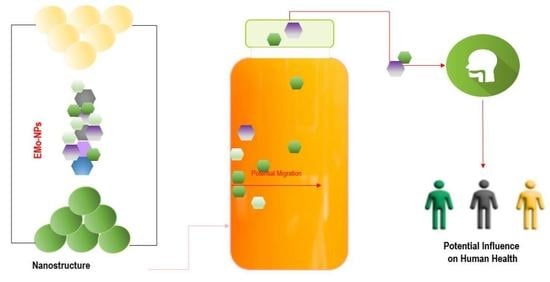
Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health
Abstract
:1. Introduction
2. Antimicrobial Mechanisms of EMo-NPs
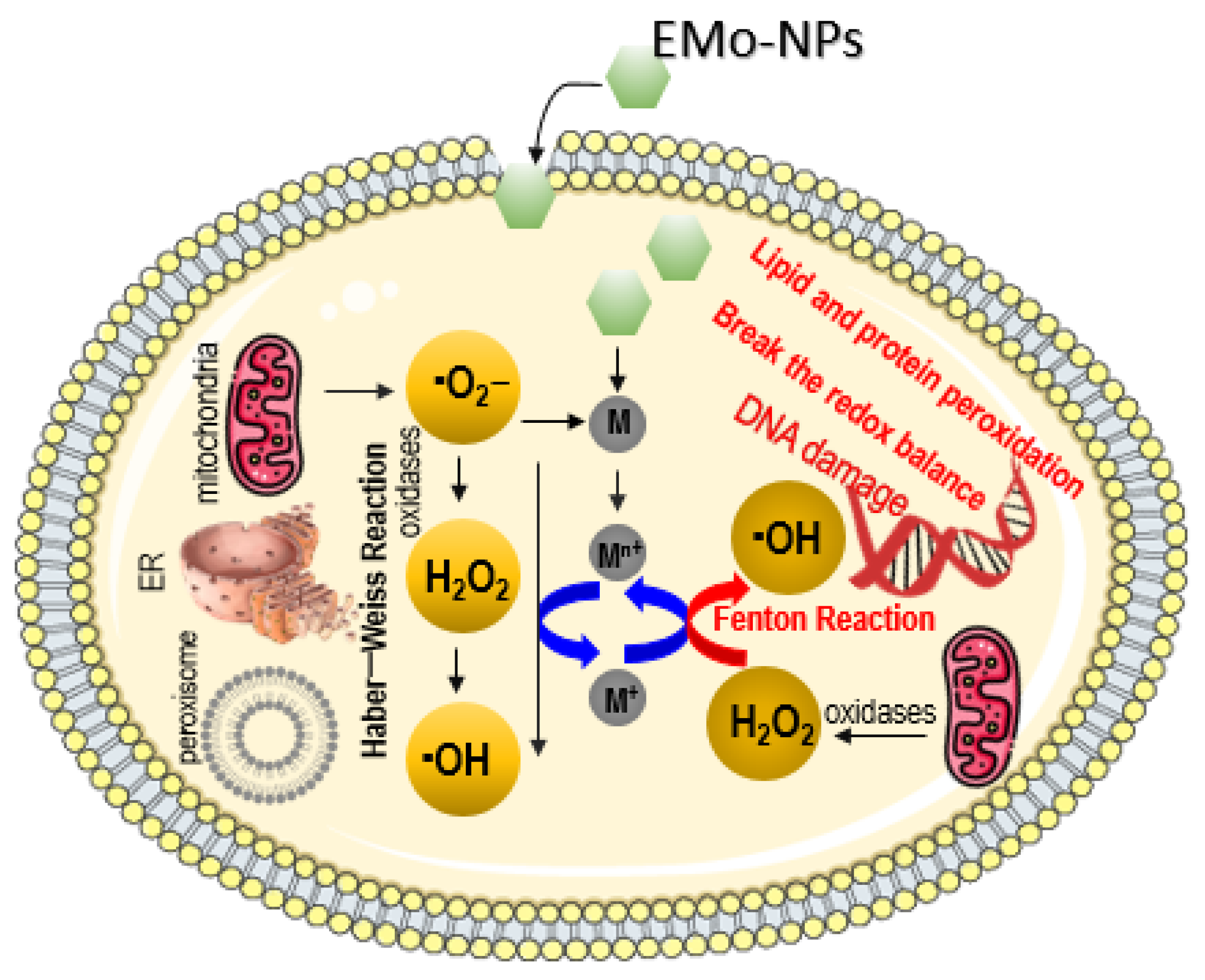
2.1. ROS Induction through Fenton-Type and Haber–Weiss Reactions
2.2. ROS Induction by EMo-NPs through Photocatalytical Reactions
3. EMo-NPs’ Route from Food/Beverage Packaging into Human GI Tract
3.1. EMo-NPs Migration from the Package Matrix into Food/Beverage Matrix: Possible Mechanisms
3.2. EMo-NPs’ Route from the Food/Beverage Matrix into the Human GI Tract
4. Influences of EMo-NPs on Human Health
4.1. Intracellular ROS Induction in Eukaryotic Cells
4.2. EMo-NPs: Main Concerns
EMo-NPs’ Immunotoxicity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J. Nanobiotechnol. 2021, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Miteva, A. Nanotechnology in sport and security. Strateg. Policy Sci. Educ. 2021, 29, 46–53. [Google Scholar] [CrossRef]
- Ghebretatios, M.; Schaly, S.; Prakash, S. Nanoparticles in the Food Industry and Their Impact on Human Gut Microbiome and Diseases. Int. J. Mol. Sci. 2021, 22, 1942. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Pal, M. Nanotechnology: A New Approach in Food Packaging. J. Food. Microbiol. Saf. Hyg. 2017, 2, 1000121. [Google Scholar] [CrossRef]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The safety of nanomaterials in food production and packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Stoica, M.; Stoica, D. Nanofillers for Food Packaging: Antimicrobial Potential of Metal-Based Nanoparticles. CNTP 2020, 1, 1–23. [Google Scholar] [CrossRef]
- Yadav, S.K.; Yadav, R.D.; Tabassum, H.; Aria, M. Recent Developments in Nanotechnology-Based Biosensors for the Diagnosis of Coronavirus. Plasmonics 2023. [Google Scholar] [CrossRef]
- Xie, X. Application of Nanomedicine in Diagnostic Technology. Highlights Sci. Eng. Technol. 2023, 40, 125–131. [Google Scholar] [CrossRef]
- Tsuzuki, T. Mechanochemical synthesis of metal oxide nanoparticles. Commun. Chem. 2021, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional Nanoparticles and Nanopesticides in Agricultural Application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 690706. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food. 2017, 1, 6. [Google Scholar] [CrossRef]
- Franz, R.; Bott, J.; Störmer, A. Considerations for and Guidance to Testing and Evaluating Migration/Release of Nanoparticles from Polymer Based Nanocomposites. J. Nanomater. 2020, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Stoica, M. Biodegradable nanomaterials for drink packaging. In Nanotechnology in the Beverage Industry: Fundamentals and Applications, 1st ed.; Abdeltif, A., Ranjendran, S., Nguyen, T.A., Assadi, A., MahdySharoba, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 609–632. [Google Scholar]
- Hasan, K.M.F.; Xiaoyi, L.; Shaoqin, Z.; Horvath, P.G.; Bak, M.; Bejo, L.; Sipos, G.; Alpar, T. Functional silver nanoparticles synthesis from sustainable point of view: 2000 to 2023—A review on game changing materials. Heliyon 2022, 8, e12322. [Google Scholar] [CrossRef]
- Lingle, R. Nanotechnology’s Favorable Future in Food Packaging. Available online: https://www.packagingdigest.com/smart-packaging/nanotechnologys-favorable-future-food-packaging (accessed on 20 April 2023).
- Sharma, A.; Ranjit, R.; Kumar, N.; Kumar, M.; Giri, B.S. Nanoparticles based nanosensors: Principles and their applications in active packaging for food quality and safety detection. Biochem. Eng. J. 2023, 193, 108861. [Google Scholar] [CrossRef]
- Perera, K.Y.; Hopkins, M.; Jaiswal, A.K.; Jaiswal, S. Nanoclays-containing bio-based packaging materials: Properties, applications, safety, and regulatory issues. J. Nanostruct. Chem. 2023. [Google Scholar] [CrossRef]
- El Gohary, H.G.; Alhagri, I.A.; Qahtan, T.F.; Al-Hkimi, A.N.; Saeed, A.; Abolaban, F.; Alshammari, E.M.; Asnag, G.M. Reinforcement of structural, thermal and electrical properties and antibacterial activity of PVA/SA blend filled with hybrid nanoparticles (Ag and TiO2NPs): Nanodielectric for energy storage and food packaging industries. Ceram. Int. 2023, in press. [Google Scholar] [CrossRef]
- Global Metal & Metal Oxide Nanoparticles Market by Type (Aluminium, Iron, Gold, Copper, Silver, Magnesium, Platinum, Zinc, Others), By Application (Chemical & Coatings, Pharma & Healthcare, Transportation, Personal Care & Cosmetics, Electrical & Electronics, Defence, Other) And By Region (North America, Latin America, Europe, Asia Pacific and Middle East & Africa), Forecast from 2022 to 2030. Available online: https://dataintelo.com/report/metal-metal-oxide-nanoparticles-market/ (accessed on 20 April 2023).
- Metal Nanoparticles Market Share, Size, Trends, Industry Analysis Report, By Metal (Platinum, Gold, Silver, Iron, Copper, Nickel); By Synthesis Method; By End-Use Industry; By Region; Segment Forecast, 2022–2030. Available online: https://www.polarismarketresearch.com/industry-analysis/metal-nanoparticles-market (accessed on 21 April 2023).
- Chen, S.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, A.; Kaushal, A.; Kaur, D.; Pandey, A.; Goyal, R.N. In situ high temperature XRD studies of ZnO nanopowder prepared via cost effective ultrasonic mist chemical vapour deposition. Bull. Mater. Sci. 2008, 31, 573–577. [Google Scholar] [CrossRef]
- Annathurai, S.; Chidambaram, S.; Baskaran, B.; Venkatesan, G.P.P. Green Synthesis and Electrical Properties of p-CuO/n-ZnO Heterojunction Diodes. J. Inorg. Organomet. Polym. Mater. 2019, 29, 535–540. [Google Scholar] [CrossRef]
- Sharifpur, M.; Meyer, J.P.; Aybar, H.S. Nanofluids Opportunities and Challenges. In Proceedings of the 11th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Kruger National Park, South Africa, 20–23 July 2015. [Google Scholar]
- Shu, Z.; Wang, S. Synthesis and Characterization of Magnetic Nanosized Fe3O4/MnO2 Composite Particles. J. Nanomater. 2009, 2009, 340217. [Google Scholar] [CrossRef]
- Efatian, H.; Ahari, H.; Shahbazzadeh, D.; Nowruzi, B.; Yousefi, S. Fabrication and characterization of LDPE/silver-copper/titanium dioxide nanocomposite films for application in Nile Tilapia (Oreochromis niloticus) packaging. J. Food Meas. Charact. 2021, 15, 2430–2439. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef]
- Golabiazar, R.; Omar, Z.A.; Ahmad, R.N.; Hasan, S.A.; Sajadi, S. Mohammad Synthesis and characterization of antibacterial magnetite-activated carbon nanoparticles. J. Chem. Res. 2020, 44, 80–87. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef]
- Xu, J.K.; Zhang, F.F.; Sun, J.J.; Sheng, J.; Wang, F.; Sun, M. Bio and nanomaterials based on Fe3O4. Molecules 2014, 19, 21506–21528. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1431 (accessed on 2 March 2023).
- Luo, Y.B.; Cao, Y.Z.; Guo, G. Effects of TiO2 nanoparticles on the photodegradation of poly (lactic acid). J. Appl. Polym. Sci. 2018, 135, 46509. [Google Scholar] [CrossRef]
- Nenavathu, B.P.; Sharma, A.; Dutta, R.J. Se doped ZnO nanoparticles with improved catalytic activity in degradation of Cholesterol. J. Water. Environ. Nanotechnol. 2018, 3, 289–300. [Google Scholar]
- Braga, N.F.; da Silva, A.P.; Arantes, T.M.; Lemes, A.P.; Cristovan, F.H. Physical-chemical properties of nanocomposites based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and titanium dioxide nanoparticles. Mater. Res. Express. 2018, 5, 015303. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra, M.J.; Pourrahimi, A.; Olsson, R.T.; Lagaron, J.M. The impact of zinc oxide particle morphology as an antimicrobial and when incorporated in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfaces applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M.N. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef]
- Jafarizadeh-Malmiri, H.; Sayyar, Z.; Anarjan, N.; Berenjian, A. Challenges for Nanobiotechnology. In Nanobiotechnology in Food: Concepts, Applications and Perspectives, 1st ed.; Jafarizadeh-Malmiri, H., Sayyar, Z., Anarjan, N., Berenjian, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 19–25. [Google Scholar]
- Lan, W.; Wang, S.; Zhang, Z.; Liang, X.; Liu, X.; Zhang, J. Development of red apple pomace extract/chitosan-based films reinforced by TiO(2) nanoparticles as a multifunctional packaging material. Int. J. Biol. Macromol. 2021, 168, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life. 2019, 22, 100420. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Stoica, M. Polymer nanocomposites for drink bottles. In Nanotechnology in the Beverage Industry: Fundamentals and Applications, 1st ed.; Abdeltif, A., Ranjendran, S., Nguyen, T.A., Assadi, A., MahdySharoba, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 633–655. [Google Scholar]
- Ahmadi, A.; Ahmadi, P.; Sani, M.A.; Ehsani, A.; Ghanbarzadeh, B. Functional biocompatible nanocomposite films consisting of selenium and zinc oxide nanoparticles embedded in gelatin/cellulose nanofiber matrices. Int. J. Biol. Macromol. 2021, 175, 87–97. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra Rovira, M.J.; Mas, L.C.; Moragas, G.S.; Lagaron, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 45673. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Freitas, F.; Reis, M.A.M.; Prieto, M.A.; Lagaron, J.M. Biosynthesis of silver nanoparticles and polyhydroxybutyrate nanocomposites of interest in antimicrobial applications. Int. J. Biol. Macromol. 2018, 108, 426–435. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Stormer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Diaz, R.M.; Cardoso-Avila, P.E.; Tavares, J.A.P.; Patakfalvi, R.; Cruz, V.V.; de Guevara, H.; Coronado, O.G.; Garibay, R.I.A.; Arroyo, Q.E.S.; Marañón-Ruiz, V.F. Two-Step Triethylamine-Based Synthesis of MgO Nanoparticles and Their Antibacterial Effect against Pathogenic Bacteria. Nanomaterials 2021, 11, 410. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.; Almeida, A. Metallic Nanoparticles in the Food Sector: A Mini-Review. Foods 2022, 11, 402. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured nanoparticles in the aquatic environmentbiochemical responses on freshwater organisms: A critical overview. Aquat. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Antibacterial Nanocomposites Based on Thermosetting Polymers Derived from Vegetable Oils and Metal Oxide Nanoparticles. Polymers 2019, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.C.; Moura, F.C.C.; Ardisson, J.D.; Fabris, J.D.; Lago, R.M. Highly active heterogeneous Fenton-like systems based on FeO/Fe3O4 composites prepared by controlled reduction of iron oxides. Appl. Catal. B 2008, 83, 131–139. [Google Scholar] [CrossRef]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association with Alzheimer’s. Disease Arch. Neurosci. 2014, 2, e20078. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, L. Magnetite (Fe3O4) nanoparticles: Are they really safe? La Granja. Rev. Cienc. Vida. 2015, 21, 77–83. [Google Scholar]
- Sun, Z.; Yathindranath, V.; Worden, M.; Thliveris, J.A.; Chu, S.; Parkinson, F.E.; Hegmann, T.; Miller, D.W. Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int. J. Nanomed. 2013, 8, 961–970. [Google Scholar] [CrossRef]
- Balaba, N.; Jaerger, S.; Horsth, D.F.L.; de O. Primo, J.; de S. Correa, J.; Bittencourt, C.; Zanette, C.M.; Anaissi, F.J. Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules 2023, 28, 142. [Google Scholar] [CrossRef]
- Maji, J.; Pandey, S.; Basu, S. Synthesis and evaluation of antibacterial properties of magnesium oxide nanoparticles. Bull. Mater. Sci. 2020, 43, 25. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.N.; et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Lin, J.; Nguyen, N.T.; Zhang, C.; Ha, A.; Liu, H.H. Antimicrobial Properties of MgO Nanostructures on Magnesium Substrates. ACS Omega 2020, 5, 24613–24627. [Google Scholar] [CrossRef]
- Mittag, A.; Schneider, T.; Westermann, M.; Glei, M. Toxicological assessment of magnesium oxide nanoparticles in HT29 intestinal cells. Arch. Toxicol. 2019, 93, 1491–1500. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef]
- Tang, Z.X.; Lv, B.F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Patiño, C.; Galotto, M.J.; Palma, J.L.; Alburquenque, D.; Escrig, J. Novel antimicrobial titanium dioxide nanotubes obtained through a combination of atomic layer deposition and electrospinning technologies. Nanomaterials 2018, 8, 128. [Google Scholar] [CrossRef]
- Radzig, M.; Koksharova, O.; Khmel, I.; Ivanov, V.; Yorov, L.; Kiwi, J.; Rtimi, S.; Tastekova, E.; Aybush, A.; Nadtochenko, V. Femtosecond Spectroscopy of Au Hot-Electron Injection into TiO2: Evidence for Au/TiO2 Plasmon Photocatalysis by Bactericidal Au Ions and Related Phenomena. Nanomaterials 2019, 9, 217. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Martinez-Garcia, M.; Hascoët, A.S.; Rodríguez-Jerez, J.J. Bactericidal efficacy of UV activated TiO2 nanoparticles against Gram-positive and Gram-negative bacteria on suspension. CYTA J. Food 2019, 17, 408–418. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Hu, Y.; Chen, A.; Zhou, L.; Gao, H.; Liu, Y.; Liu, S. Study on Photocatalytic Antibacterial and Sustained-Release Properties of Cellulose/TiO2/β-CD Composite Hydrogel. J. Nanomater. 2019, 2019, 2326042. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S. Mechanisms of the Antibacterial Effects of TiO2–FeOx under Solar or Visible Light: Schottky Barriers versus Surface Plasmon Resonance. Coatings 2018, 8, 391. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Dell’Edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, K.; Kiwi, J. Recent Developments in Accelerated Antibacterial Inactivation on 2D Cu-Titania Surfaces under Indoor Visible Light. Coatings 2017, 7, 20. [Google Scholar] [CrossRef]
- Duncan, T.V.; Pillai, K. Release of Engineered Nanomaterials from Polymer Nanocomposites: Diffusion, Dissolution, and Desorption. ACS Appl. Mater. Interfaces 2014, 7, 2–19. [Google Scholar] [CrossRef]
- Stoica, M.; Stoica, D.; Ivan, A.S.; Bălănică Dragomir, C.M. Biopolymers: Regulatory and legislative issues. In Biopolymers Recent Updates, Challenges and Opportunities, 1st ed.; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer: Cham, Switzerland, 2022; pp. 55–71. [Google Scholar]
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015. OJ L 327, 11.12.2015. pp. 1–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015R2283 (accessed on 4 March 2023).
- Arab-Tehrany, E.; Gonzalez, L.S. Transfer Phenomena in Food/Packaging System. In Functional Polymers in Food Science from Technology to Biology, 1st ed.; Cirillo, G., Spizzirri, U.G., Iemma, F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 67–94. [Google Scholar]
- Ghaani, M.; Farris, S. Migration of Primary Aromatic Amines From Food Packaging Materials. In Reference Module in Food Science; Robertson, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 154–196. [Google Scholar]
- Sharma, J.; Tewari, K.; Aryac, R.J. Diffusion in polymeric systems-A review on free volume theory. Prog. Org. Coat. 2017, 111, 83–92. [Google Scholar] [CrossRef]
- Stoica, M.; Borda, D. Flexible Packaging Structures for High-Pressure Thermal Processing (HPTP). In Reference Module in Food Science; Robertson, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; 8p. [Google Scholar]
- Fasano, E.; Cirillo, T.; Esposito, F.; Lacorte, S. Migration of monomers and plasticizers from packed foods and heated microwave foods using QuEChERS sample preparation and gas chromatography/mass spectrometry. LWT—Food Sci. Technol. 2015, 64, 1015–1021. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Li, X.; Zhou, W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Nerín, C.; Aznar, M.; Carrizo, D. Food contamination during food process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Pocas, F. Migration From Packaging and Food Contact Materials Into Foods. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wyser, Y.; Adams, M.; Avella, M.; Carlander, D.; Garcia, L.; Pieper, G.; Rennen, M.; Schuermans, J.; Weiss, J. Outlook and challenges of nanotechnologies for food packaging. Packag. Technol. Sci. 2016, 29, 615–648. [Google Scholar] [CrossRef]
- Ahari, H.; Lahijani, L.K. Migration of Silver and Copper Nanoparticles from Food Coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Brandelli, A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness 2020, 9, 8–20. [Google Scholar] [CrossRef]
- Grasso, A.; Ferrante, M.; Moreda-Pineiro, A.; Arena, G.; Magarini, R.; Conti, G.O.; Cristaldi, A.; Copat, C. Dietary exposure of zinc oxide nanoparticles (ZnO-NPs) from canned seafood by single particle ICP-MS: Balancing of risks and benefits for human health. Ecotoxicol. Environ. Saf. 2022, 231, 113217. [Google Scholar] [CrossRef]
- Peng, C.; Lu, W.; Fang, Y. An insight into the effect of food nanoparticles on the metabolism of intestinal cells. Curr. Opin. Food Sci. 2022, 43, 174–182. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of nanotechnology in food packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Vitulo, M.; Gnodi, E.; Meneveri, R.; Barisani, D. Interactions between Nanoparticles and Intestine. Int. J. Mol. Sci. 2022, 23, 4339. [Google Scholar] [CrossRef]
- Heringa, M.B.; Peters, R.J.B.; Bleys, R.L.A.W.; van der Lee, M.K.; Tromp, P.C.; van Kesteren, P.C.E.; van Eijkeren, J.C.H.; Undas, A.K.; Oomen, A.G.; Bouwmeester, H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018, 15, 15. [Google Scholar] [CrossRef]
- Diao, J.; Xia, Y.; Jiang, X.; Qiu, J.; Cheng, S.; Su, J.; Duan, X.; Gao, M.; Qin, X.; Zhang, J.; et al. Silicon dioxide nanoparticles induced neurobehavioral impairments by disrupting microbiota–gut–brain axis. J. Nanobiotechnol. 2021, 19, 174. [Google Scholar] [CrossRef]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review from the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity of Metallic Nanoparticles on Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef]
- Jagtiani, E. Advancements in nanotechnology for food science and industry. Food Front. 2022, 3, 56–82. [Google Scholar] [CrossRef]
- Szarka, A.; Lorincz, T.; Hajdinák, P. Friend or Foe: The Relativity of (Anti)oxidative Agents and Pathways. Int. J. Mol. Sci. 2022, 23, 5188. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.A.; Machado, A.R.T.; Ogunjimi, A.T.; Alberici, L.C.; Antunes, L.M.G.; Barbosa, F. Cytotoxicity, mutagenicity, oxidative stress and mitochondrial impairment in human hepatoma (HepG2) cells exposed to copper oxide, copper-iron oxide and carbon nanoparticles. Ecotoxicol. Environ. Saf. 2020, 189, 109982. [Google Scholar] [CrossRef] [PubMed]
- Anreddy, R.N.R. Copper Oxide Nanoparticles Induces Oxidative Stress and Liver Toxicity in Rats Following Oral Exposure. Toxicol. Rep. 2018, 5, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Bugata, L.S.P.; Pitta Venkata, P.; Gundu, A.R.; Mohammed Fazlur, R.; Reddy, U.A.; Kumar, J.M.; Reddy Mekala, V.; Bojja, S.; Mahboob, M. Acute and Subacute Oral Toxicity of Copper Oxide Nanoparticles in Female Albino Wistar Rats. J. Appl. Toxicol. 2019, 39, 702–716. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; De Rijk, E.; Bonetto, A.; Wohlleben, W.; Stone, V.; Brunelli, A.; Badetti, E.; Marcomini, A.; Gosens, I.; Cassee, F.R. Toxicity of Copper Oxide and Basic Copper Carbonate Nanoparticles After Short-Term Oral Exposure in Rats. Nanotoxicology 2019, 13, 50–72. [Google Scholar] [CrossRef]
- Elkhateeb, S.A.; Ibrahim, T.R.; El-Shal, A.S.; Abdel Hamid, O.I. Ameliorative Role of Curcumin on Copper Oxide Nanoparticles-Mediated Renal Toxicity in Rats: An Investigation of Molecular Mechanisms. J. Biochem. Mol. Toxicol. 2020, 34, e22593. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Ebrahim, N.M.; Gaber, M.H. In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J. Trace Elem. Med. Biol. 2020, 60, 126481. [Google Scholar] [CrossRef]
- He, H.; Zou, Z.; Wang, B.; Xu, G.; Chen, C.; Qin, X.; Yu, C.; Zhang, J. Copper Oxide Nanoparticles Induce Oxidative DNA Damage and Cell Death via Copper Ion-Mediated P38 MAPK Activation in Vascular Endothelial Cells. Int. J. Nanomed. 2020, 15, 3291–3302. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Ali, G.A.M.; Hassanein, A.; Attia, A.M.; Marzouk, E.R. Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant. Sustainability 2022, 14, 4914. [Google Scholar] [CrossRef]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Shin, N.R.; Shin, I.S.; Moon, C.; Kim, J.K.; Kim, H.C.; Kim, J.C. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part Fibre Toxicol. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Tulinska, J.; Mikusova, M.L.; Liskova, A.; Busova, M.; Masanova, V.; Uhnakova, I.; Rollerova, E.; Alacova, R.; Krivosikova, Z.; Wsolova, L.; et al. Copper Oxide Nanoparticles Stimulate the Immune Response and Decrease Antioxidant Defense in Mice After Six-Week Inhalation. Front. Immunol. 2022, 13, 874253. [Google Scholar] [CrossRef]
- Eskin, A.N.; Öztürk, S.; Eskin, A. The Effects of Magnetic Iron oxide Nanoparticles (Fe3O4) on Some Biological Aspects of Galleria mellonella L. (Lepidoptera: Pyralidae). Celal Bayar Univ. J. Sci. 2021, 17, 319–324. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; p. 660. [Google Scholar]
- McMillen, S.A.; Dean, R.; Dihardja, E.; Ji, P.; Lönnerdal, B. Benefits and Risks of Early Life Iron Supplementation. Nutrients 2022, 14, 4380. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Saafane, A.; Girard, D. Interaction between iron oxide nanoparticles (Fe3O4 NPs) and human neutrophils: Evidence that Fe3O4 NPs possess some pro-inflammatory activities. Chem. Biol. Interact. 2022, 365, 110053. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Available online: https://nap.nationalacademies.org/read/10026/chapter/11 (accessed on 7 March 2023).
- Wu, L.; Wen1, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Shen, Y.; Xiao, Y.; Gao, L.; Shi, S. Cytotoxicity studies of Fe3O4 nanoparticles in chicken macrophage cells. R. Soc. Open Sci. 2020, 7, 191561. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P. Magnesium Oxide Nanoparticles: Effective Antilarvicidal and Antibacterial Agents. ACS Omega 2023, 8, 5225–5233. [Google Scholar]
- Ammulu, M.A.; Vinay Viswanath, K.; Giduturi, A.K.; Vemuri, P.K.; Mangamuri, U.; Poda, S. Phytoassisted synthesis of magnesium oxide nanoparticles from Pterocarpus marsupium rox.b heartwood extract and its biomedical applications. J. Genet. Eng. Biotechnol. 2021, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Andreadelli, A.; Petrakis, S.; Tsoureki, A.; Tsiolas, G.; Michailidou, S.; Baltzopoulou, P.; Merkestein, R.V.; Hodgson, P.; Sceats, M.; Karagiannakis, G.; et al. Effects of Magnesium Oxide and Magnesium Hydroxide Microparticle Foliar Treatment on Tomato PR Gene Expression and Leaf Microbiome. Microorganisms 2021, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Fahmy, H.M.; El-Hakim, M.H.; Nady, D.S.; Elkaramany, Y.; Mohamed, F.A.; Yasien, A.M.; Moustafa, M.A.; Elmsery, B.E.; Yousef, H.A. Review on MgO nanoparticles multifunctional role in the biomedical field: Properties and applications. Nanomed. J. 2022, 9, 1–14. [Google Scholar]
- Mohammed, R.S.; Aadim, K.A.; Ahmed, K.A. Estimation of in vivo toxicity of MgO/ZnO core/shell nanoparticles synthesized by eco-friendly non-thermal plasma technology. Appl. Nanosci. 2022, 12, 3783–3795. [Google Scholar] [CrossRef]
- Rangrazi, A.; Daneshmand, M.S.; Ghazvini, K.; Shafaee, H. Effects of Magnesium Oxide Nanoparticles Incorporation on Shear Bond Strength and Antibacterial Activity of an Orthodontic Composite: An In Vitro Study. Biomimetics 2022, 7, 133. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace. Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Çesmeli, S.; Biray Avci, C. Application of Titanium Dioxide (TiO2) Nanoparticles in Cancer Therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef]
- Freyre-Fonseca, V.; Medina-Reyes, E.I.; Téllez-Medina, D.I.; Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Vaca-Paniagua, F.; Delgado-Buenrostro, N.L.; Flores-Flores, J.O.; López-Villegas, E.O.; Gutiérrez-López, G.F.; et al. Influence of shape and dispersion media of titanium dioxide nanostructures on microvessel network and ossification. Colloids Surf. B Biointerfaces 2018, 162, 193–201. [Google Scholar] [CrossRef]
- Gojznikar, J.; Zdravkovic, B.; Vidak, M.; Leskošek, B.; Ferk, P. TiO2 Nanoparticles and Their Effects on Eukaryotic Cells: A Double-Edged Sword. Int. J. Mol. Sci. 2022, 23, 12353. [Google Scholar] [CrossRef]
- Heidari, Z.; Mohammadipour, A.; Haeri, P.; Ebrahimzadeh-bideskan, A. The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran. J. Basic Med. Sci. 2019, 22, 745–751. [Google Scholar]
- Hwang, J.-S.; Yu, J.; Kim, H.-M.; Oh, J.-M.; Choi, S.-J. Food Additive Titanium Dioxide and Its Fate in Commercial Foods. Nanomaterials 2019, 9, 1175. [Google Scholar] [CrossRef]
- Jensen, D.M.; Løhr, M.; Sheykhzade, M.; Lykkesfeldt, J.; Wils, R.S.; Loft, S.; Møller, P. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis 2019, 34, 203–214. [Google Scholar] [CrossRef]
- Jovanović, B.; Jovanović, N.; Cvetković, V.J.; Matić, S.; Stanić, S.; Whitley, E.M.; Mitrović, T.L. The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster—A 20 generation dietary exposure experiment. Sci. Rep. 2018, 8, 17922. [Google Scholar] [CrossRef]
- Korábková, E.; Kašpárková, V.; Jasenská, D.; Moricová, D.; Dad’ová, E.; Truong, T.H.; Capáková, Z.; Vícha, J.; Pelková, J.; Humpolícek, P. Behaviour of Titanium Dioxide Particles in Artificial Body Fluids and Human Blood Plasma. Int. J. Mol. Sci. 2021, 22, 10614. [Google Scholar] [CrossRef]
- Notter, T.; Aengenheister, L.; Weber-Stadlbauer, U.; Naegeli, H.; Wick, P.; Meyer, U.; Buerki-Thurnherr, T. Prenatal exposure to TiO2 nanoparticles in mice causes behavioral deficits with relevance to autism spectrum disorder and beyond. Transl. Psychiatry 2018, 8, 193. [Google Scholar] [CrossRef]
- Papp, A.; Horváth, T.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M.; Berkesi, D.S.; Kozma, G.; Kónya, Z.; Wilhelm, I.; Patai, R.; et al. Presence of Titanium and Toxic Effects Observed in Rat Lungs, Kidneys, and Central Nervous System in vivo and in Cultured Astrocytes in vitro on Exposure by Titanium Dioxide Nanorods. Int. J. Nanomed. 2020, 15, 9939–9960. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Jetten, M.J.; Jonkhout, M.C.M.; Garduño-Balderas, L.G.; Briedé, J.J.; de Kok, T.M.; Chirino, Y.; van Loveren, H. Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171). Food Chem. Toxicol. 2018, 111, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Raja, G.; Cao, S.; Kim, D.-H.; Kim, T.-J. Mechanoregulation of Titanium Dioxide Nanoparticles in Cancer Therapy. Mater. Sci. Eng. C 2020, 107, 110303. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escamilla, J.C.; Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Flores-Flores, J.O.; Ganem-Rondero, A.; Rodríguez-Sosa, M.; Terrazas, L.I.; Delgado-Buenrostro, N.L.; Chirino, Y.I. Food-grade titanium dioxide (E171) by solid or liquid matrix administration induces inflammation, germ cells sloughing in seminiferous tubules and blood-testis barrier disruption in mice. J. Appl. Toxicol. 2019, 39, 1586–1605. [Google Scholar] [CrossRef]
- Suker, D.K.; Jasim, F.A. Liver histopathological alteration after repeated intra-tracheal instillation of titanium dioxide in male rats. Gastroenterol. Hepatol. Bed Bench 2018, 11, 159–168. [Google Scholar]
- Zdravković, T.P.; Zdravković, B.; Lunder, M.; Ferk, P. The effect of micro-sized titanium dioxide on WM-266-4 metastatic melanoma cell line. Bosn. J. Basic Med. Sci. 2019, 19, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, W.-K.; Xu, Q.-Y.; Zhou, X.; Wang, Y.; Yue, Z.-H.; Song, B. Nec-1 Attenuates Neurotoxicity Induced by Titanium Dioxide Nanomaterials on Sh-Sy5y Cells Through RIP1. Nanoscale Res. Lett. 2020, 15, 65. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Othman, H.H.; Abdullah, R.; Edin, H.Y.A.S.; AL-Haj, N.A. Beneficial and toxicological aspects of zinc oxide nanoparticles in animals. Vet. Med. Sci. 2022, 8, 1769–1779. [Google Scholar] [CrossRef]
- Pei, X.; Jiang, H.; Xu, G.; Li, C.; Li, D.; Tang, S. Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study. Int. J. Mol. Sci. 2022, 23, 6724. [Google Scholar] [CrossRef] [PubMed]
- Shkal, K.E.M.; Azab, A.E.; Attia, A.M.; El-Banna, S.G.; Yahya, R.A.M. Zinc oxide nanoparticles attenuate the oxidative damage and disturbance in antioxidant defense system induced by cyclophosphamide in male albino rats. Insights Biol. Med. 2020, 4, 001–008. [Google Scholar]
- Wang, C.; Wang, H.; Lin, M.; Hu, X. ZnO nanoparticles induced cytotoxicity on human pulmonary adenocarcinoma cell line LTEP-a-2. Process. Saf. Environ. Prot. 2015, 93, 265–273. [Google Scholar] [CrossRef]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef]
- Bi, J.; Mo, C.; Li, S.; Huang, M.; Lin, Y.; Yuan, P.; Liu, Z.; Jia, B.; Xu, S. Immunotoxicity of metal and metal oxide nanoparticles: From toxic mechanisms to metabolism and outcomes. Biomater. Sci. 2023, 1–76. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The interaction between nanoparticles and immune system: Application in the treatment of inflammatory diseases. J. Nanobiotechnol. 2022, 20, 127. [Google Scholar] [CrossRef]
- Goncalves, D.M.; De Liz, R.; Girard, D. Activation of neutrophils by nanoparticles. Sci. World J. 2011, 11, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Kagan, V.E. The role of nanotoxicology in realizing the ‘helping without harm’ paradigm of nanomedicine: Lessons from studies of pulmonary effects of single-walled carbon nanotubes. J. Intern. Med. 2010, 267, 106–118. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, J.; Zhang, J.; Timashev, P.; Ma, X.; Liang, X.-J. Adaptive changes induced by noble-metal nanostructures in vitro and in vivo. Theranostics 2020, 10, 5649–5670. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Almatroudi, A.; Almatroodi, S.A.; Mahzari, A.; Alsahli, M.A.; Rahmani, A.H. Endoplasmic Reticulum Stress Provocation by Different Nanoparticles: An Innovative Approach to Manage the Cancer and Other Common Diseases. Molecules 2020, 25, 5336. [Google Scholar] [CrossRef]
- Manshian, B.B.; Pokhrel, S.; Mädler, L.; Soenen, S.J. The impact of nanoparticle-driven lysosomal alkalinization on cellular functionality. J Nanobiotechnol. 2018, 16, 85. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, J.; Tripathi, B.N.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Park, E.J.; Choi, D.-H.; Kim, Y.; Lee, E.-W.; Song, Y.; Cho, M.-H.; Kim, J.-H.; Kim, S.-W. Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and ER stress in RAW264.7 cells. Toxicol. Vitr. 2014, 28, 1402–1412. [Google Scholar] [CrossRef]
- Maysinger, D.; Gran, E.R.; Bertorelle, F.; Fakhouri, H.; Antoine, R.; Kaul, E.S.; Samhadaneh, D.M.; Stochaj, U. Gold nanoclusters elicit homeostatic perturbations in glioblastoma cells and adaptive changes of lysosomes. Theranostics 2020, 10, 1633–1648. [Google Scholar] [CrossRef]
- Wang, F.; Salvati, A.; Boya, P. Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 2018, 8, 170271. [Google Scholar] [CrossRef]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug. Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
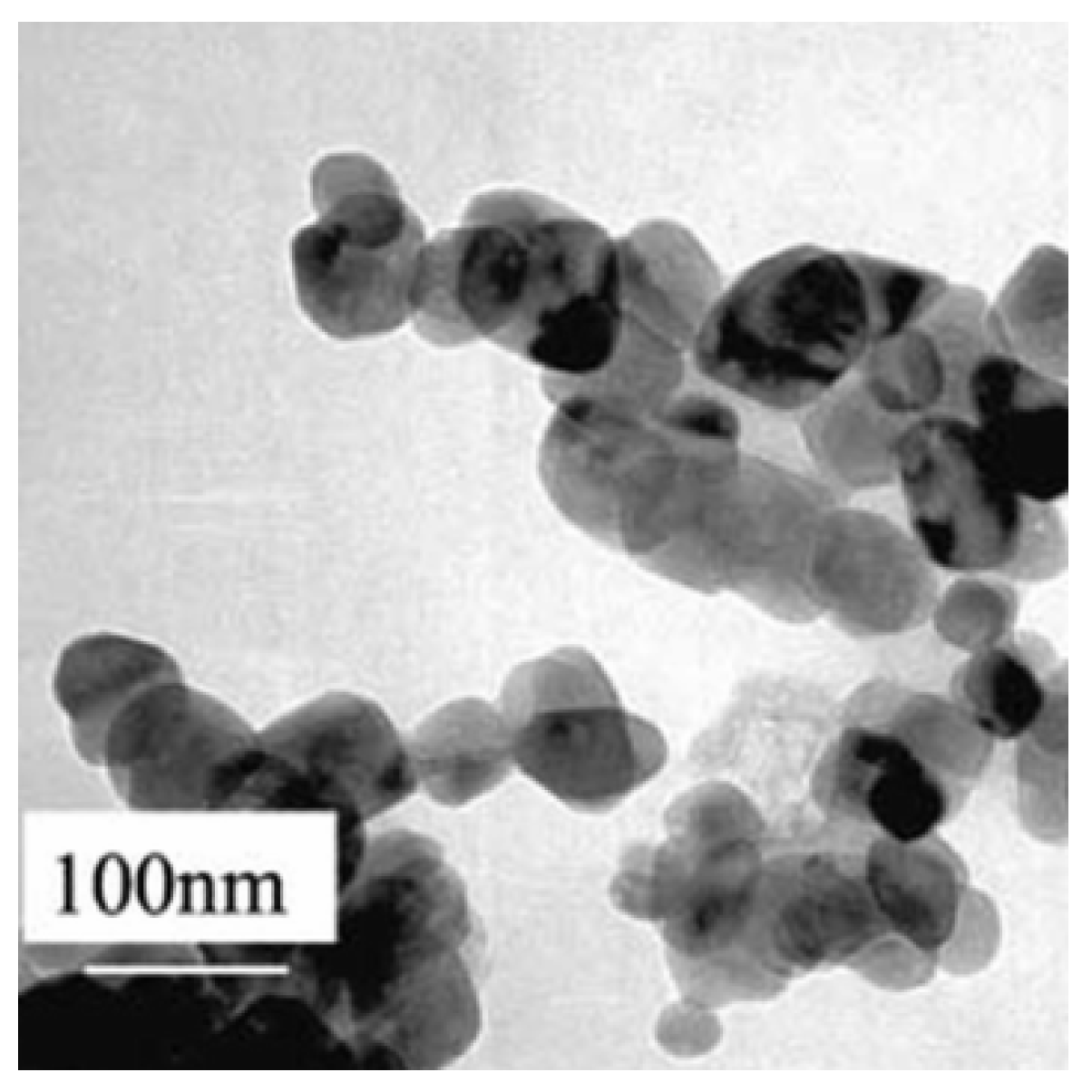
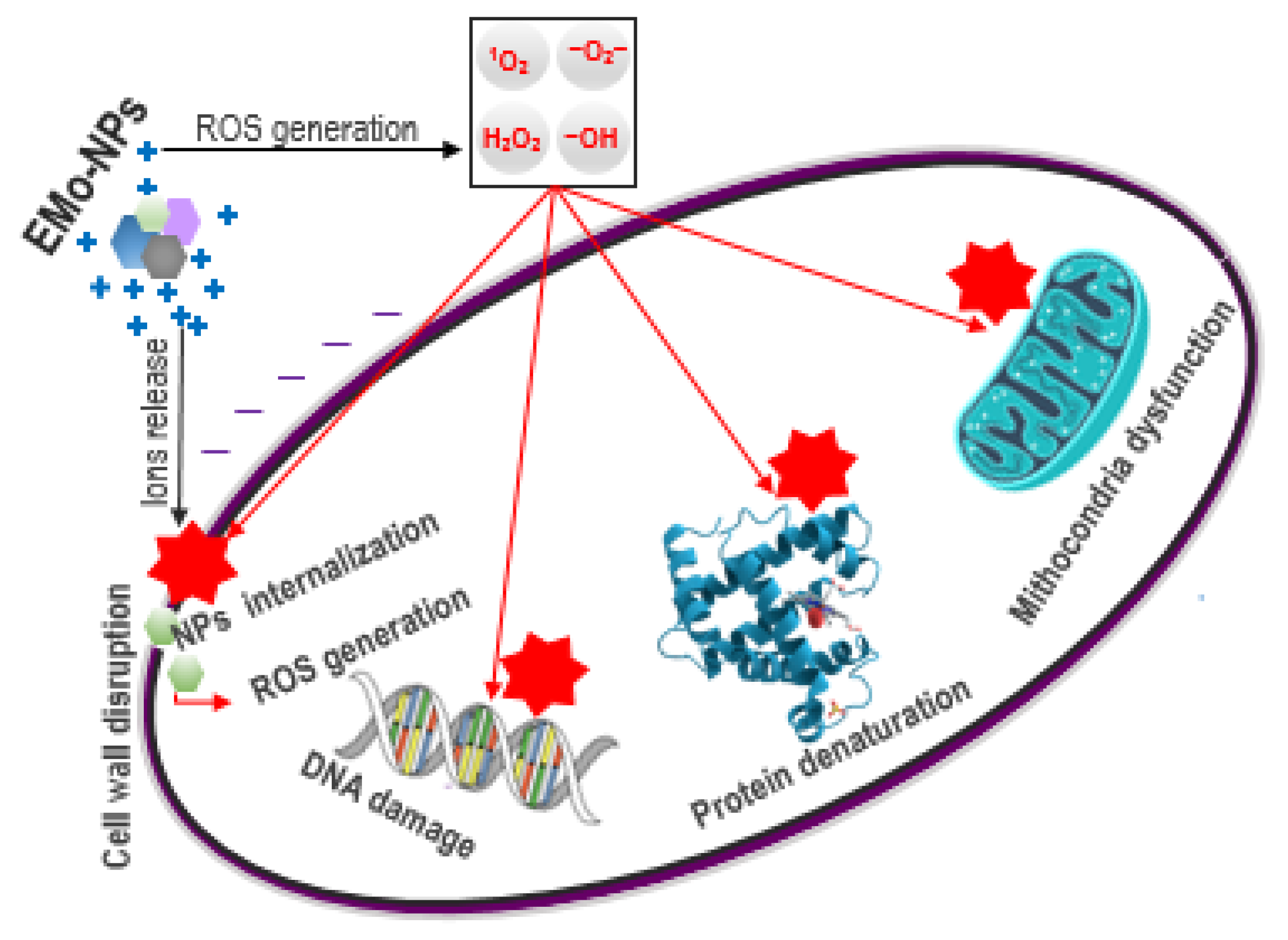
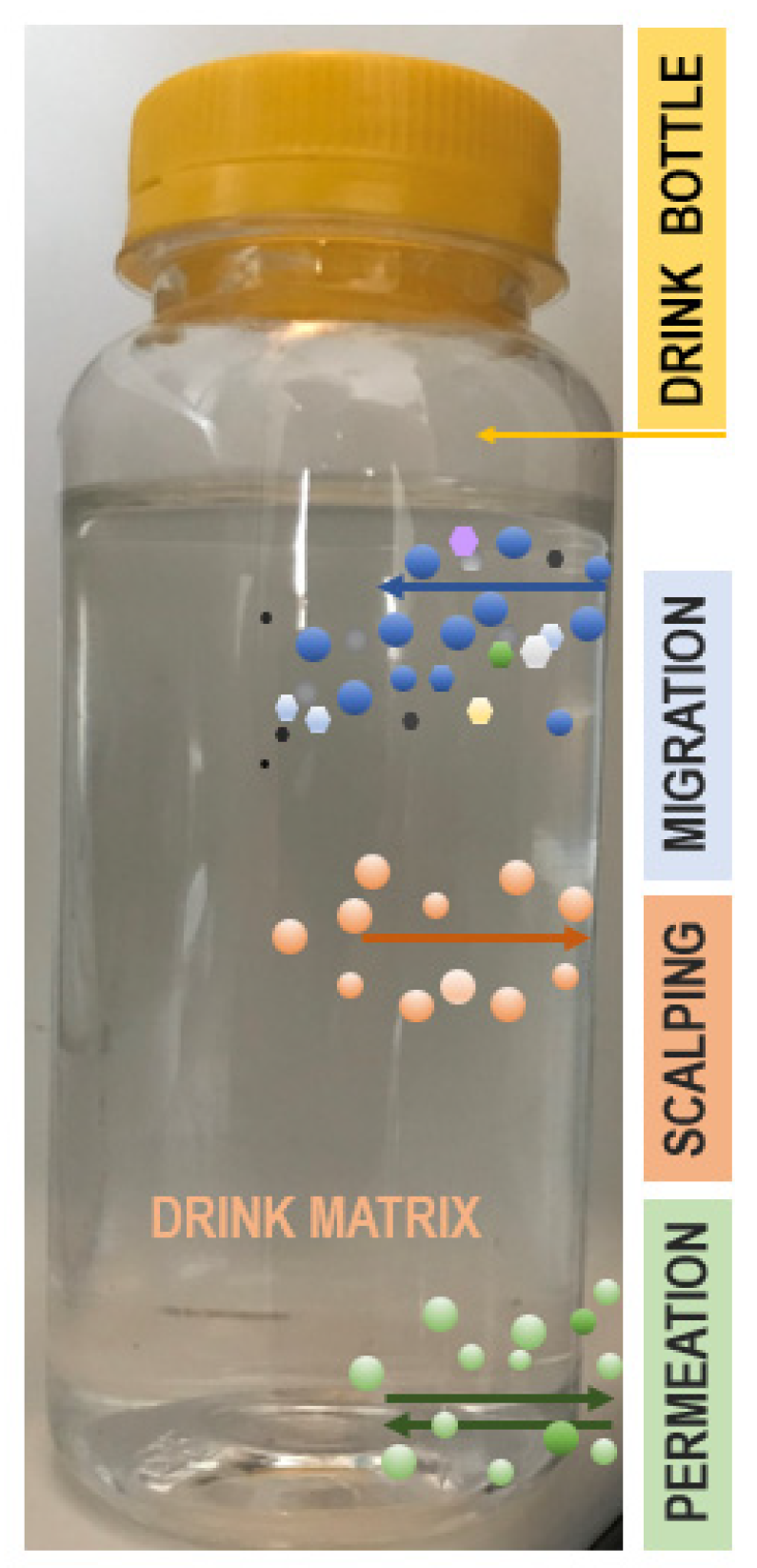

| Type | Benefits | References |
|---|---|---|
| CuO-NPs | Excellent antibacterial (against Gram-positive and Gram-negative bacteria), antifungal, and antiviral agent; good reinforcing NPs of polymer/biopolymer matrix | [1,3,4,9,17,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] |
| Fe3O4-NPs | Distinct type of NPs with superior properties compared to α-Fe2O3 (ferric oxide–hematite) and γ-Fe2O3 (ferric oxide–maghemite); possesses strong antibacterial characteristics; source of bioavailable iron; mineral-fortified supplement | |
| MgO-NPs | Strong antimicrobial action similar to nanosilver (depending on their sizes; when the size is smaller than 15 nm, MgO-NPs have a powerful biocidal efficacy); nanoscale MgO is a polymer/biopolymer reinforcement agent; dietary supplement | |
| TiO2-NPs | Top EMo-NPs (major promising NPs player in the food industry, extensively used); photocatalytic antimicrobial activities against bacteria, yeast, and fungi; UV-protective food nanopackaging; enhancer of mechanical and thermal stability of food packaging; oxygen scavenger; biosensor for volatile organic compounds; food coloring agent | |
| ZnO-NPs | Excellent photocatalytical and photocorrosion antimicrobial against bacteria, yeast, and fungi; UV light absorber in food packaging; source of zinc in food supplements |
| EMo-NPs | Main Concerns | References |
|---|---|---|
| CuO-NPs | Cu, a trace element, is vitally important, playing a significant role in numerous biological activities (hemoglobin production, iron metabolism, hormone synthesis, etc.). However, a high level of Cu ions could be toxic. Cu ions are redox-active, affecting biological systems. Their reactivity is wholly dependent on extrinsic and intrinsic factors (size, shape, surface charge, concentration of NPs). The smaller the size, the more toxic they are. NPs smaller than 40 nm can directly enter cell nuclei from the circulatory system, while NPs greater than 100 nm can cross the cell membrane. Spherical ones are more reactive. CuO-NPs are Trojan horse-type carriers, releasing Cu ions inside the cells. Compared with Fe3O4-NPs, ZnO-NPs, and TiO2-NPs, CuO-NPs are the most potent in terms of cytotoxicity. CuO-NPs’ uptake in many organs (spleen, liver, kidneys, brain, lungs, blood, heart, stomach, bones, marrow), and they could exert: oxidative stress genotoxicity cytotoxicity immunotoxicity inflammation | [104,105,106,107,108,109,110,111,112,113,114] |
| Fe3O4-NPs | Fe is an essential biological trace element not only for human beings but for all other life forms. In the human body, the majority of Fe is in hemoglobin (50–60%); 25% is in an easily mobilizable store, and the remaining 15% is in myoglobin and in numerous enzymes involved in oxidative metabolism and many other cellular activities. In addition, Fe3O4-NPs is used in various biomedical applications (cancer, diabetes, diagnosis of contrast substances, magnetic resonance imaging, inflammatory diseases, targeted drug delivery, gene therapy, biosensors, etc.). Although Fe-based supplements are highly effective for improving iron status and Fe3O4-NPs-based biomedical applications have good potential, there are controversial results regarding the cytotoxic effects and the overall integrity of the cells, once the engineered Fe3O4-NPs are inside the cells. Fe3O4-NPs are less toxic than other metal oxide NPs, but they could effectively enter the cell nucleus. Along similar lines as CuO-NPs, Fe3O4-NPs reactivity is linked to surface modification, concentration, size, shape, dose dependency, obtainment method, etc., and could induce: disruptions of the oxidative balance cytotoxicity immunotoxicity neurobehavioral toxicity inflammation ferroptosis fibrosis/cirrhosis and loss of liver function | [61,115,116,117,118,119,120,121,122,123,124] |
| MgO-NPs | Mg2+ is an important cation for human health. In the human body, the majority of Mg is mainly stored in bones (50–65%) while 34–39% is in muscle, soft tissues, and organs and 1–2% is in blood and extracellular fluids. It plays a significant function in many physiological processes. MgO-NPs are used in a wide range of biomedical applications (cancer therapy, medical imaging, nanocryosurgery, bone regeneration, biosensor, tissue engineering, dental implants, bioactive glasses, etc.). However, MgO-NPs’ toxicity is controversial, depending on the physical and chemical characteristics of the NPs and tested cell type. Concerns about their safety remain (at high concentrations), and refer to: oxidative stress hemolytic activity arteriosclerosis inflammation neronal apoptosis hepatocytotoxicity | [125,126,127,128,129,130,131] |
| TiO2-NPs | TiO2-NPs are one of the most commonly used NPs in consumer products (foods, medicines). The exposure of humans to TiO2-NPs via the oral route is inevitable. The potential toxicity of TiO2-NPs is addressed by multiple studies and is wholly dependent on their size, shape, surface charge, concentration, and solubility. Due to their smaller size, TiO2-NPs are more easily absorbed into cells, where they can become involved in: oxidative stress genotoxicity cytotoxicity immunotoxicity inflammation TiO2-NPs promote photochemical reactions, as they are more active in UV light, but they can induce ROS under dark conditions. If ROS induction can occur under dark conditions, it can likely take place inside the human body. This may lead TiO2-NPs to be toxicologically potent than previously known. | [52,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149] |
| ZnO-NPs | Zn is one of the most essential trace minerals and possesses exceptional properties such as the capacity to modulate immune responses, improve fertility and metabolism, scavenge free radicals, etc. ZnO-NPs are one of the most prevalent metal oxide NPs and possess wide biomedical applications (treatment of various kinds of cancers, drug delivery, etc.). The exposure of humans to ZnO-NPs is very frequent and constitutes an issue of concern to health. The potential toxicity of ZnO-NPs is entirely dependent on their size, shape, surface charge, concentration, and solubility. Studies of their toxicology in in vivo models indicate that ZnO-NPs may cause: oxidative stress genotoxicity cytotoxicity inflammation hepatotoxicity | [150,151,152,153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuparu-Cretu, M.; Braniste, G.; Necula, G.-A.; Stanciu, S.; Stoica, D.; Stoica, M. Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health. Foods 2023, 12, 1882. https://doi.org/10.3390/foods12091882
Stuparu-Cretu M, Braniste G, Necula G-A, Stanciu S, Stoica D, Stoica M. Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health. Foods. 2023; 12(9):1882. https://doi.org/10.3390/foods12091882
Chicago/Turabian StyleStuparu-Cretu, Mariana, Gheorghe Braniste, Gina-Aurora Necula, Silvius Stanciu, Dimitrie Stoica, and Maricica Stoica. 2023. "Metal Oxide Nanoparticles in Food Packaging and Their Influence on Human Health" Foods 12, no. 9: 1882. https://doi.org/10.3390/foods12091882