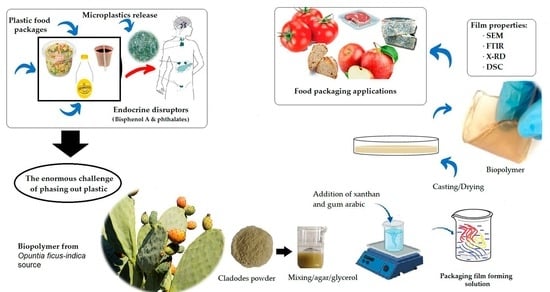
Development of Eco-Friendly Biocomposite Films Based on Opuntia ficus-indica Cladodes Powder Blended with Gum Arabic and Xanthan Envisaging Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
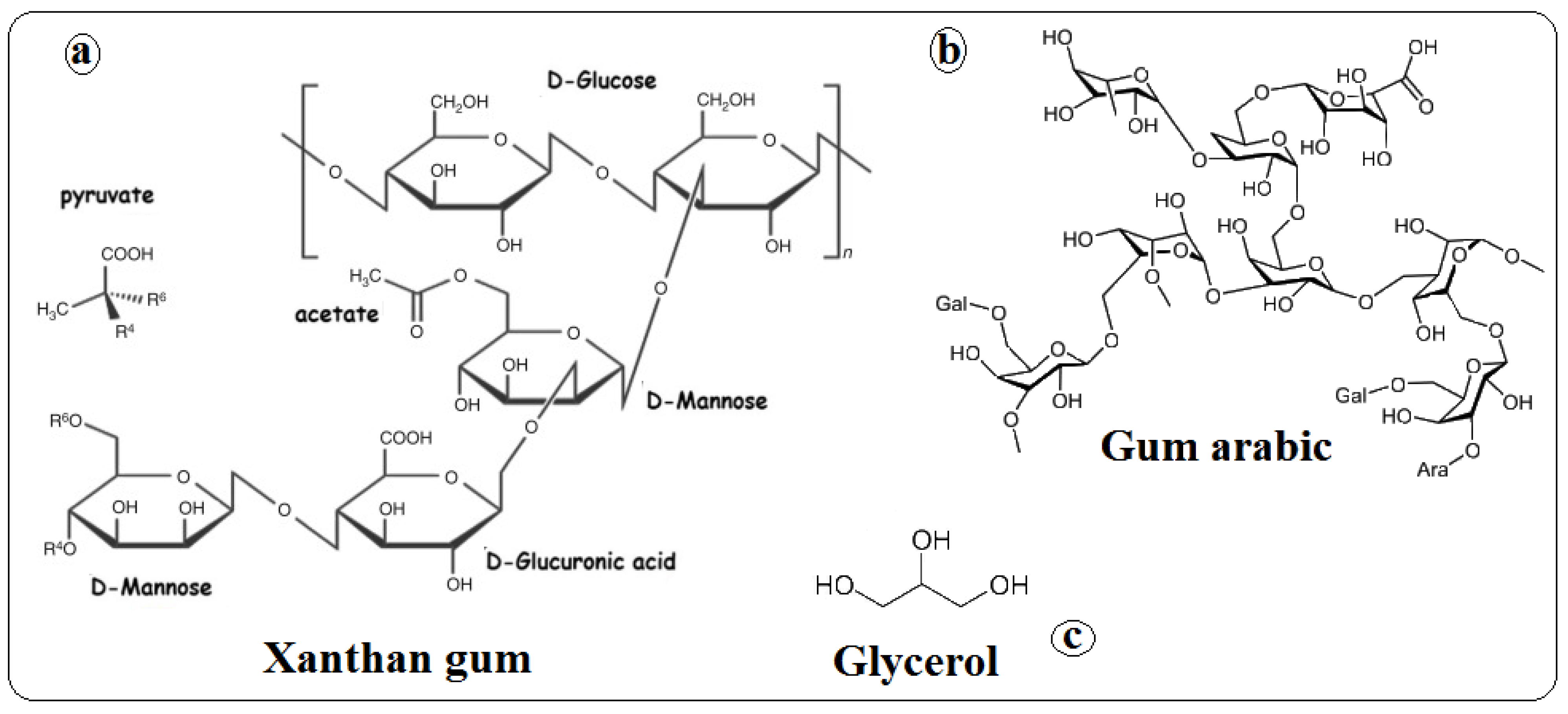
2.1. Materials
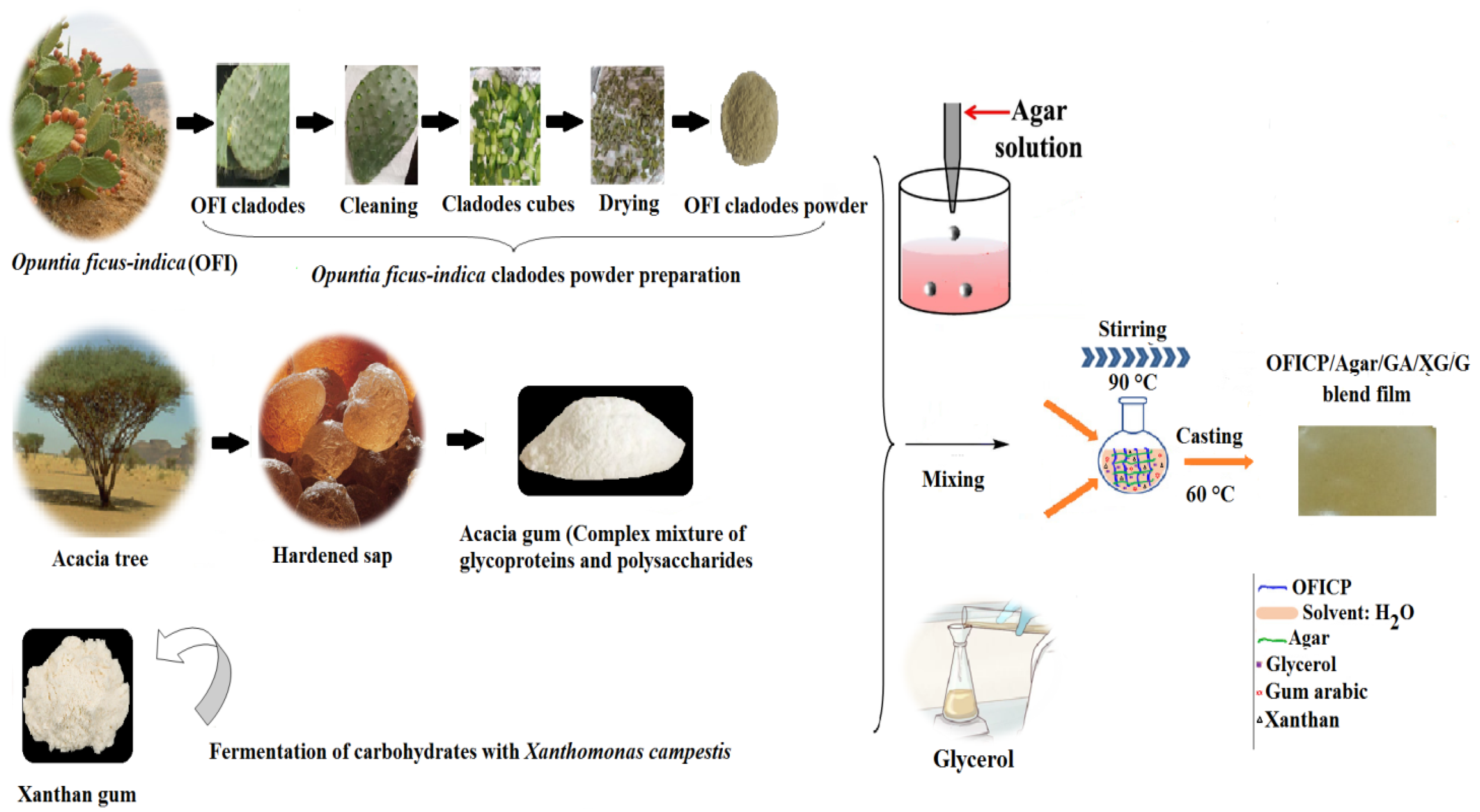
2.2. Preparation of Opuntia ficus-indica Cladodes Powder (OFICP)
2.3. Blend Films Preparation
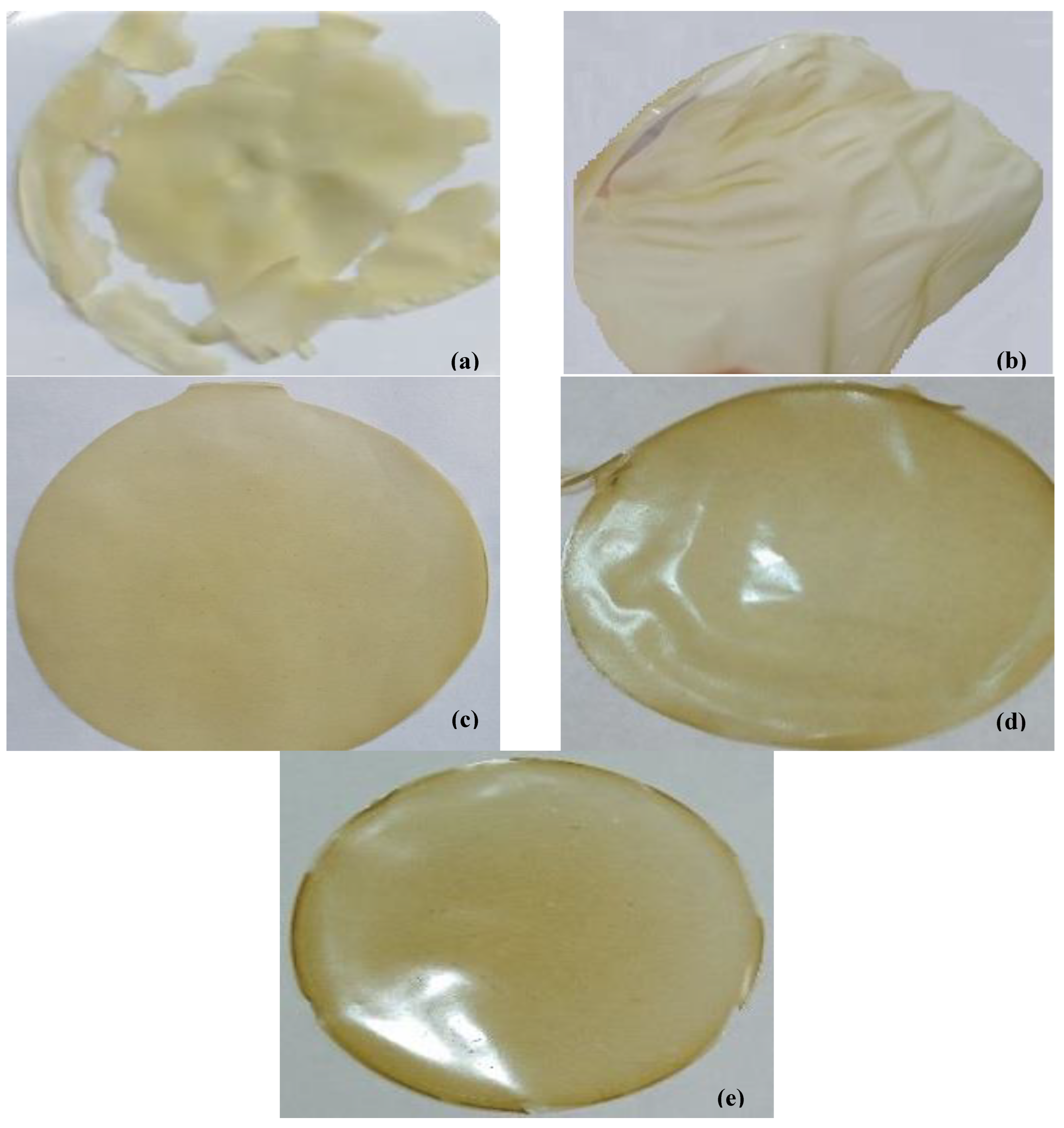
2.4. Visual Control
2.5. Film Thickness Measurement
2.6. Scanning Electron Microscope (SEM)
2.7. Moisture Content
2.8. Water Solubility
2.9. Fourier Transform Infrared (FTIR) Spectroscopy
2.10. X-ray Diffraction (XRD)
2.11. Differential Scanning Calorimeter (DSC)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Film Appearance
3.2. Film Thickness
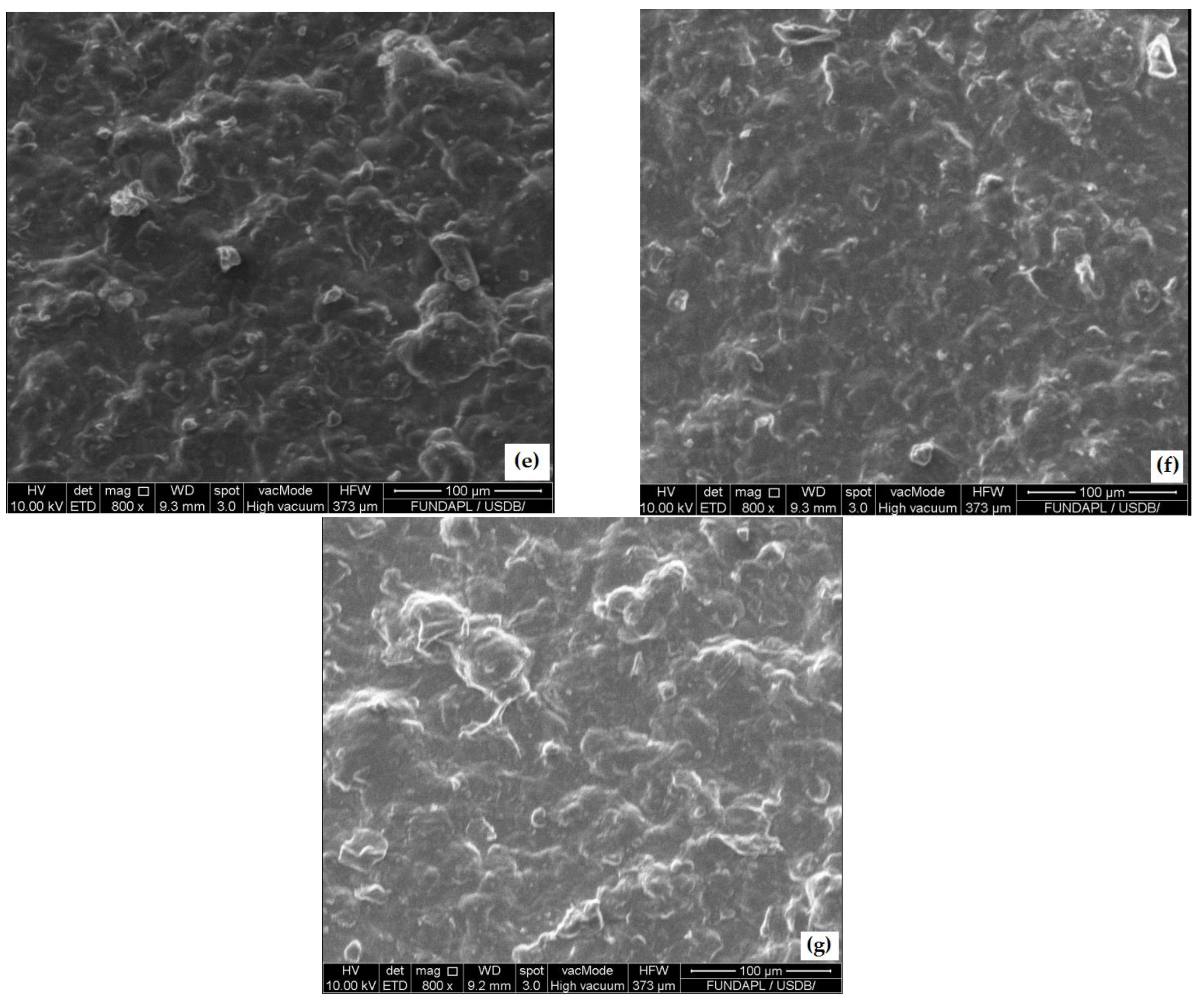
3.3. Film Morphology
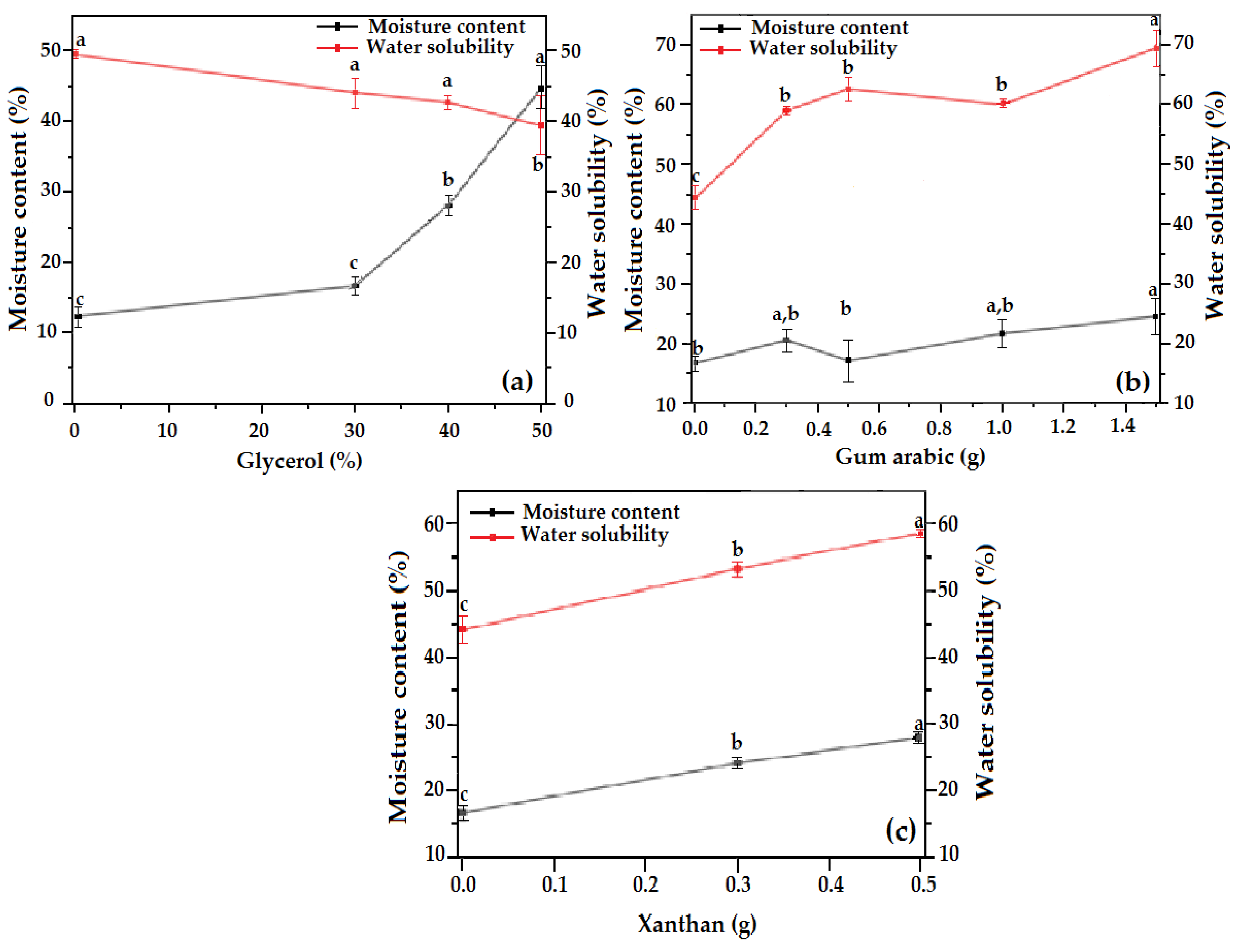
3.4. Moisture Content and Water Solubility
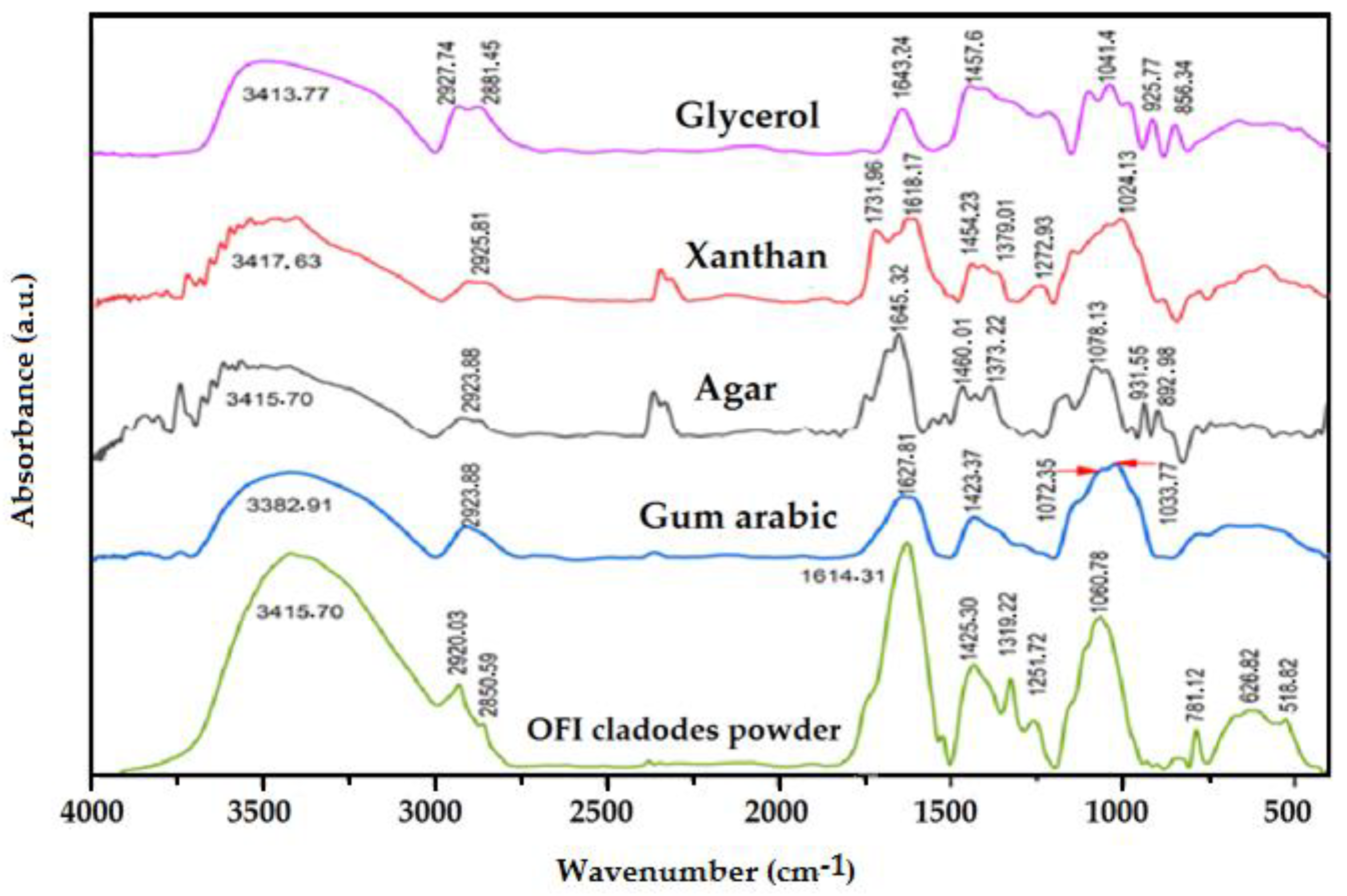
3.5. Chemical Composition and Molecular Interaction by FTIR
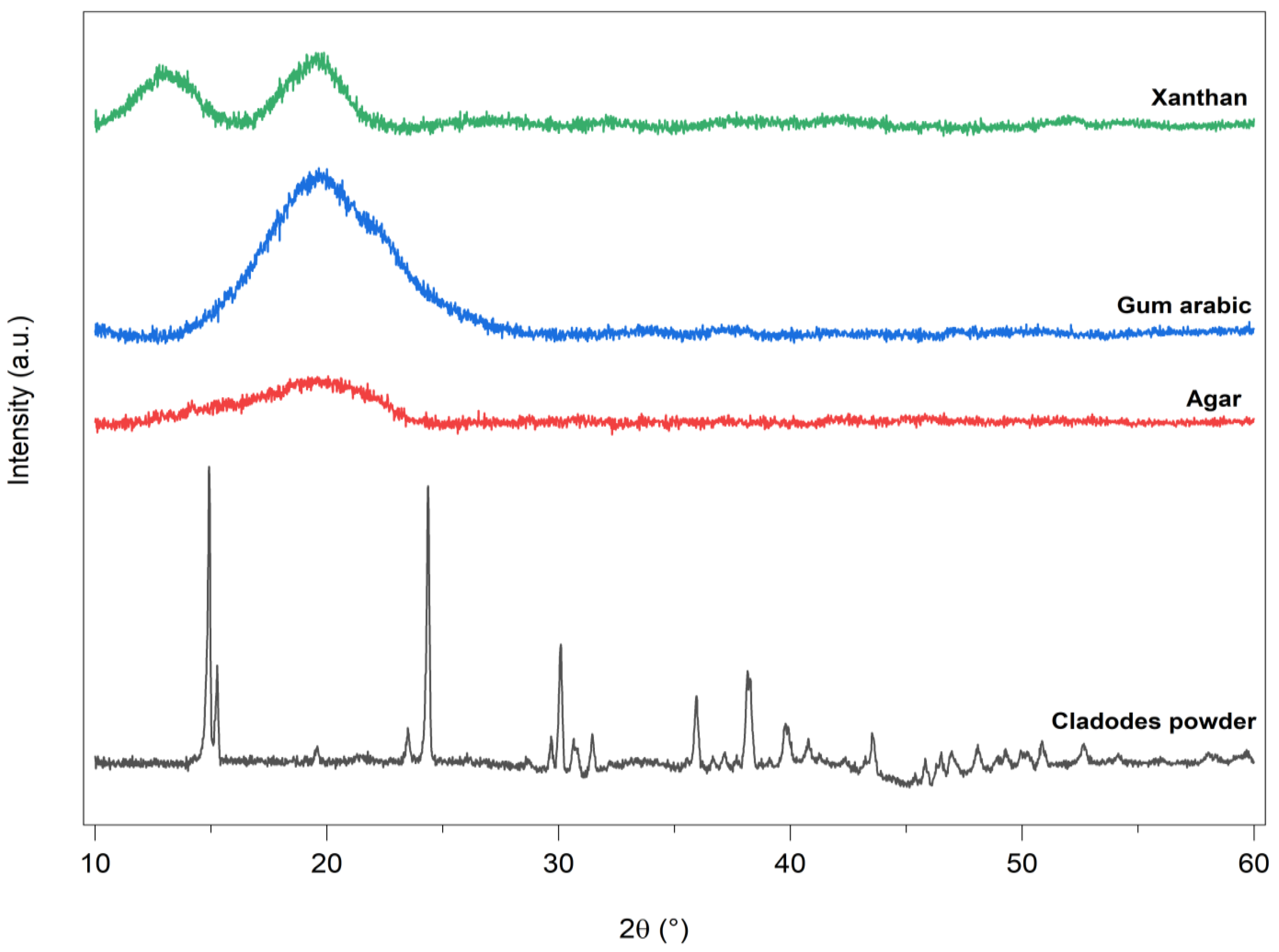
3.6. X-ray Diffraction Analysis
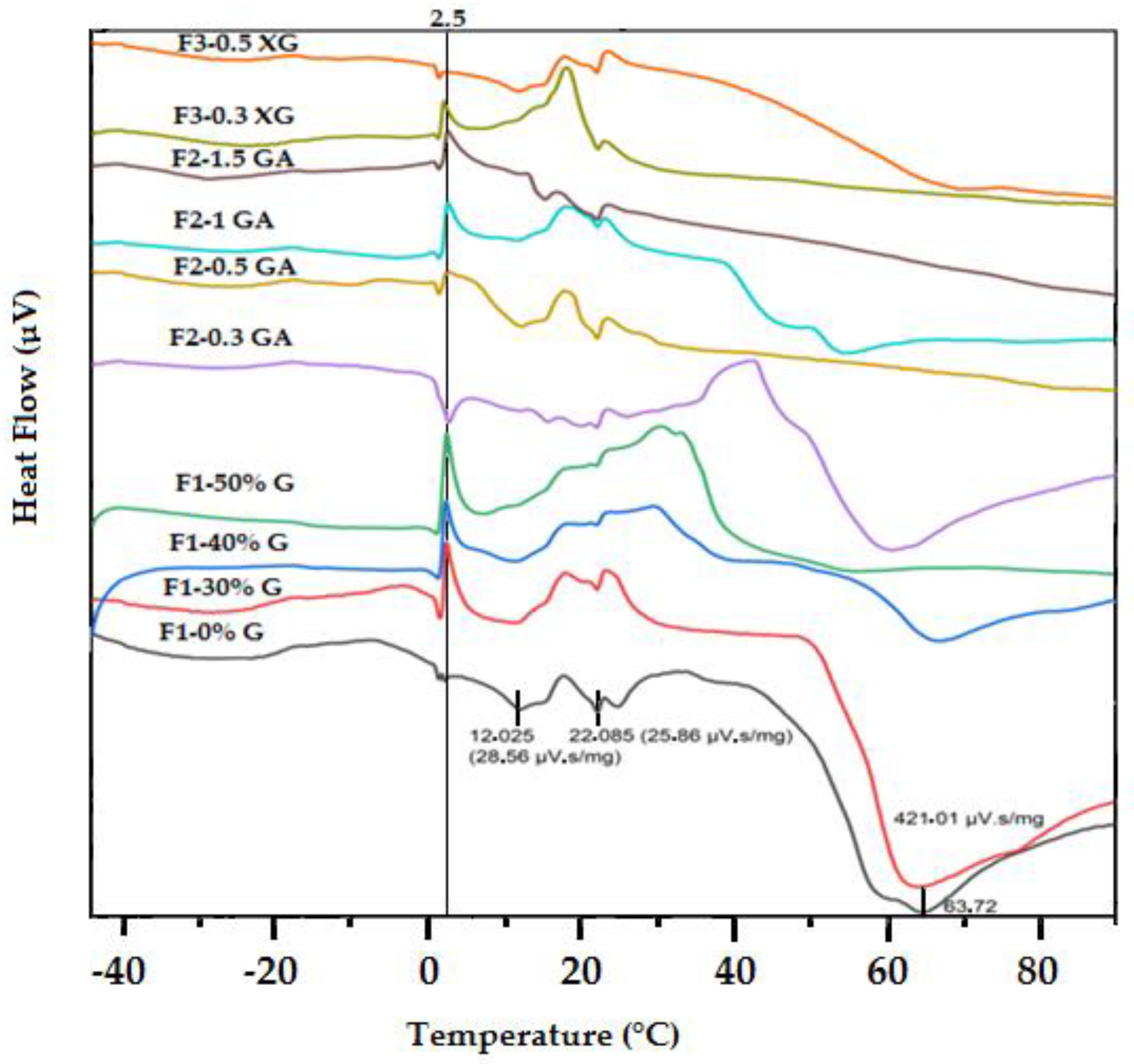
3.7. Thermal Properties by Differential Scanning Calorimeter (DSC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Namratha, K.; Ilyas, S.; Sultana, A.; Hezam, A.; Sunil, L.; Surmeneva, M.A.; Surmenev, R.A.; Nayan, M.B.; Ramakrishna, S.; et al. Emerging Trends for ZnO Nanoparticles and Their Applications in Food Packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Chen, X.; Ma, W.; Hu, W.; Liu, Z.; Wang, H.; Chen, Y.; Li, L. Progress in release-activated food packaging films. Packag. Technol. Sci. 2023, 36, 889–902. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 15, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Miller, S.A. Environmental impacts of plastic packaging of food products. Resour. Conserv. Recycl. 2022, 180, 106156. [Google Scholar] [CrossRef]
- Real, L.E.P. Plastics Statistics: Production, Recycling, and Market Data. In Recycled Materials for Construction Applications; Springer: Cham, Switzerland, 2023; pp. 103–113. [Google Scholar]
- Oudir, M.; Chader, H.; Bouzid, B.; Bendisari, K.; Latreche, B.; Boudalia, S.; Iguer-Ouada, M. Male rat exposure to low dose of di (2-ethylhexyl) phthalate during pre-pubertal, pubertal and post-pubertal periods: Impact on sperm count, gonad histology and testosterone secretion. Reprod. Toxicol. 2018, 75, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltrán, J.A.; Roncalés, P. Development of a new antioxidant active packaging for fresh meat. J. Agric. Food Chem. 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Djenane, D.; Beltrán, J.A.; Camo, J.; Roncalés, P. Influence of vacuum at different ageing times and subsequent retail display on shelf life of beef cuts packaged with active film under high O2. J. Food Sci. Technol. 2016, 53, 4244–4257. [Google Scholar] [CrossRef]
- Lammi, S.; Le Moigne, N.; Djenane, D.; Gontard, N.; Angellier Coussy, H. Dry fractionation of olive pomace for the development of food packaging biocomposites. Ind. Crops Prod. 2018, 120, 250–261. [Google Scholar] [CrossRef]
- Ahmed, W.; Azmat, R.; Khojah, E.; Ahmed, R.; Qayyum, A.; Shah, A.N.; Abbas, A.; Moin, S.; Samra, B.N. The development of a green innovative bioactive film for industrial application as a new emerging technology to protect the quality of fruits. Molecules 2022, 27, 486. [Google Scholar] [CrossRef] [PubMed]
- Narasagoudr, S.S.; Masti, S.P.; Hegde, V.G.; Chougale, R.B. Cetrimide crosslinked chitosan/guar gum/gum ghatti active biobased films for food packaging applications. J. Polym. Environ. 2023, 31, 579–594. [Google Scholar] [CrossRef]
- Ait Ouahioune, L.; Wrona, M.; Becerril, R.; Salafranca, J.; Nerín, C.; Djenane, D. Ceratonia siliqua L. kibbles, seeds and leaves as a source of volatile bioactive compounds for antioxidant food biopackaging applications. Food Packag. Shelf Life 2022, 31, 100764. [Google Scholar] [CrossRef]
- Ratna; Ulfariati, C.; Yusmanizar; Aprilia, S.; Rahmiati; Munawar, A.A. Development of biocomposite edible film food packaging based on gelatin from chicken claw waste. Case Stud. Chem. Environ. Eng. 2023, 8, 100371. [Google Scholar] [CrossRef]
- Gürler, N.; Torğut, G. Physicomechanical, thermal and dielectric properties of eco-friendly starch-microcrystalline cellulose-clay nanocomposite films for food packaging and electrical applications. Packag. Technol. Sci. 2022, 35, 473–483. [Google Scholar] [CrossRef]
- Freitas, E.E.S.; Dias, Ê.R.; Albuquerque, M.M.S.; Biondi, I.B.; Branco, C.R.C.; Cruz, R.S.; Branco, A.; Camilloto, G.P. Antioxidant films based on poly(lactic acid) incorporated with crude extract from Malpighia emarginata DC pomace for use in food packaging. Packag. Technol. Sci. 2024, 37, 39–50. [Google Scholar] [CrossRef]
- Martins, M.; Ribeiro, M.H.; Almeida, C.M.M. Physicochemical, nutritional, and medicinal properties of Opuntia ficus-indica (L.) Mill. and its main agro-industrial use: A review. Plants 2023, 12, 1512. [Google Scholar] [CrossRef]
- Dick, M.; Limberger, C.; Thys, R.C.S.; de Oliveira Rios, A.; Flôres, S.H. Mucilage and cladode flour from cactus (Opuntia monacantha) as alternative ingredients in gluten-free crackers. Food Chem. 2020, 314, 126178. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization. Élaboration D’une Stratégie de Développement de la Filière du Figuier de Barbarie (Opuntia ficus-indica L.) en Algérie—TCP/ALG/3702. 2022. Available online: www.fao.org (accessed on 2 November 2023).
- Maaoui, A.; Trabelsi, A.B.H.; Hamdi, M.; Chagtmi, R.; Jamaaoui, F.; Lopez, G.; Cortazar, M.; Olazar, M. Towards local circular economy through Opuntia Ficus-indica cladodes conversion into renewable biofuels and biochars: Product distribution and kinetic modelling. Fuel 2023, 332, 126056. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; de los Angeles Aguilera-Barreiro, M.; Contreras-Padilla, M.; Pérez-Torrero, E.; Rodriguez-Garcia, M.E. Nopal cladodes (Opuntia ficus indica): Nutritional properties and functional potential. J. Funct. Foods 2022, 95, 105183. [Google Scholar] [CrossRef]
- De Andrade Vieira, É.; de Magalhães Cordeiro, A.M.T. Bioprospecting and potential of cactus mucilages: A bibliometric review. Food Chem. 2022, 401, 134121. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A.; Izzo, E.; Rizza, L.; Bonina, A.; Bonina, F. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, E347–E352. [Google Scholar] [CrossRef] [PubMed]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Kim, S.R.; Lee, W.Y. Application of plant mucilage polysaccharides and their techno-functional properties’ modification for fresh produce preservation. Carbohydr. Polym. 2021, 272, 118371. [Google Scholar] [CrossRef] [PubMed]
- Gheribi, R.; Khwaldia, K. Cactus mucilage for food packaging applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef]
- Makhloufi, N.; Chougui, N.; Rezgui, F.; Benramdane, E.; Silvestre, A.J.; Freire, C.S.; Vilela, C. Polysaccharide-based films of cactus mucilage and agar with antioxidant properties for active food packaging. Polym. Bull. 2022, 79, 11369–11388. [Google Scholar] [CrossRef]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia ficus indica edible parts: A food and nutritional security perspective. Food Rev. Int. 2022, 38, 930–952. [Google Scholar] [CrossRef]
- Msaddak, L.; Abdelhedi, O.; Kridene, A.; Rateb, M.; Belbahri, L.; Ammar, E.; Nasri, M.; Zouari, N. Opuntia ficus-indica cladodes as a functional ingredient: Bioactive compounds profile and their effect on antioxidant quality of bread. Lipids Health Dis. 2017, 16, 32. [Google Scholar] [CrossRef]
- Makhloufi, N.; Chougui, N.; Rezgui, F.; Benramdane, E.; Freire, C.S.; Vilela, C.; Silvestre, A.J. Bio-based sustainable films from the Algerian Opuntia ficus-indica cladodes powder: Effect of plasticizer content. J. Appl. Polym. Sci. 2021, 138, 50450. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Megna, B. Structure-property relationship of PLA-Opuntia Ficus-indica biocomposites. Compos. Part B Eng. 2019, 167, 199–206. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Chacon, W.D.C.; Paz-Arteaga, S.L.; Torres-León, C.; Valencia, G.A. Gums-Based Coatings Applied to Extend the Shelf Life of Foods: A Review. J. Polym. Environ. 2023, 31, 433–446. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, J.; Duan, A.; Li, X. Pectin/sodium alginate/xanthan gum edible composite films as the fresh-cut package. Int. J. Biol. Macromol. 2021, 181, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Xiao, Y.; Guo, X.; Huang, A.; Xu, H. Development of gum arabic-based nanocomposite films reinforced with cellulose nanocrystals for strawberry preservation. Food Chem. 2021, 350, 129199. [Google Scholar] [CrossRef] [PubMed]
- Rukmanikrishnan, B.; Rajasekharan, S.K.; Lee, J.; Lee, J. Biocompatible agar/xanthan gum composite films: Thermal, mechanical, UV, and water barrier properties. Polym. Adv. Technol. 2019, 30, 2750–2758. [Google Scholar] [CrossRef]
- Tahsiri, Z.; Mirzaei, H.; Hosseini, S.M.H.; Khalesi, M. Gum arabic improves the mechanical properties of wild almond protein film. Carbohydr. Polym. 2019, 222, 114994. [Google Scholar] [CrossRef]
- Zibaei, R.; Hasanvand, S.; Hashami, Z.; Roshandel, Z.; Rouhi, M.; de Toledo Guimarães, J.; Mortazavian, A.M.; Sarlak, Z.; Mohammadi, R. Applications of emerging botanical hydrocolloids for edible films: A review. Carbohydr. Polym. 2021, 256, 117554. [Google Scholar] [CrossRef]
- De Farias, P.M.; de Vasconcelos, L.B.; da Silva Ferreira, M.E.; Alves Filho, E.G.; De Freitas, V.A.; Tapia-Blácido, D.R. Nopal cladode as a novel reinforcing and antioxidant agent for starch-based films: A comparison with lignin and propolis extract. Int. J. Biol. Macromol. 2021, 183, 614–626. [Google Scholar] [CrossRef]
- Yoksan, R.; Dang, K.M. The effect of polyethylene glycol sorbitan monostearate on the morphological characteristics and performance of thermoplastic starch/biodegradable polyester blend films. Int. J. Biol. Macromol. 2023, 231, 123332. [Google Scholar] [CrossRef]
- De Farias, P.M.; de Vasconcelos, L.B.; Ferreira, M.E.; Pascall, M.; Tapia-Blácido, D.R. Nopal cladode (Opuntia ficus-indica) flour: Production, characterization, and evaluation for producing bioactive film. Food Packag. Shelf Life 2021, 29, 100703. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Ben Miri, Y.; Ariño, A. Use of algerian type Ras El-Hanout spices mixture with marination to increase the sensorial quality, shelf life, and safety of whole rabbit carcasses under low-O2 modified atmosphere packaging. Foods 2023, 12, 2931. [Google Scholar] [CrossRef] [PubMed]
- Ait Ouahioune, L.; Wrona, M.; Nerín, C.; Djenane, D. Novel active biopackaging incorporated with macerate of carob (Ceratonia siliqua L.) to extend shelf-life of stored Atlantic salmon fillets (Salmo salar L.). LWT Food Sci. Technol. 2022, 156, 113015. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Khazaei, N.; Ghasemlou, M.; HadiNezhad, M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr. Polym. 2013, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.B.; Choi, Y.-G.; Kim, J.-Y.; Lim, S.-T. Improvement of water solubility and humidity stability of tapioca starch film by incorporating various gums. LWT Food Sci. Technol. 2015, 64, 475–482. [Google Scholar] [CrossRef]
- Ratna; Aprilia, S.; Arahman, N.; Bilad, M.R.; Suhaimi, H.; Munawar, A.A.; Nasution, I.S. Bio-nanocomposite based on edible gelatin film as active packaging from Clarias gariepinus fish skin with the addition of cellulose nanocrystalline and nanopropolis. Polymers 2022, 14, 3738. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Khodaei, D.; Oltrogge, K.; Hamidi-Esfahani, Z. Preparation and characterization of blended edible films manufactured using gelatin, tragacanth gum and, Persian gum. LWT Food Sci. Technol. 2020, 117, 108617. [Google Scholar]
- Tessaro, L.; Lourenço, R.V.; Martelli-Tosi, M.; do Amaral Sobral, P.J. Gelatin/chitosan based films loaded with nanocellulose from soybean straw and activated with “Pitanga” (Eugenia uniflora L.) leaf hydroethanolic extract in W/O/W emulsion. Int. J. Biol. Macromol. 2021, 186, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ustunol, Z. Solubility and moisture sorption isotherms of whey-protein-based edible films as influenced by lipid and plasticizer incorporation. J. Agric. Food Chem. 2001, 49, 4388–4391. [Google Scholar] [CrossRef] [PubMed]
- Arham, R.; Salengke, S.; Metusalach, M.; Mulyati, M. Optimization of agar and glycerol concentration in the manufacture of edible film. Int. Food Res. J. 2018, 25, 1845–1851. [Google Scholar]
- Hazirah, M.N.; Isa, M.; Sarbon, N. Effect of xanthan gum on the physical and mechanical properties of gelatin-carboxymethyl cellulose film blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Haq, M.A.; Hasnain, A.; Azam, M. Characterization of edible gum cordia film: Effects of plasticizers. LWT Food Sci. Technol. 2014, 55, 163–169. [Google Scholar] [CrossRef]
- Arismendi, C.; Chillo, S.; Conte, A.; Del Nobile, M.A.; Flores, S.; Gerschenson, L.N. Optimization of physical properties of xanthan gum/tapioca starch edible matrices containing potassium sorbate and evaluation of its antimicrobial effectiveness. LWT Food Sci. Technol. 2013, 53, 290–296. [Google Scholar] [CrossRef]
- Bertasa, M.; Botteon, A.; Brambilla, L.; Riedo, C.; Chiantore, O.; Poli, T.; Sansonetti, A.; Scalarone, D. Cleaning materials: A compositional multi-analytical characterization of commercial agar powders. J. Anal. Appl. Pyrolysis 2017, 125, 310–317. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Shahidi-Noghabi, M.; Dovom, A.M. Investigating the fabrication and functional properties of new composite hydrogels containing gellan/alginate/xanthan gum. J. Sol-Gel Sci. Technol. 2023, 105, 637–649. [Google Scholar] [CrossRef]
- Karaaslan, M.; Şengün, F.; Cansu, Ü.; Başyiğit, B.; Sağlam, H.; Karaaslan, A. Gum arabic/maltodextrin microencapsulation confers peroxidation stability and antimicrobial ability to pepper seed oil. Food Chem. 2021, 337, 127748. [Google Scholar] [CrossRef]
- Bokovets, S.P.; Pertsevoi, F.V.; Murlykina, N.V.; Smetanska, I.M.; Borankulova, A.S.; Ianchyk, M.V.; Omelchenko, S.B.; Grinchenko, O.O.; Grychenko, N.G.; Dikhtyar, A.M. Investigation of infrared spectra of agar-based gel systems for the production of jelly bars. J. Chem. Technol. 2023, 31, 92–103. [Google Scholar]
- Wanchoo, R.; Sharma, P. Viscometric study on the compatibility of some water-soluble polymer–polymer mixtures. Eur. Polym. J. 2003, 39, 1481–1490. [Google Scholar] [CrossRef]
- Jaderi, Z.; Tabatabaee Yazdi, F.; Mortazavi, S.A.; Koocheki, A. Effects of glycerol and sorbitol on a novel biodegradable edible film based on Malva sylvestris flower gum. Food Sci. Nutr. 2023, 11, 991–1000. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef]
- Plota, A.; Masek, A. Lifetime prediction methods for degradable polymeric materials—A short review. Materials 2020, 13, 4507. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Toker, O.S.; Tornuk, F. Effect of xanthan and locust bean gum synergistic interaction on characteristics of biodegradable edible film. Int. J. Biol. Macromol. 2017, 102, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Padilla, M.; Rivera-Muñoz, E.M.; Gutiérrez-Cortez, E.; Del López, A.R.; Rodríguez-García, M.E. Characterization of crystalline structures in Opuntia ficus-indica. J. Biol. Phys. 2015, 41, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, J.; Tan, K.B.; Chen, M.; Zhu, Y. Development of hydroxypropyl methylcellulose film with xanthan gum and its application as an excellent food packaging bio-material in enhancing the shelf life of banana. Food Chem. 2022, 374, 131794. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; John, M.; Misra, M.; Menezes, P.L. Nanocrystalline Materials: Synthesis, Characterization, Properties, and Applications. Crystals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Bijalwan, P.; Dutta, M.; Banerjee, A.; Laha, T. Fe-based amorphous/nanocrystalline composite coating by plasma spraying: Effect of heat input on morphology, phase evolution and mechanical properties. J. Alloys Compd. 2019, 771, 827–837. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- de Morais Lima, M.; Carneiro, L.C.; Bianchini, D.; Dias, A.R.G.; Zavareze, E.d.R.; Prentice, C.; Moreira, A.d.S. Structural, thermal, physical, mechanical, and barrier properties of chitosan films with the addition of xanthan gum. J. Food Sci. 2017, 82, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, R.; Hong, S.Y.; Lee, J.; Seo, Y.-K.; Nam, J.-D.; Suhr, J. MXene-xanthan nanocomposite films with layered microstructure for electromagnetic interference shielding and Joule heating. Chem. Eng. J. 2021, 410, 128348. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Viguié, J.; Molina-Boisseau, S.; Dufresne, A. Processing and characterization of waxy maize starch films plasticized by sorbitol and reinforced with starch nanocrystals. Macromol. Biosci. 2007, 7, 1206–1216. [Google Scholar] [CrossRef]
- Chaudhary, D.S.; Adhikari, B.P.; Kasapis, S. Glass-transition behaviour of plasticized starch biopolymer system—A modified Gordon–Taylor approach. Food Hydrocoll. 2011, 25, 114–121. [Google Scholar] [CrossRef]
- Aphibanthammakit, C.; Nigen, M.; Gaucel, S.; Sanchez, C.; Chalier, P. Surface properties of Acacia senegal vs Acacia seyal films and impact on specific functionalities. Food Hydrocoll. 2018, 82, 519–533. [Google Scholar] [CrossRef]
- Manhivi, V.E.; Venter, S.; Amonsou, E.O.; Kudanga, T. Composition, thermal and rheological properties of polysaccharides from amadumbe (Colocasia esculenta) and cactus (Opuntia spp.). Carbohydr. Polym. 2018, 195, 163–169. [Google Scholar] [CrossRef]
- Rivera-Corona, J.L.; Rodríguez-González, F.; Rendón-Villalobos, R.; García-Hernández, E.; Solorza-Feria, J. Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition. LWT Food Sci. Technol. 2014, 59, 806–812. [Google Scholar] [CrossRef]



| Formulation Number | Codification | Ratio OFICP:A:GA:XG | OFICP (g) | Agar (A) (g) | Gum Arabic (GA) (g) | Xanthan (XG) (g) | Glycerol (G) (%) |
|---|---|---|---|---|---|---|---|
| 1st formulation (F1) | F1-0%G | 2:2:0:0 | 2 | 2 | 0 | 0 | 0 |
| F1-30%G | 2:2:0:0 | 2 | 2 | 0 | 0 | 30 | |
| F1-40%G | 2:2:0:0 | 2 | 2 | 0 | 0 | 40 | |
| F1-50%G | 2:2:0:0 | 2 | 2 | 0 | 0 | 50 | |
| 2nd formulation (F2) | F2-0.3GA | 2:1.7: 0.3:0 | 2 | 1.7 | 0.3 | 0 | 30 |
| F2-0.5GA | 2:1.5: 0.5:0 | 2 | 1.5 | 0.5 | 0 | 30 | |
| F2-1GA | 2:1:1:0 | 2 | 1 | 1 | 0 | 30 | |
| F2-1.5GA | 2:0.5:1.5:0 | 2 | 0.5 | 1.5 | 0 | 30 | |
| 3rd formulation (F3) | F3-0.3XG | 2:1.7:0:0.3 | 2 | 1.7 | 0 | 0.3 | 30 |
| F3-0.5XG | 2:1.5:0:0.5 | 2 | 1.5 | 0 | 0.5 | 30 | |
| F3-1XG | 2:1:0:1 | 2 | 1 | 0 | 1 | 30 |
| Formulation Number | Samples Code | Thickness (mm) |
|---|---|---|
| 1st formulation (F1) | F1-0%G | 0.056 ± 0.013 c |
| F1-30%G | 0.069 ± 0.016 b | |
| F1-40%G | 0.084 ± 0.009 a | |
| F1-50%G | 0.095 ± 0.023 a | |
| 2nd formulation (F2) | F2-0.3GA | 0.123 ± 0.058 a |
| F2-0.5GA | 0.124 ± 0.048 a | |
| F2-1GA | 0.098 ± 0.029 a,b | |
| F2-1.5GA | 0.090 ± 0.016 b | |
| 3rd formulation (F3) | F3-0.3XG | 0.105 ± 0.03 a |
| F3-0.5XG | 0.101 ± 0.02 a |
| Sample | Peak Position (°) | Crystallinity (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OFICP | 14.92 | 15.27 | 23.49 | 24.37 | 29.67 | 35.95 | 38.15 | 25.89 | ||||||
| F1-0%G | 12.70 | 13.74 | 15.33 | 23.49 | 24.45 | 27.63 | 30.14 | |||||||
| F1-30%G | 14.34 | 23.62 | 29.26 | 35.19 | 37.35 | 6.20 | ||||||||
| F1-40%G | 14.49 | 23.80 | 29.55 | 35.35 | 37.66 | 9.63 | ||||||||
| F1-50%G | 14.49 | 23.80 | 29.52 | 35.37 | 37.66 | 8.64 | ||||||||
| F2-0.3GA | 14.65 | 24.19 | 29.89 | 35.79 | 38.02 | 3.72 | ||||||||
| F2-0.5GA | 13.63 | 15.12 | 24.53 | 30.38 | 38.41 | 4.64 | ||||||||
| F2-1GA | 14.83 | 15.61 | 24.99 | 30.59 | 38.67 | 4.49 | ||||||||
| F2-1.5GA | 14.55 | 24.01 | 25.67 | 26.66 | 28.59 | 29.70 | 7.60 | |||||||
| F3-0.3XG | 14.70 | 24.21 | 29.89 | 35.76 | 38.13 | 5.051 | ||||||||
| F3-0.5XG | 14.44 | 23.88 | 24.09 | 29.57 | 35.42 | 37.79 | 5.033 | |||||||
| Samples Code | Glass Transition (°C)/ ∆C p (µV) | |||
|---|---|---|---|---|
| Tig | Tg (Peak) | Teg | ∆C p (µV) | |
| F1-0%G | 03.59 | 01.89 | 0.966 | 1.079 |
| F1-30%G | ND | ND | ND | ND |
| F1-40%G | 16.45 | 15.42 | 15.073 | 0.576 |
| F1-50%G | 16,80 | 15.67 | 16.246 | 0.29 |
| F2-0.3GA | 11.36 | 08.87 | 9.633 | 1.019 |
| F2-0.5GA | 11.62 | 11.45 | 11.018 | 0.174 |
| F2-1GA | 15.98 | 15.29 | 16.423 | 0.013 |
| F2-1.5GA | 23.22 | 31.31 | 31.175 | 1.079 |
| F3-0.3XG | 27.60 | 27.70 | 25.573 | 0.932 |
| F3-0.5XG | 16.981 | 16.381 | 15.34 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudir, M.; Ait Mesbah, Z.; Lerari, D.; Issad, N.; Djenane, D. Development of Eco-Friendly Biocomposite Films Based on Opuntia ficus-indica Cladodes Powder Blended with Gum Arabic and Xanthan Envisaging Food Packaging Applications. Foods 2024, 13, 78. https://doi.org/10.3390/foods13010078
Oudir M, Ait Mesbah Z, Lerari D, Issad N, Djenane D. Development of Eco-Friendly Biocomposite Films Based on Opuntia ficus-indica Cladodes Powder Blended with Gum Arabic and Xanthan Envisaging Food Packaging Applications. Foods. 2024; 13(1):78. https://doi.org/10.3390/foods13010078
Chicago/Turabian StyleOudir, Malha, Zohra Ait Mesbah, Djahida Lerari, Nadia Issad, and Djamel Djenane. 2024. "Development of Eco-Friendly Biocomposite Films Based on Opuntia ficus-indica Cladodes Powder Blended with Gum Arabic and Xanthan Envisaging Food Packaging Applications" Foods 13, no. 1: 78. https://doi.org/10.3390/foods13010078