Effects of Mactra chinenesis Peptides on Alcohol-Induced Acute Liver Injury and Intestinal Flora in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MCP
2.3. Determination of Molecular Weight Distribution and Total Amino Acid Composition
2.4. Measurement of MCP-Mediated Liver-Protective Effects in Mice
Experimental Animals and Treatment
2.5. LORR and Liver Index Assay
2.6. Determination of Biochemical Indicators
2.7. Histopathologic Analysis
2.8. qRT-PCR Assay
2.9. Western Blotting
2.10. High-Throughput Sequencing of Intestinal Flora
2.11. Statistical Analysis
3. Results
3.1. Molecular Weight Distribution and Amino Acid Compositions of Extracted Peptides
3.2. Effects of MCPs on Acute Alcohol Intoxication Tolerance and Body Weight in Mice
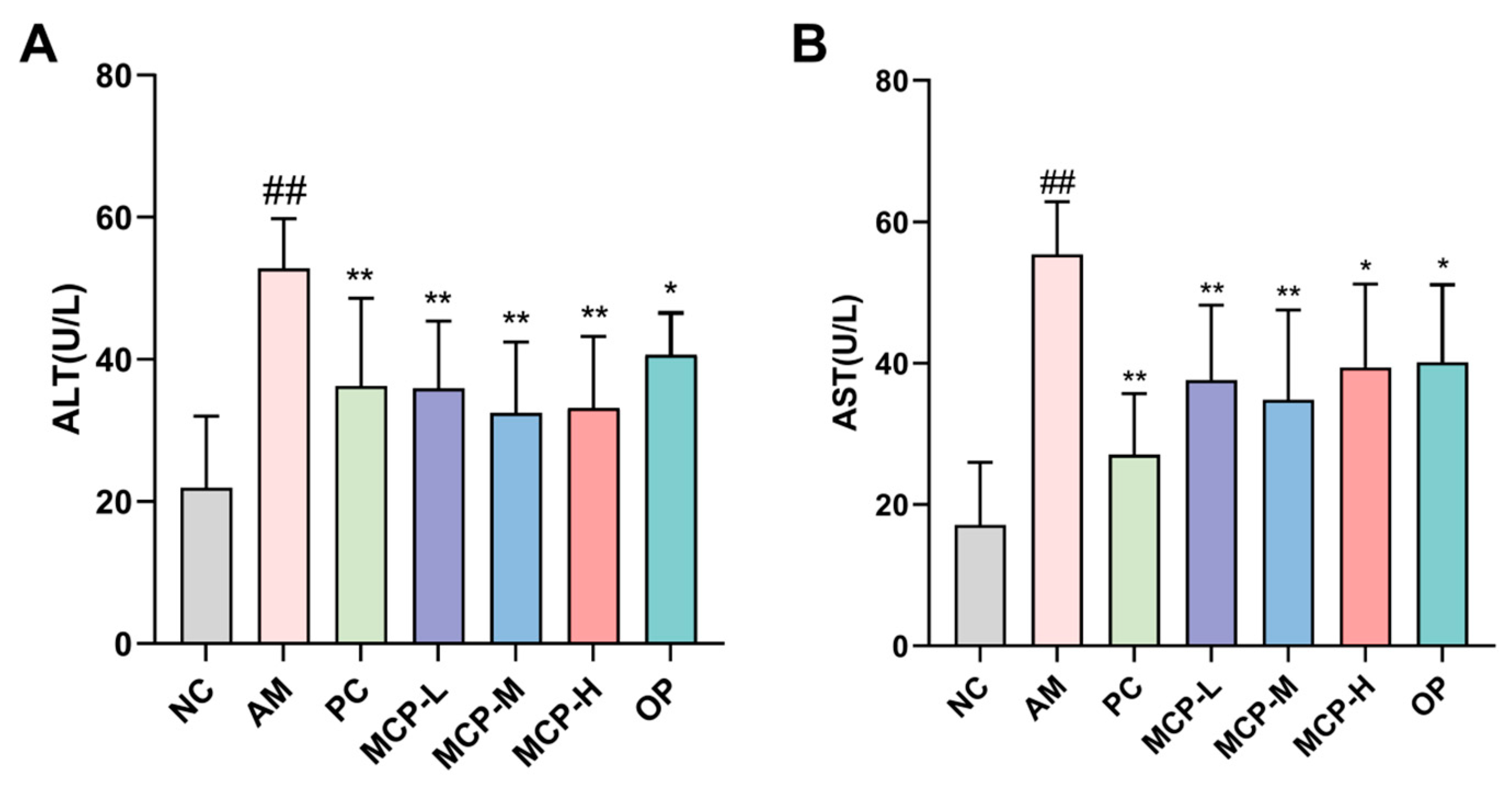
3.3. Effects of MCPs on the ALT and AST Levels in Serum
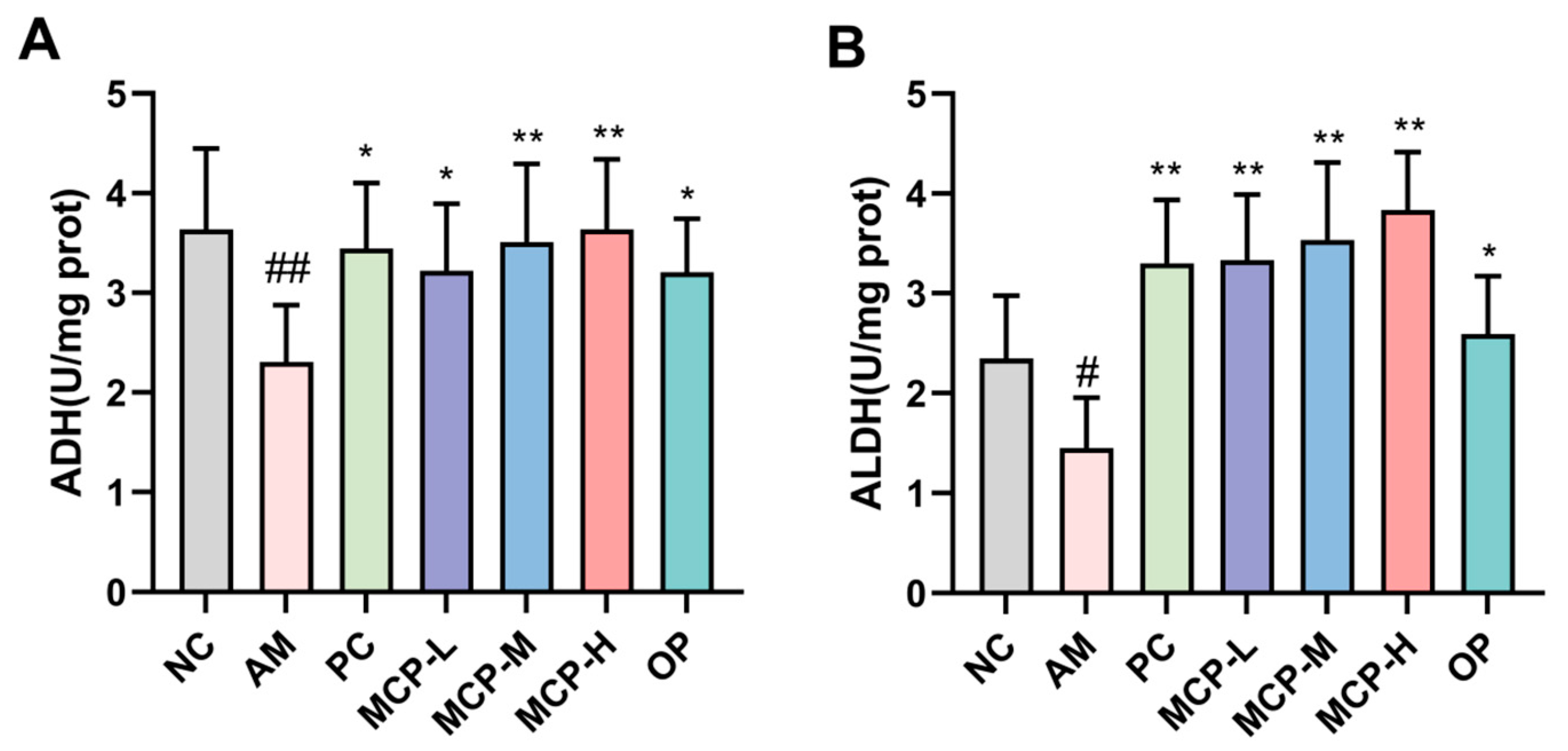
3.4. Effects of MCPs on Alcohol-Metabolizing Enzymes
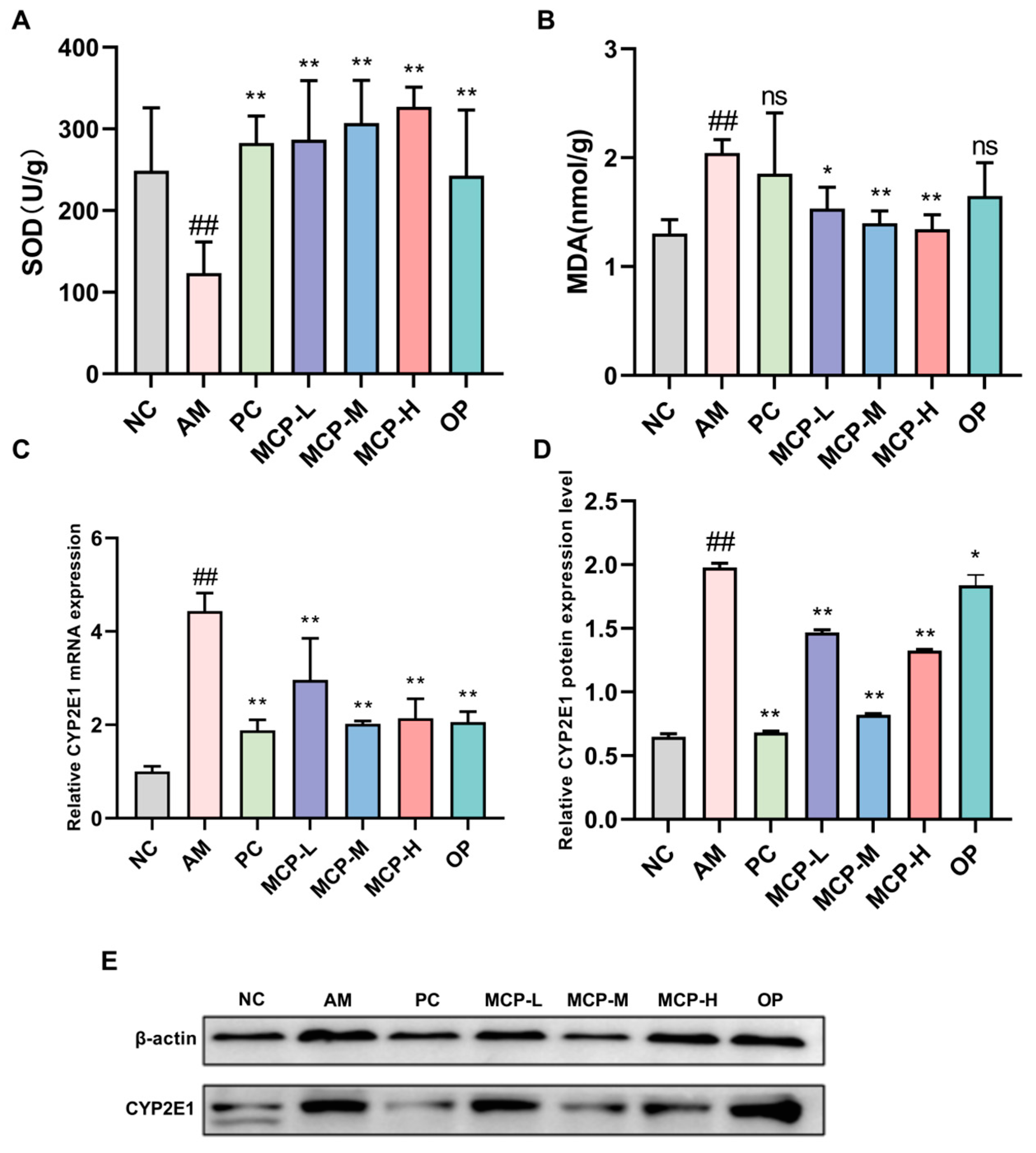
3.5. MCPs Improve Oxidative Stress in ALD Mice
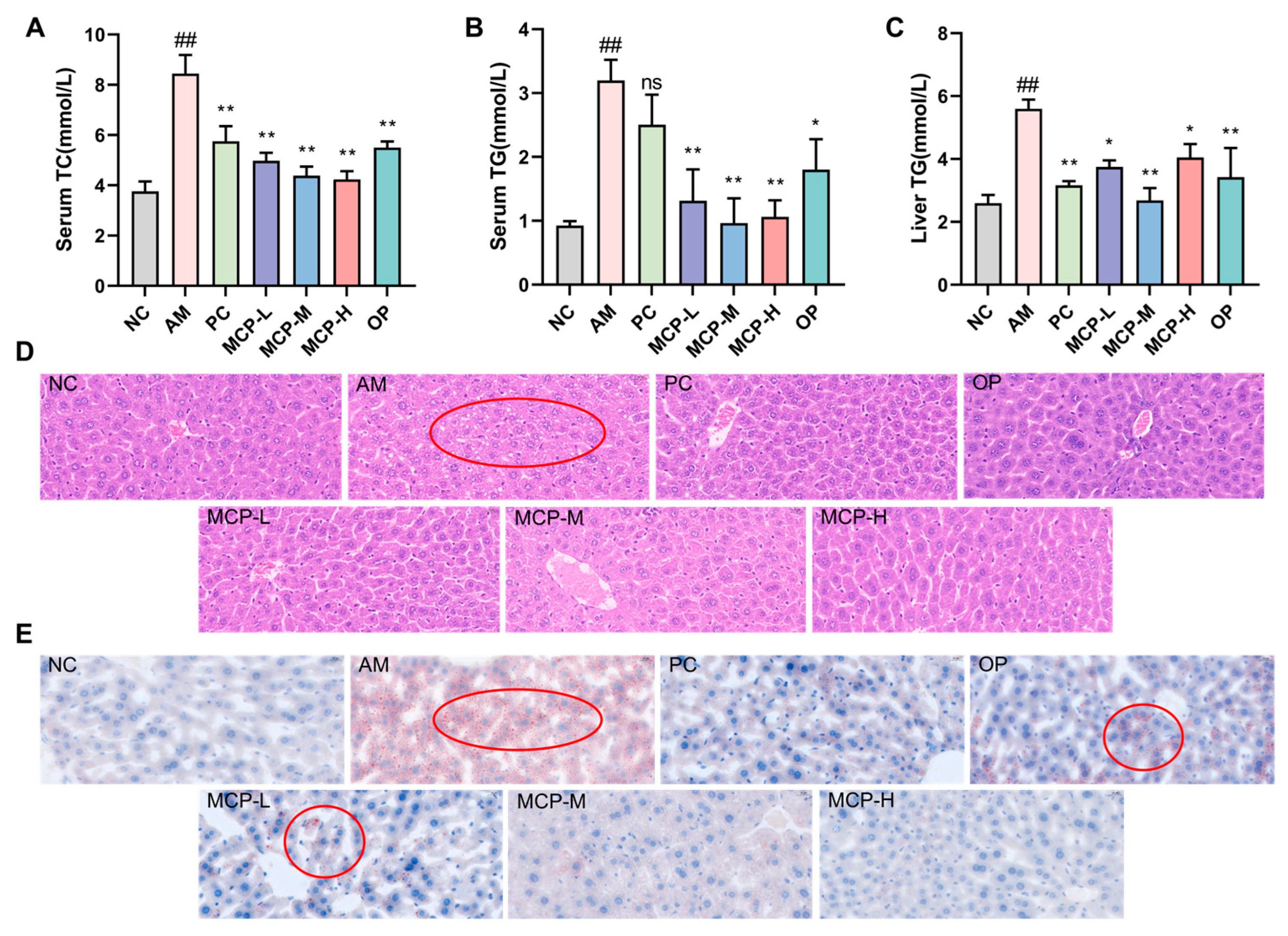
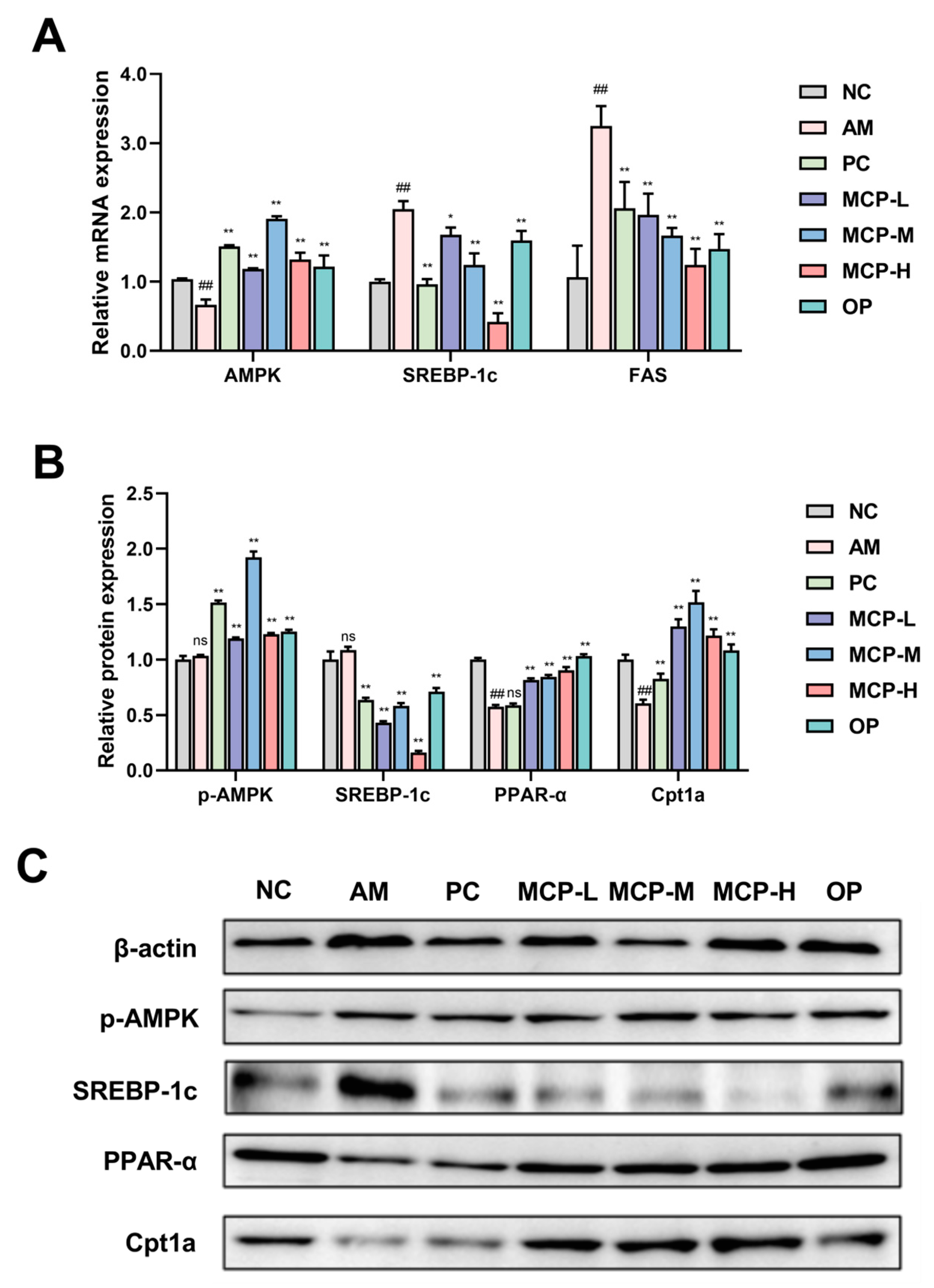
3.6. MCPs Improve Abnormal Lipid Metabolism in ALD Mice
3.7. MCPs Improve Inflammatory Response in ALD Mice
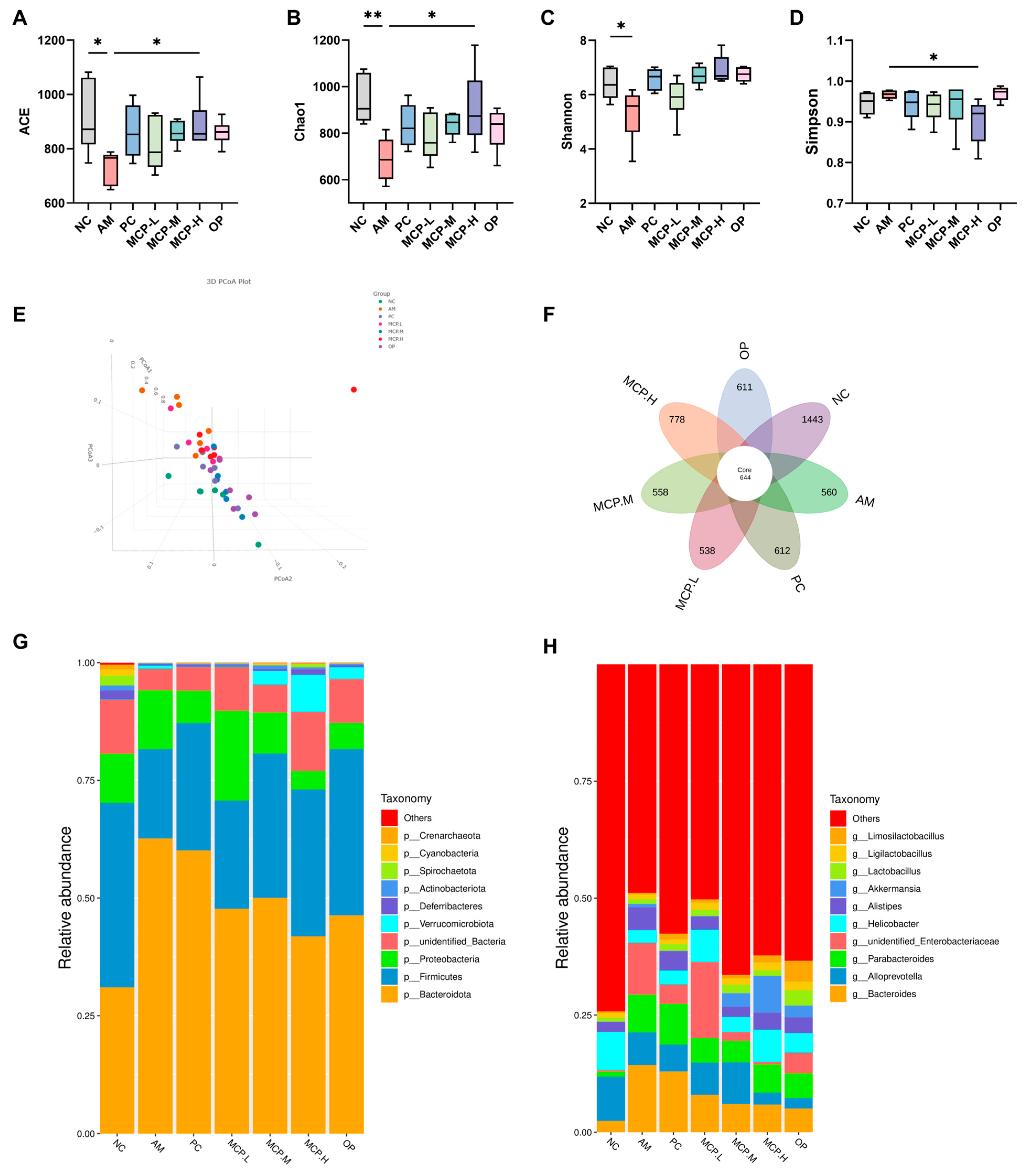
3.8. MCPs Improve Gut Microbiota Composition in ALD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Huo, C.; Qian, Y.; Ren, D.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Massey, V.L.; Arteel, G.E. Acute alcohol-induced liver injury. Front. Physiol. 2012, 3, 193. [Google Scholar] [CrossRef]
- Guo, M.; Mao, B.; Ahmed Sadiq, F.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.; Lee, Y.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Foods 2020, 70, 103995. [Google Scholar] [CrossRef]
- Dai, W.; Chen, C.; Feng, H.; Li, G.; Peng, W.; Liu, X.; Yang, J.; Hu, X. Protection of Ficus pandurata Hance against acute alcohol-induced liver damage in mice via suppressing oxidative stress, inflammation, and apoptosis. J. Ethnopharmacol. 2021, 275, 114140. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Zheng, X.; Qu, Y. Antagonistic effect of the glycopeptide from zein on acute alcohol-induced liver injury in mice. J. Funct. Foods 2022, 92, 105062. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, L.; Gao, J.; Jia, R.; Zheng, Y.; Zhao, S.; Zhao, M.; Toldrá, F. Musculus senhousei as a promising source of bioactive peptides protecting against alcohol-induced liver injury. Food Chem. Toxicol. 2023, 174, 113652. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Wang, Y.; Zhou, Q.; Lu, Y.; Zhang, Y.; Zhang, J. Protective effect of kinsenoside on acute alcohol-induced liver injury in mice. Rev. Bras. De Farmacogn. 2019, 29, 637–643. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, M.Y.; Baik, S.K. Alcoholic liver disease: Treatment. World J. Gastroenterol. Wjg 2014, 20, 12934. [Google Scholar] [CrossRef]
- Karsan, H.A.; Parekh, S. Management of alcoholic hepatitis: Current concepts. World J. Hepatol. 2012, 4, 335. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, W.; Cui, W.; Jia, L.; Zhang, J. A polysaccharide of PFP-1 from Pleurotus geesteranus attenuates alcoholic liver diseases via Nrf2 and NF-κB signaling pathways. Food Funct. 2021, 12, 4591–4605. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Zhou, K.; Wang, Y.; Wang, Z.M.; Xie, Y.; Zhou, H.; Xu, B.C. Isolation and identification of bioactive peptides from Xuanwei ham that rescue oxidative stress damage induced by alcohol in HHL-5 hepatocytes. Food Funct. 2020, 11, 9710–9720. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Park, S.; Park, T.G.; Park, H.; Kim, Y.; Kim, E.J.; Shin, W.; Kim, A.; Yoo, H.; Kweon, M.; et al. Noni fruit extract ameliorates alcohol-induced hangover symptoms by reducing the concentrations of alcohol and acetaldehyde in a Sprague Dawley rat model and a human intervention study. Food Funct. 2023, 14, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kang, K.; Li, Y.; Sang, L.; Chang, B. Tea polyphenols protect mice from acute ethanol-Induced liver injury by modulating the gut microbiota and short-chain fatty acids. J. Funct. Foods 2021, 87, 104865. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Sun, M.; Liang, Y.; Wang, X.; Qi, D.; Han, C. The Saggy Ink Cap Medicinal Mushroom, Coprinus comatus (Agaricomycetes), Protein Attenuates Acute Alcoholic Liver Injury in Association with Changes in the Gut Microbiota of Mice. Int. J. Med. Mushrooms 2021, 23, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liao, A.; Cui, Y.; Yu, G.; Hou, Y.; Pan, L.; Chen, W.; Zheng, S.; Li, X.; Ma, J.; et al. Wheat embryo globulin protects against acute alcohol-induced liver injury in mice. Food Chem. Toxicol. 2021, 153, 112240. [Google Scholar] [PubMed]
- YU, G.; LI, J.; HE, H.; HUANG, W.; ZHANG, W. Ultrafiltration preparation of potent bioactive corn peptide as alcohol metabolism stimulator in vivo and study on its mechanism of action. J. Food Biochem. 2013, 37, 161–167. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.; Shin, E.; Woo, M.S.; Kim, J.; Kim, J.H.; Lee, D.K.; Hahm, J.R.; Kim, H.J.; et al. Dipeptide YA is Responsible for the Positive Effect of Oyster Hydrolysates on Alcohol Metabolism in Single Ethanol Binge Rodent Models. Mar. Drugs 2020, 18, 512. [Google Scholar] [CrossRef]
- Chen, M.; Gong, F.; Zhang, Y.Y.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z. Preventive effect of YGDEY from tilapia fish skin gelatin hydrolysates against alcohol-induced damage in HepG2 cells through ROS-mediated signaling pathways. Nutrients 2019, 11, 392. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, F.; Wu, Y.; Dai, L.; Feng, Y.; Chen, S.; Wang, G.; Ma, H.; Li, X.; Dai, C. Identification of a novel peptide that activates alcohol dehydrogenase from crucian carp swim bladder and how it protects against acute alcohol-induced liver injury in mice. J. Pharm. Biomed. Anal. 2022, 207, 114426. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Y.; Liu, X.; Sun, J.; Wang, X.; Sang, Y. Improvement in the gelling properties of myofibrillar protein from the razor clam (Sinonovacula constricta) through phosphorylation and structural characterization of the modified protein. Food Chem. X 2023, 20, 101006. [Google Scholar] [CrossRef]
- Bai, J.; Chen, Y.; Ning, Z.; Liu, S.; Xu, C.; Yan, J. Proteoglycan isolated from Corbicula fluminea exerts hepato-protective effects against alcohol-induced liver injury in mice. Int. J. Biol. Macromol. 2020, 142, 1–10. [Google Scholar] [CrossRef]
- Liang, J.; Li, Q.; Lin, B.; Yu, Y.; Ding, Y.; Dai, X.; Li, Y. Comparative studies of oral administration of marine collagen peptides from Chum Salmon (Oncorhynchus keta) pre- and post-acute ethanol intoxication in female Sprague-Dawley rats. Food Funct. 2014, 5, 2078–2085. [Google Scholar] [CrossRef]
- Sugimoto, K.; Takei, Y. Pathogenesis of alcoholic liver disease. Hepatol. Res. 2017, 47, 70–79. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, R.; Zhang, Q.; Sun, Y.; Chen, H. Effect of natural polysaccharides on alcoholic liver disease: A review. Int. J. Biol. Macromol. 2023, 251, 126317. [Google Scholar] [CrossRef]
- Xiao, C.; Zhou, F.; Zhao, M.; Su, G.; Sun, B. Chicken breast muscle hydrolysates ameliorate acute alcohol-induced liver injury in mice through alcohol dehydrogenase (ADH) activation and oxidative stress reduction. Food Funct. 2018, 9, 774–784. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldrá, F.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Peptides Extracted from Meat By-products: A Review. Food Anal. Methods 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Honda, T.; Ishigami, M.; Luo, F.; Lingyun, M.; Ishizu, Y.; Kuzuya, T.; Hayashi, K.; Nakano, I.; Ishikawa, T.; Feng, G.; et al. Branched-chain amino acids alleviate hepatic steatosis and liver injury in choline-deficient high-fat diet induced NASH mice. Metabolism 2017, 69, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Wang, Y.; Lin, D.; Liu, J. Hepatoprotective Effect of Albumin Peptides from Corn Germ Meal on Chronic Alcohol-Induced Liver Injury in Mice. J. Food Sci. 2017, 82, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, S.; Song, C.; Sun, X.; Liu, X. Black soybean-derived peptides exerted protective effect against alcohol-induced liver injury in mice. J. Funct. Foods 2021, 87, 104828. [Google Scholar] [CrossRef]
- Murakami, H.; Ito, M.; Furukawa, Y.; Komai, M. Leucine accelerates blood ethanol oxidation by enhancing the activity of ethanol metabolic enzymes in the livers of SHRSP rats. Amino Acids 2012, 43, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Zheng, X.; Qu, Y.; Shi, Y. Preparation of corn glycopeptides and evaluation of their antagonistic effects on alcohol-induced liver injury in rats. J. Funct. Foods 2020, 66, 103776. [Google Scholar] [CrossRef]
- Correa, M.; Sanchis-Segura, C.; Aragon, C.M.G. Influence of brain catalase on ethanol-induced loss of righting reflex in mice. Drug Alcohol. Depend. 2001, 65, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Q.; Cao, L.; Zhao, B. Hepatoprotective effect of Gan Kang Yuan against chronic liver injury induced by alcohol. J. Ethnopharmacol. 2017, 208, 1–7. [Google Scholar] [CrossRef]
- Cai, Z.; Song, L.; Qian, B.; Xu, W.; Ren, J.; Jing, P.; Oey, I. Understanding the effect of anthocyanins extracted from purple sweet potatoes on alcohol-induced liver injury in mice. Food Chem. 2018, 245, 463–470. [Google Scholar] [CrossRef]
- Mir, S.M.; Sahu, B.D.; Koneru, M.; Kuncha, M.; Jerald, M.K.; Ravuri, H.G.; Kanjilal, S.; Sistla, R. Supplementation of oat (Avena sativa L.) extract abates alcohol-induced acute liver injury in a mouse model. Nutr. Res. 2018, 54, 80–92. [Google Scholar] [CrossRef]
- Zelner, I.; Matlow, J.N.; Natekar, A.; Koren, G. Synthesis of fatty acid ethyl esters in mammalian tissues after ethanol exposure: A systematic review of the literature. Drug Metab. Rev. 2013, 45, 277–299. [Google Scholar] [CrossRef]
- Haseba, T.; Tomita, Y.; Kurosu, M.; Ohno, Y. Dose and time changes in liver alcohol dehydrogenase (ADH) activity during acute alcohol intoxication involve not only class I but also class III ADH and govern elimination rate of blood ethanol. Leg. Med. 2003, 5, 202–211. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Yang, C.; Zhong, Y.; Wu, D.; Shi, L.; Chen, L.; Li, Y. Hepatoprotective effects of Methyl ferulic acid on alcohol-induced liver oxidative injury in mice by inhibiting the NOX4/ROS-MAPK pathway. Biochem. Biophys. Res. Commun. 2017, 493, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Cai, Z.; Ge, H.; Ma, S.; Yu, Y.; Liu, J.; Zhang, T. Transcriptome analysis reveals the hepatoprotective mechanism of soybean meal peptides against alcohol-induced acute liver injury mice. Food Chem. Toxicol. 2021, 154, 112353. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, J.; He, H.; Yu, G.; Du, J. Antihepatotoxic effect of corn peptides against Bacillus Calmette-Guerin/lipopolysaccharide-induced liver injury in mice. Food Chem. Toxicol. 2009, 47, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Z.; Yang, M.; Wang, Y.; Cao, J.; Khan, A.; Liu, Y.; Cheng, G. Protective effects of E Se tea extracts against alcoholic fatty liver disease induced by high fat/alcohol diet: In vivo biological evaluation and molecular docking study. Phytomedicine 2022, 101, 154113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure–activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Kong, L.; Chandimali, N.; Han, Y.; Lee, D.; Kim, J.; Kim, S.; Kim, T.; Jeong, D.K.; Sun, H.; Lee, D.S.; et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int. J. Mol. Sci. 2019, 20, 2712. [Google Scholar] [CrossRef] [PubMed]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update1. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.Q.; Ajmo, J.M.; You, M. Adiponectin and alcoholic fatty liver disease. Iubmb Life 2008, 60, 790–797. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, C.; Liu, T.; Tian, M.; Wu, N.; Yu, Z.; Zhao, F.; Qi, J.; Zhu, Q. FNDC3B protects steatosis and ferroptosis via the AMPK pathway in alcoholic fatty liver disease. Free Radic. Biol. Med. 2022, 193, 808–819. [Google Scholar] [CrossRef]
- Yu, H.; Yi, X.; Gao, X.; Ji, J.; Liu, Z.; Xia, G.; Li, C.; Zhang, X.; Shen, X. Tilapia-Head Chondroitin Sulfate Protects against Nonalcoholic Fatty Liver Disease via Modulating the Gut-Liver Axis in High-Fat-Diet-Fed C57BL/6 Mice. Foods 2022, 11, 922. [Google Scholar] [CrossRef]
- Kang, O.; Kim, S.; Mun, S.; Seo, Y.; Hwang, H.; Lee, Y.; Lee, H.; Kang, D.; Kwon, D. Puerarin ameliorates hepatic steatosis by activating the PPARα and AMPK signaling pathways in hepatocytes. Int. J. Mol. Med. 2015, 35, 803–809. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.H.; Yu, H.S.; Park, H.G.; Kang, U.G.; Ahn, Y.M.; Kim, Y.S. The effect of clozapine on the AMPK-ACC-CPT1 pathway in the rat frontal cortex. Int. J. Neuropsychopharmacol. 2012, 15, 907–917. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Zi, Y.; Zhao, H.; Wang, H.; Zhang, Y. Functional coix seed protein hydrolysates as a novel agent with potential hepatoprotective effect. Food Funct. 2020, 11, 9495–9502. [Google Scholar] [CrossRef]
- Yakovleva, T.; Bazov, I.; Watanabe, H.; Hauser, K.F.; Bakalkin, G. Transcriptional control of maladaptive and protective responses in alcoholics: A role of the NF-κB system. Brain Behav. Immun. 2011, 25, S29–S38. [Google Scholar] [CrossRef]
- Kono, H.; Wheeler, M.D.; Rusyn, I.; Lin, M.; Seabra, V.; Rivera, C.A.; Bradford, B.U.; Forman, D.T.; Thurman, R.G. Gender differences in early alcohol-induced liver injury: Role of CD14, NF-κB, and TNF-α. Am. J. Physiol.-Gastroint. Liver Physiol. 2000, 278, G652–G661. [Google Scholar] [CrossRef]
- Nie, W.; Du, Y.; Xu, F.; Zhou, K.; Wang, Z.; Al-Dalali, S.; Wang, Y.; Li, X.; Ma, Y.; Xie, Y.; et al. Oligopeptides from Jinhua ham prevent alcohol-induced liver damage by regulating intestinal homeostasis and oxidative stress in mice. Food Funct. 2021, 12, 10053–10070. [Google Scholar] [CrossRef]
- Hritz, I.; Mandrekar, P.; Velayudham, A.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Kurt-Jones, E.; Szabo, G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 2008, 48, 1224–1231. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef]
- Yan, A.W.; E. Fouts, D.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; E. Nelson, K.; A. Brenner, D.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef]

| Molecular Weight (Da) | MCPs (%) | OP (%) |
|---|---|---|
| >10,000 | 0.54 | 0.04 |
| 10,000~5000 | 0.13 | 0.05 |
| 5000~3000 | 0.30 | 0.27 |
| 3000~2000 | 0.61 | 0.76 |
| 2000~1000 | 4.15 | 6.63 |
| 1000~500 | 20.04 | 21.98 |
| 500~180 | 62.01 | 53.66 |
| <180 | 12.20 | 16.60 |
| Amino Acids | Content (g/100 g) | |
|---|---|---|
| MCP | OP | |
| Asp | 2.68 ± 0.12 | 2.75 ± 0.08 |
| Glu | 16.72 ± 0.51 | 14.01 ± 0.48 |
| Ser | 2.51 ± 0.08 | 1.96 ± 0.11 |
| His 4 | 1.51 ± 0.13 | 1.70 ± 0.09 |
| Gly 3 | 8.86 ± 0.55 | 9.69 ± 0.81 |
| Thr 1 | 4.35 ± 0.02 | 3.01 ± 0.17 |
| Arg | 10.03 ± 0.71 | 13.09 ± 1.03 |
| Ala 3 | 9.36 ± 0.69 | 8.25 ± 0.16 |
| Tyr 4 | 4.18 ± 0.10 | 4.84 ± 0.13 |
| Cys | 0.50 ± 0.12 | 0.26 ± 0.08 |
| Val 1,2,3 | 3.18 ± 0.12 | 3.40 ± 0.11 |
| Met 1,3,4 | 6.19 ± 0.49 | 3.53 ± 0.24 |
| Phe 1,3 | 5.35 ± 0.38 | 5.76 ± 0.16 |
| Ile 1,2,3 | 3.01 ± 0.12 | 3.01 ± 0.20 |
| Leu 1,2,3 | 13.71 ± 0.83 | 12.17 ± 0.96 |
| Lys 1,4 | 7.19 ± 0.64 | 11.13 ± 0.77 |
| Pro 3 | 0.84 ± 0.13 | 1.31 ± 0.20 |
| Essential amino acid | 42.98 | 42.01 |
| Branched chain amino acid | 19.90 | 18.58 |
| Hydrophobic amino acids | 50.50 | 47.12 |
| Treatment Groups | LORR Rate (%) | Latency of LORR (min) | Duration of LORR (min) | Body Weight (g) | Liver Weight (g) | Liver Index (%) |
|---|---|---|---|---|---|---|
| NC | - | - | - | 44.75 ± 3.32 a | 1.685 ± 0.228 b | 3.735 ± 0.451 g |
| AM | 80 | 29.39 ± 21.71 f | 434.83 ± 99.42 a | 37.99 ± 6.09 c | 1.691 ± 0.181 a | 4.175 ± 0.193 a |
| PC | 70 | 30.70 ± 30.30 e | 316.14 ± 100.06 e | 38.61 ± 2.86 bc | 1.511 ± 0.196 g | 3.881 ± 0.374 e |
| OP | 70 | 35.21 ± 11.63 c | 318.85 ± 82.60 d | 39.04 ± 4.97 bc | 1.574 ± 0.283 e | 3.939 ± 0.583 c |
| MCP-L | 60 | 30.83 ± 10.45 d | 334.24 ± 45.67 b | 38.66 ± 3.07 bc | 1.603 ± 0.175 d | 4.054 ± 0.246 b |
| MCP-M | 60 | 37.54 ± 12.57 b | 321.33 ± 85.57 c | 37.62 ± 4.97 c | 1.641 ± 0.201 c | 3.936 ± 0.229 d |
| MCP-H | 60 | 42.21 ± 15.61 a | 312.40 ± 26.15 f | 40.52 ± 3.11 b | 1.549 ± 0.159 f | 3.814 ± 0.271 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Cheng, M.; Yi, X.; Xia, G.; Liu, Z.; Shi, H.; Shen, X. Effects of Mactra chinenesis Peptides on Alcohol-Induced Acute Liver Injury and Intestinal Flora in Mice. Foods 2024, 13, 1431. https://doi.org/10.3390/foods13101431
Wu D, Cheng M, Yi X, Xia G, Liu Z, Shi H, Shen X. Effects of Mactra chinenesis Peptides on Alcohol-Induced Acute Liver Injury and Intestinal Flora in Mice. Foods. 2024; 13(10):1431. https://doi.org/10.3390/foods13101431
Chicago/Turabian StyleWu, Dong, Ming Cheng, Xiangzhou Yi, Guanghua Xia, Zhongyuan Liu, Haohao Shi, and Xuanri Shen. 2024. "Effects of Mactra chinenesis Peptides on Alcohol-Induced Acute Liver Injury and Intestinal Flora in Mice" Foods 13, no. 10: 1431. https://doi.org/10.3390/foods13101431






