Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties
Abstract
:1. Introduction
2. Chemical Composition
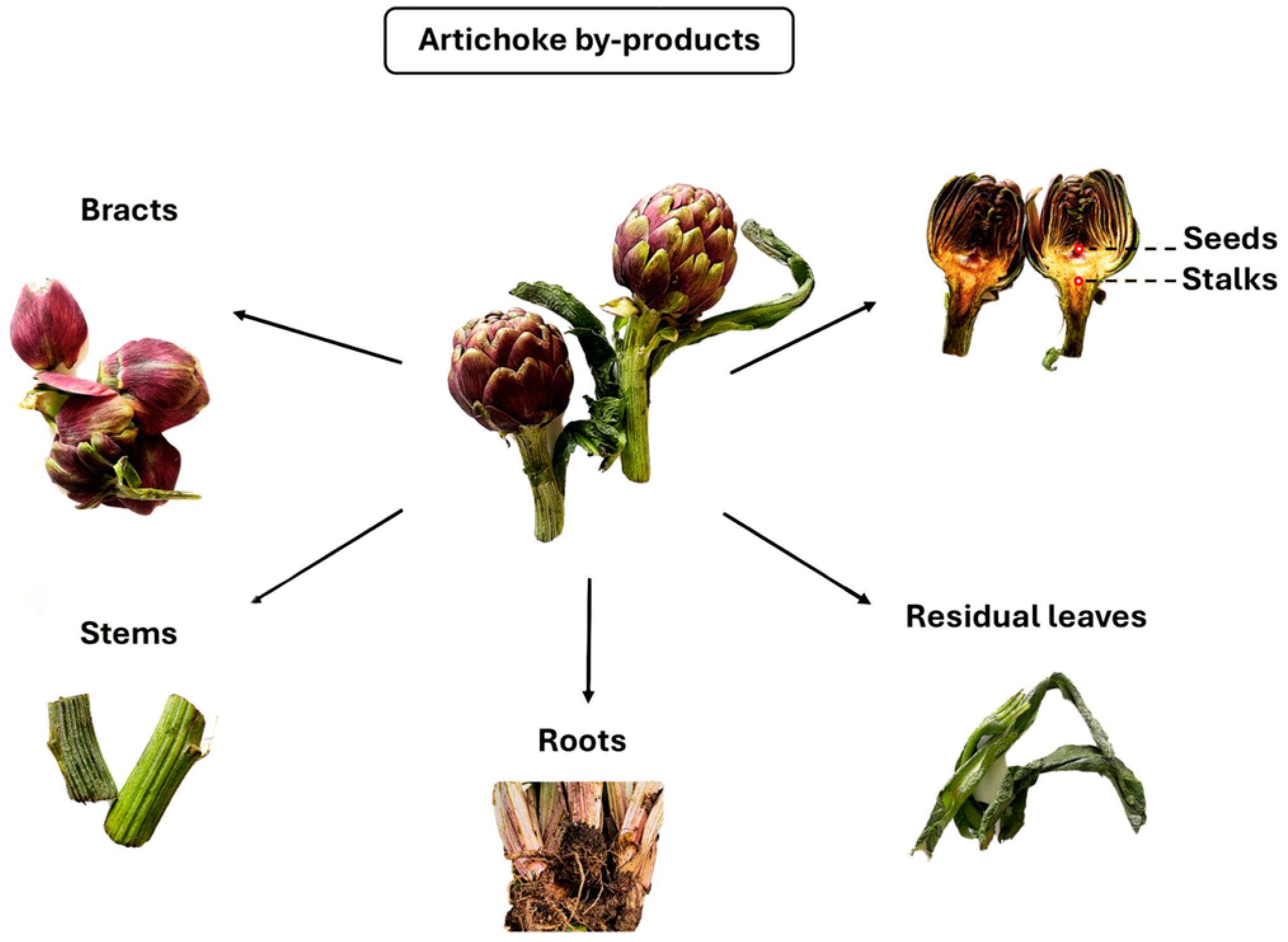
2.1. Globe Artichoke Main By-Products: Bracts and Stems
2.2. Other Globe Artichoke By-Products: Residual Leaves, Stalks, Roots, and Seeds
3. Artichoke By-Product Processing
4. Biological Properties
4.1. Hypolipidemic Activity
4.2. Hypoglycemic and Antiglycative Activity
4.3. Anti-Inflammatory and Antioxidant Activities
4.4. Anti-Proliferative Activity
4.5. Antimicrobial Activity
4.6. Prebiotic Activity
5. Functional Properties
6. Food Additives
7. Functional Food and Food Supplements Ingredients
8. Future Prospectives of Artichoke By-Products Use
8.1. Health and Nutrition Claims
8.2. Novel Foods
9. Animal Feed
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT. Crops and Livestock Products. 2022. Available online: https://fenix.fao.org/faostat/internal/en/#data/QCL (accessed on 5 February 2024).
- Rana, R.L.; Bux, C.; Lombardi, M. Trends in scientific literature on the environmental sustainability of the artichoke (Cynara cardunculus L. spp.) supply chain. Br. Food J. 2023, 125, 2315–2332. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenerg. 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; United Nations: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 5 February 2024).
- The World Bank. World Development Report. 2018. Available online: https://www.worldbank.org/en/publication/wdr2018/brief/world-development-report-2018-data (accessed on 5 February 2024).
- European Commission. A European Strategy for Smart, Sustainable and Inclusive Growth. 2020. Available online: https://commission.europa.eu/index_en (accessed on 5 February 2024).
- Elimelech, E.; Ofira, A.; Eyal, E. What gets measured gets managed: A new method of measuring household food waste. Waste Manag. 2018, 76, 68–81. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: From food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crops Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Mejri, F.; Baati, T.; Martins, A.; Selmi, S.; Serralheiro, M.L.; Falé, P.L.; Rauter, A.; Casabianca, H.; Hosni, K. Phytochemical analysis and in vitro and in vivo evaluation of biological activities of artichoke (Cynara scolymus L.) floral stems: Towards the valorization of food by-products. Food Chem. 2020, 333, 127506. [Google Scholar] [CrossRef]
- Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods 2021, 10, 459. [Google Scholar] [CrossRef]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar]
- Feiden, T.; Valduga, E.; Zeni, J.; Steffens, J. Bioactive compounds from artichoke and application potential. Food Technol. Biotechnol. 2023, 61, 312–327. [Google Scholar] [CrossRef]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; de Falco, E.; Rastrelli, L. Chemical profile and cellular antioxidant activity of artichoke by-products. Food Funct. 2016, 7, 4841–4850. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Dhen, N.; Gargouri, M.; Boulila, A. Chemical composition, antioxidant potential and enzymes inhibitory properties of globe artichoke by-products. Chem. Biodivers. 2020, 17, e2000073. [Google Scholar] [CrossRef]
- Almela, L.; Rodríguez, T.; Roca, M.J.; Martínez, D.; Fernández-López, J.A. Quantity and quality of proteins in artichoke by-products (Cynara scolymus L.). Acta Hortic. 2005, 681, 505–510. [Google Scholar] [CrossRef]
- Silva, S.V.; Malcata, F.X. Studies pertaining to coagulant and proteolytic activities of plant proteases from Cynara cardunculus. Food Chem. 2005, 89, 19–26. [Google Scholar] [CrossRef]
- Mejiri, F.; Karmali, A.; Jaoued, N.; Casabianca, H.; Hosni, K. Purification and partial characterization of peroxidases from three food waste by-products: Broad bean pods, pea pods, and artichoke stems. Appl. Biochem. Biotechnol. 2019, 189, 576–588. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. BioMed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef]
- Meneses, M.; Martínez-Marín, A.L.; Madrid, J.; Martínez-Teruel, A.; Hernández, F.; Megías, M.D. Ensilability, in vitro and in vivo values of the agro-industrial by-products of artichoke and broccoli. Environ. Sci. Pollut. Res. 2020, 27, 2919–2925. [Google Scholar] [CrossRef]
- Domingo, C.S.; Rojas, A.M.; Fissore, E.N.; Gerschenson, L.N. Rheological behavior of soluble dietary fiber fractions isolated from artichoke residues. Eur. Food Res. Technol. 2019, 245, 1239–1249. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT-Food Sci. Technol. 2020, 132, 109883. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, K.; Ban, L.; Mao, Y.; Hou, C.; Li, J. Silage Fermentation: A potential biological approach for the long-term preservation and recycling of polyphenols and terpenes in globe artichoke (Cynara scolymus L.) by-products. Molecules 2020, 25, 3302. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. In vitro fermentability of globe artichoke by-product by Lactobacillus acidophilus and Bifidobacterium bifidum. Bioact. Carbohydr. Diet. Fibre 2021, 26, 120086. [Google Scholar] [CrossRef]
- Kollia, E.; Markaki, P.; Zoumpoulakis, P.; Proestos, C. Antioxidant activity of Cynara scolymus L. and Cynara cardunculus L. extracts obtained by different extraction techniques. Nat. Prod. Res. 2017, 31, 1163–1167. [Google Scholar] [CrossRef]
- Stumpf, B.; Künne, M.; Ma, L.; Xua, M.; Yana, F.; Piepho, H.P.; Honermeier, B. Optimization of the extraction procedure for the determination ofphenolic acids and flavonoids in the leaves of globe artichoke (Cynara cardunculus var. scolymus L.). J. Pharm. Biomed. Anal. 2020, 177, 112879. [Google Scholar] [CrossRef]
- Monllor, P.; Romero, G.; Sendra, E.; Atzori, A.S.; Díaz, J.R. Short-term effect of the inclusion of silage artichoke by-products in diets of dairy goats on milk quality. Animals 2020, 10, 339. [Google Scholar] [CrossRef]
- Muelas, R.; Romero, G.; Díaz, J.R.; Monllor, P.; Fernández-López, J.; Viuda-Martos, M.; Cano-Lamadrid, M.; Sendra, E. Quality and functional parameters of fermented milk obtained from goat milk fed with broccoli and artichoke plant by-products. Foods 2022, 11, 2601. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of chlorogenic acids from agri-food wastes: Updates on green extraction techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef]
- Ozkan, G. Valorization of artichoke outer petals by using ultrasound-assisted extraction and natural deep eutectic solvents (NADES) for the recovery of phenolic compounds. J. Sci. Food Agric. 2024, 104, 2744–2749. [Google Scholar] [CrossRef]
- Fissore, E.N.; Santo Domingo, C.; Pujol, C.A.; Damonte, E.B.; Rojas, A.M.; Gerschenson, L.N. Upgrading of residues of bracts, stems and hearts of Cynara cardunculus L. var. scolymus to functional fractions enriched in soluble fiber. Food Funct. 2014, 5, 463–470. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef] [PubMed]
- López-Salas, L.; Borrás-Linares, I.; Quintin, D.; García Gomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; Lozano-Sánchez, J. Artichoke by-products as natural source of phenolic food ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Pagliari, S.; Cannavacciuolo, C.; Celano, R.; Carabetta, S.; Russo, M.; Labra, M.; Campone, L. Valorisation, green extraction development, and metabolomic analysis of wild artichoke by-product using pressurised liquid extraction UPLC–HRMS and multivariate data analysis. Molecules 2022, 27, 7157. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; Russo, M.; Rastrelli, L. Pressurized hot water extraction of bioactive compounds from artichoke by-products. Electrophoresis 2018, 39, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Reche, C.; Rosselló, C.; Umaña, M.M.; Eim, V.; Simal, S. Mathematical modelling of ultrasound-assisted extraction kinetics of bioactive compounds from artichoke by-products. Foods 2021, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, R.S.; Machado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- Punzi, R.; Paradiso, A.; Fasciano, C.; Trani, A.; Faccia, M.; de Pinto, M.C.; Gambacorta, G. Phenols and antioxi-dant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke by-products. Nat. Prod. Commun. 2014, 9, 1315–1318. [Google Scholar]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.A.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from in-dustrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Arthicoke (Cynara cardunuculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef]
- Salem, M.B.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid.-Based Complement. Alternat. Med. 2017, 2017, 4951937. [Google Scholar] [PubMed]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultra-sound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017, 52, 2078–2087. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N.; Martín-Belloso, O. Comparison of dietary fibre from by-products of processing fruits and greens and from cereals. LWT-Food Sci. Technol. 1999, 32, 503–508. [Google Scholar] [CrossRef]
- Vaz, A.A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Physicochemical properties and bioaccessibility of phenolic compounds of dietary fibre concentrates from vegetable by-products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crops Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Fissore, E.N.; Santo Domingo, C.; Gerschenson, L.N.; Giannuzzi, L. A study of the effect of dietary fiber fractions obtained from artichoke (Cynara cardunculus L. var. scolymus) on the growth of intestinal bacteria associated with health. Food Funct. 2015, 6, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Cavini, S.; Guzzetti, L.; Givoia, F.; Regonesi, M.E.; Di Gennaro, P.; Magoni, C.; Campone, L.; Labra, M.; Bruni, I. Artichoke (Cynara cardunculus var. scolymus L.) by-products as a source of inulin: How to valorise an agricultural supply chain extracting an added-value compound. Nat. Prod. Res. 2020, 36, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nakashima, S.; Nakamura, S.; Hattori, Y.; Ando, T.; Matsuda, H. Inhibitory effects of cynaropicrin and related sesquiterpene lactones from leaves of artichoke (Cynara scolymus L.) on induction of iNOS in RAW264.7 cells and its high-affinity proteins. J. Nat. Med. 2021, 75, 381–392. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Dejonghe, W.; Mariniello, L.; Porta, R. Enzymatic milk clotting activity in artichoke (Cynara scolymus) leaves and alpine thistle (Carduus defloratus) flowers. Immobilization of alpine thistle aspartic protease. Food Chem. 2016, 204, 115–121. [Google Scholar] [CrossRef]
- Bravo Bolívar, M.S.; Pasini, F.; Marzocchi, S.; Ravagli, C.; Tedeschi, P. Future perspective and technological innovation in cheese making using artichoke (Cynara scolymus) as vegetable rennet: A review. Foods 2023, 12, 3032. [Google Scholar] [CrossRef] [PubMed]
- Raccuia, S.A.; Melilli, M.G. Cynara cardunculus L., a potential source of inulin in the Mediterranean environment: Screening of genetic variability. Aust. J. Agric. Res. 2004, 55, 693–698. [Google Scholar] [CrossRef]
- Ehsani, J.; Mohesenzadeh, M.; Khomeiri, M.; Ghasemnezhad, A. Chemical characteristics, and effect of inulin extracted from artichoke (Cynara scolymus L.) root on biochemical properties of synbiotic yogurt at the end of fermentation. Iran. J. Chem. Chem. Eng. Int. Engl. Ed. 2018, 37, 219–230. [Google Scholar]
- Castellino, M.; Renna, M.; Leoni, B.; Calasso, M.; Difonzo, G.; Santamaria, P.; Gambacorta, G.; Caponio, F.; De Angelis, M.; Paradiso, V.M. Conventional and unconventional recovery of inulin rich extracts for food use from the roots of globe artichoke. Food Hydrocoll. 2020, 107, 105975. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from globe artichoke roots: A promising ingredient for the production of functional fresh pasta. Foods 2022, 11, 3032. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, W.M.; Bisar, G.H.; Aamer, R.A. Impact of inulin extracted, purified from (Chicory and Globe Artichoke) roots and the combination with maltodextrin as prebiotic dietary fiber on the functional properties of stirred bio-yogurt. Food Nutr. Sci. 2023, 14, 70–89. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakisc, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Bradkorb, A.; Cattenoz, T.; Clemente, A.; Comi, I.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. Infogest static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1040. [Google Scholar] [CrossRef]
- Menard, O.; Lesmes, U.; Shani-Levi, C.S.; Araiza Calahorra, A.; Lavoisier, A.; Morzel, M.; Rieder, A.; Feron, G.; Nebbia, S.; Mashiah, L.; et al. Static in vitro digestion model adapted to the general older adult population: An INFOGEST international consensus. Food Funct. 2023, 14, 4569–4582. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Ferron, L.; Frosi, I.; Papetti, A. Advances in static in vitro digestion models after the COST action Infogest consensus protocol. Food Funct. 2021, 12, 7619–7636. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R.P.A. A Human Gastric Simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The design, operation, and application of a dynamic gastric model. Dissolution Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Mazzei, D.; Giusti, S.; Sbrana, T.; Ahluwalia, A. Multicompartmental modular bioreactor as innovative system for dynamic cell cultures and co-cultures. In Bioreactors: Design, Properties and Applications; Antolli, P.G., Liu, Z., Eds.; Nova Science Inc.: Hauppauge, NY, USA, 2011; pp. 159–178. [Google Scholar]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The effect of artichoke on lipid profile: A review of possible mechanisms of action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Suárez, M.J.; Mateos-Aparicio, I.; Pérez-Cózar, M.L.; Yokoyama, W.; Redondo-Cuenca, A. Hypolipidemic effects of dietary fiber from an artichoke by-product in Syrian hamsters. J. Funct. Foods 2019, 56, 156–162. [Google Scholar] [CrossRef]
- Shimoda, H.; Ninomiya, K.; Nishida, N.; Yoshino, T.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Anti-hyperlipidemic sesquiterpenes and new sesquiterpene glycosides from the leaves of artichoke (Cynara scolymus L.): Structure requirement and mode of action. Bioorg. Med. Chem. Lett. 2003, 13, 223–228. [Google Scholar] [CrossRef]
- Benkhoud, H.; Baâti, T.; Njim, L.; Selmi, S.; Hosni, K. Antioxidant, antidiabetic, and antihyperlipidemic activities of wheat flour-based chips incorporated with omega-3-rich fish oil and artichoke powder. J. Food Biochem. 2021, 45, 13297. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Monteferrario, F.; Perna, S.; Faliva, M.A.; Opizzi, A. Health-promoting properties of artichoke in preventing cardiovascular disease by its lipidic and glycemic-reducing action. Monaldi Arch. Chest Dis. 2013, 80, 17–26. [Google Scholar] [CrossRef]
- Colombo, R.; Paolillo, M.; Frosi, I.; Ferron, L.; Papetti, A. Effect of the simulated digestion process on the chlorogenic acid trapping activity against methylglyoxal. Food Funct. 2023, 14, 541–549. [Google Scholar] [CrossRef]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Augimeri, G.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and anti-Inflammatory effects of extracts from pulsed electric field-treated artichoke by-products in lipopolysaccharide-stimulated human THP-1 macrophages. Foods 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Biel, W.; Witkowicz, R.; Piątkowska, E.; Podsiadło, C. Proximate composition, minerals and antioxidant activity of artichoke leaf extracts. Biol. Trace Elem. Res. 2020, 194, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Azam, M.; Basra, M.A.R. Impact of natural antioxidants on biological systems. LGU J. Life Sci. 2020, 4, 139–162. [Google Scholar]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Rodríguez, D.; Soto-Maldonado, C.; Torres-Alarcón, C.; Pastrana-Castro, L.; Weinstein-Oppenheimer, C.; Zúñiga-Hansen, M.E. Valorization of Globe Artichoke (Cynara scolymus) agro-industrial discards, obtaining an extract with a selective effect on viability of cancer cell lines. Processes 2020, 8, 715. [Google Scholar] [CrossRef]
- Moselhy, M.; Abd-Elhafez, K.; El-Kholany, E.; Gohar, M.; Nasr, N. Antimicrobial, antioxidant and anticancer properties of globe artichoke and grape by-products as a source of the bio-active phenolic compounds. Egypt. J. Chem. 2023, 66, 609–624. [Google Scholar] [CrossRef]
- Salama, A.A.; El-Baz, F.K. Antioxidant and antiproliferative effects on human liver HePG2 epithelial cells from artichoke (Cynara scolymus L.) by-products. J. Nat. Sci. Res. 2013, 3, 17–24. [Google Scholar]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin. Ther. Pat. 2020, 30, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Kachrimanidou, V.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In vitro fermentation properties of pectins and enzymatic-modified pectins obtained from different renewable bioresources. Carbohydr. Polym. 2018, 199, 482–491. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Frutos, M.J.; Hernández-Herrero, J.A.; Pérez-Llamas, F.; Zamora, S. Effect of chlorophyll removal and particle size upon the nutritional and technological properties of powdered by-products from artichoke (Cynara scolymus L.) industrial canning. Int. J. Food Sci. Technol. 2015, 50, 2383–2390. [Google Scholar] [CrossRef]
- Domingo, C.S.; Gonzàlez, C.O.; Navarro, D.; Stortz, C.; Rojas, A.M.; Gerschenson, L.N.; Fissore, E.N. Enzyme assisted extraction of pectin and inulin enriched fractions isolated from microwave treated Cynara cardunculus tissues. Int. J. Food Sci. Technol. 2021, 56, 242–249. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; Van Der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.; Wawrzyniak, P.; Rosselló, C.; Llavata, B.; Simal, S. Evaluation of the addition of artichoke by-products to O/W emulsions for oil microencapsulation by spray drying. LWT-Food Sci. Technol. 2021, 151, 444–454. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Valadez-Blanco, R.; Hernández-Carlos, B.; Guadarrama-Mendoza, P.C. Natural antioxidant extracts as food preservatives. Acta Sci. Pol. Technol. Aliment. 2017, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Claus, T.; Maruyama, S.A.; Palombini, S.V.; Montanher, P.F.; Bonafé, E.G.; de Oliveira Santos Junior, O.; Matsushita, M.; Visentainer, J.V. Chemical characterization and use of artichoke parts for protection from oxidative stress in canola oil. LWT-Food Sci. Technol. 2015, 61, 346–351. [Google Scholar] [CrossRef]
- Larrosa, M.; Llorach, R.; Espìn, J.C.; Tomás-Barberán, F.A. Increase of antioxidant activity of tomato juice upon functionalisation with vegetable byproduct extracts. LWT-Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Ehsani, J.; Mohammad Mortazavian, A.; Khomeiri, M.; Ghasem Nejad, A. Effects of artichoke (Cynara scolymus L.) extract addition on microbiological and physico-chemical properties of probiotic yogurt. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 536–541. [Google Scholar] [CrossRef]
- Llorente, B.E.; Brutti, C.B.; Caffini, N.O. Purification and characterization of a milk-clotting aspartic proteinase from globe artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2004, 52, 8182–8189. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Lucera, A.; Marinelli, V.; Del Nobile, M.A.; Conte, A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef] [PubMed]
- Frutos, M.J.; Guilabert-Antón, L.; Tomás-Bellido, A.; Hernández-Herrero, J.A. Effect of artichoke (Cynara scolymus L.) fiber on textural and sensory qualities of wheat bread. Food Sci. Technol. Int. 2008, 14, 49–55. [Google Scholar] [CrossRef]
- Boubaker, M.; Omri, A.E.L.; Blecker, C.; Bouzouita, N. Fibre concentrate from artichoke (Cynara scolymus L.) stem by-products: Characterization and application as a bakery product ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from artichoke processing industry: Reuse in bread-making and evaluation of the physico-chemical characteristics of the final product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.; Sanfilippo, R.; Strano, M.C.; Amenta, M.; Allegra, M.; Proetto, I.; Papa, M.; Palmeri, R.; Todaro, A.; Spina, A. Artichoke industrial waste in durum wheat bread: Effects of two different preparation and drying methods of flours and evaluation of quality parameters during short storage. Foods 2023, 12, 3419. [Google Scholar] [CrossRef] [PubMed]
- San José, F.J.; Collado-Fernández, M.; López, R. Sensory evaluation of biscuits enriched with artichoke fiber-rich powders (Cynara scolymus L.). Food Sci. Nutr. 2018, 6, 160–167. [Google Scholar] [CrossRef] [PubMed]
- San José, F.J.; Collado-Fernández, M.; Álvarez-Castellanos, P.P. Variation, during shelf life, of functional properties of biscuits enriched with fibers extracted from artichoke (Cynara scolymus L.). Nutrients 2023, 15, 3329. [Google Scholar] [CrossRef]
- Amoriello, T.; Mellara, F.; Ruggeri, S.; Ciorba, R.; Ceccarelli, D.; Ciccoritti, R. Artichoke by-products valorization for phenols-enriched fresh egg pasta: A sustainable food design project. Sustainability 2022, 14, 14778. [Google Scholar] [CrossRef]
- Dadalı, C. Artichoke bracts as fat and wheat flour replacer in cake: Optimization of reduced fat and reduced wheat flour cake formulation. J. Food Meas. Charact. 2023, 17, 98–107. [Google Scholar] [CrossRef]
- Ergezer, H.; Serdaroğlu, M. Antioxidant potential of artichoke (Cynara scolymus L.) byproducts extracts in raw beef patties during refrigerated storage. J. Food Meas. Charact. 2018, 12, 982–991. [Google Scholar] [CrossRef]
- Ergezer, H.; Kaya, H.I.; ŞiMşek, Ö. Antioxidant and antimicrobial potential of artichoke (Cynara scolymus L.) extract in beef patties. Czech J. Food Sci. 2018, 36, 154–162. [Google Scholar] [CrossRef]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative efficacy of agricultural by-products in sequestering mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-free bread enriched with artichoke leaf extract in vitro exerted antioxidant and anti-inflammatory properties. Antioxidants 2023, 12, 845. [Google Scholar] [CrossRef]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Functionalisation of commercial chicken soup with enriched polyphenol extract from vegetable by-products. Eur. Food Res. Technol 2005, 220, 31–36. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and vegetable by-products to fortify spreadable cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Gatmiri, S.M.; Khadem, E.; Fakhrian, T.; Kamalinejad, M.; Hosseini, H.; Ghorat, F.; Alamdari, A.; Naderi, N. The effect of artichoke leaf extract supplementation on lipid profile of chronic kidney disease patients; a double-blind, randomized clinical trial. J. Renal Inj. Prev. 2019, 8, 225–229. [Google Scholar] [CrossRef]
- Rangboo, V.; Noroozi, M.; Zavoshy, R.; Rezadoost, S.A.; Mohammadpoorasl, A. The effect of artichoke leaf extract on alanine aminotransferase and aspartate aminotransferase in the patients with nonalcoholic steatohepatitis. Int. J. Hepatol. 2016, 3, 4030476. [Google Scholar] [CrossRef]
- Ministero Della Salute. Available online: https://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?id=1544&menu=notizie (accessed on 22 April 2024).
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 22 April 2024).
- Commission Regulation (EU) No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:en:PDF (accessed on 22 April 2024).
- European Commissione. EU Register of Health Claims. Available online: https://ec.europa.eu/food/food-feed-portal/screen/health-claims/eu-register (accessed on 22 April 2024).
- Commission Regulation (EU) No. 648/2023 of 20 March 2023 Authorising a Health Claim Made on Foods and Referring to the Reduction of Disease Risk. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R0648 (accessed on 22 April 2024).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to the combination of artichoke leaf dry extract standardised in caffeoylquinic acids, monacolin K in red yeast rice, sugar-cane derived policosanols, OPC from French maritime pine bark, garlic dry extract standardised in allicin, d-α-tocopheryl hydrogen succinate, riboflavin and inositol hexanicotinate in Limicol® and reduction of blood LDL-cholesterol concentrations pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2013, 11, 3327. [Google Scholar]
- European Medicine Agency. Available online: https://www.ema.europa.eu/ (accessed on 22 April 2024).
- Regulation (EC) No. 258/97 of the European Parliament and of the Council of 27 January 1997 Concerning Novel Foods and Novel Food Ingredients. Available online: https://eur-lex.europa.eu/eli/reg/1997/258/oj (accessed on 22 April 2024).
- Regulation EU 205/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R2283 (accessed on 22 April 2024).
- Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 22 April 2024).
- Meneses, M.; Madrid, M.; Martínez-Teruel, A.; Hernández, F. Ensiling capacity, chemical composition and multiresidue evaluation of fresh artichoke (Cynara scolymus L.) by-product to be used in ruminant feeding. Options Mediterr. 2005, 67, 351–354. [Google Scholar]
- Muelas, R.; Monllor, P.; Romero, G.; Sayas-Barberá, E.; Navarro, C.; Díaz, J.R.; Sendra, E. Milk technological properties as affected by including artichoke by-products silages in the diet of dairy goats. Foods 2017, 6, 112. [Google Scholar] [CrossRef]
- Monllor, P.; Zemzmi, J.; Muelas, R.; Roca, A.; Sendra, E.; Romero, G.; Díaz, J.R. Long-term feeding of dairy goats with 40% artichoke by-product silage preserves milk yield, nutritional composition and animal health status. Animals 2023, 13, 3585. [Google Scholar] [CrossRef] [PubMed]

| Artichoke By-Product | Biological Property | Reference |
|---|---|---|
| Anti-inflammatory | [45] | |
| Antioxidant | [16] | |
| Prebiotic | [21] | |
| Bract | Hypolipidemic | [73] |
| Antiglycative | [44] | |
| Antimicrobial | [14] | |
| Antiproliferative | [82] | |
| Antioxidant | [81] | |
| Anti-inflammatory | [77] | |
| Stem | Prebiotic | [21] |
| Antiglycative | [44] | |
| Antimicrobial | [12] | |
| Antiproliferative | [82] | |
| Antioxidant | [16] | |
| Residual | Anti-inflammatory | [52] |
| Leaf | Hypoglycemic | [74] |
| Artichoke By-Product | Rheological Property | Color Parameters | Reference |
|---|---|---|---|
| Bract | Emulsifier property | a*, b* | [34,89,90] |
| Gelling property | |||
| WHC a | |||
| OHC b | |||
| Stem | Emulsifier property | a*, b*, L* | [34,89,90] |
| Gelling property | |||
| WHC a | |||
| OHC b | |||
| Root | Gelling property | [58] |
| Artichoke By-Product | Food Additive | Functional Food | Food Supplement | Animal Feed | Reference |
|---|---|---|---|---|---|
| Bract | Rheological additive | Wheat-flour-based chips | - | Ensilage | [29,73,102,104] |
| Wheat replacer | Chicken soup | - | - | [106,111] | |
| Preservative | - | - | [102,105,107] | ||
| Biosorbent | - | - | [109] | ||
| Stem | Reological additive | Bread | - | Ensilage | [29,53,100] |
| Preservative | Spreadable cheese | - | - | [100,105,112] | |
| Biosorbent | - | - | - | [109] | |
| Modifier, stabilizer, off-flavor sequestrant | - | - | - | [20] | |
| Residual leaf | Thickener | Gluten-free bread | Hypolipidemic ingredient | - | [96,110,113] |
| Stabilizer | - | - | - | [96] | |
| Milk-clotting agent | - | - | - | [97] | |
| biosorbent | - | - | - | [109] | |
| Root | Rheological additive | Fresh pasta | - | - | [59] |
| Stabilizer | - | - | - | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, R.; Moretto, G.; Pellicorio, V.; Papetti, A. Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties. Foods 2024, 13, 1427. https://doi.org/10.3390/foods13101427
Colombo R, Moretto G, Pellicorio V, Papetti A. Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties. Foods. 2024; 13(10):1427. https://doi.org/10.3390/foods13101427
Chicago/Turabian StyleColombo, Raffaella, Giulia Moretto, Vanessa Pellicorio, and Adele Papetti. 2024. "Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties" Foods 13, no. 10: 1427. https://doi.org/10.3390/foods13101427







