1 (cm) | Trial 2 (cm) | Trial 3 (cm) | Average | |
| 0.125 | 1.00 | 1.10 | 1.00 | 1.03 ± 0.05 bc |
| 0.25 | 1.10 | 1.30 | 1.30 | 1.23 ± 0.11 c |
| 0.50 | 1.50 | 1.50 | 1.50 | 1.50 ± 0.00 d |
| 0.75 | 1.70 | 2.00 | 2.00 | 1.90 ± 0.17 e |
| 1.00 | 2.00 | 2.00 | 1.90 | 1.96 ± 0.05 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebirim, R.I.; Long, W., III. Evaluation of Antimicrobial and Preservative Effects of Cinnamaldehyde and Clove Oil in Catfish (Ictalurus punctatus) Fillets Stored at 4 °C. Foods 2024, 13, 1445. https://doi.org/10.3390/foods13101445
Ebirim RI, Long W III. Evaluation of Antimicrobial and Preservative Effects of Cinnamaldehyde and Clove Oil in Catfish (Ictalurus punctatus) Fillets Stored at 4 °C. Foods. 2024; 13(10):1445. https://doi.org/10.3390/foods13101445
Chicago/Turabian StyleEbirim, Rosemary I., and Wilbert Long, III. 2024. "Evaluation of Antimicrobial and Preservative Effects of Cinnamaldehyde and Clove Oil in Catfish (Ictalurus punctatus) Fillets Stored at 4 °C" Foods 13, no. 10: 1445. https://doi.org/10.3390/foods13101445





