Impact of Longkong Pericarp Extract on the Physicochemical Properties of Alginate-Based Edible Nanoparticle Coatings and Quality Maintenance of Shrimp (Penaeus monodon) during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Chemicals, and Reagents
2.2. Preparation of Longkong Pericarp Extract (LPE)
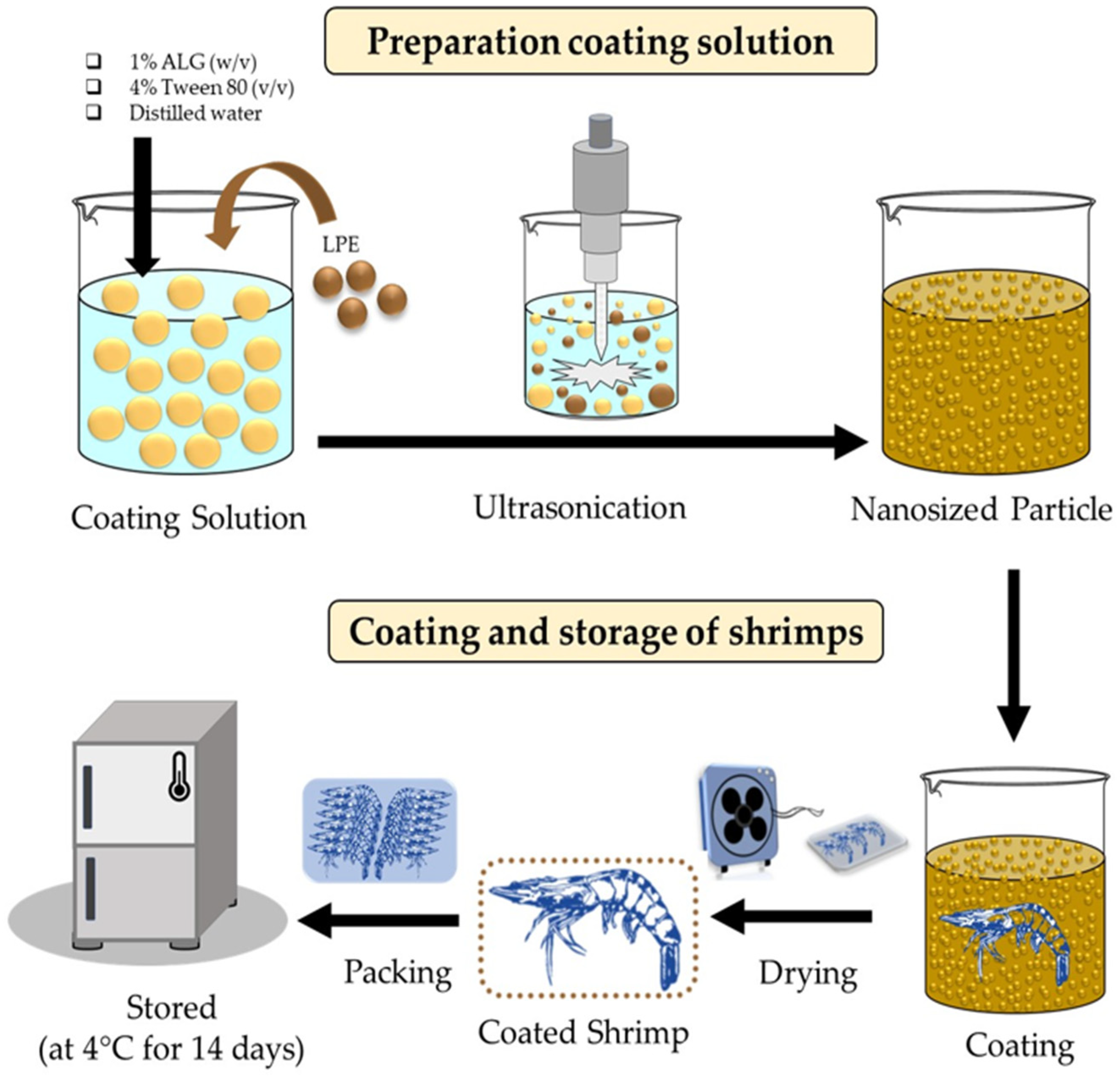
2.3. Preparation of Coating Solution
2.4. Physicochemical Analysis of Coating Solutions
2.4.1. pH
2.4.2. Viscosity
2.4.3. Turbidity
2.4.4. Whiteness Index
2.4.5. Particle Size and Polydispersity Index (PDI)
2.5. Coating and Storage of Shrimps
2.6. Shelf-Life Analyses of NP-ALG-LPE Coated Shrimp during Storage
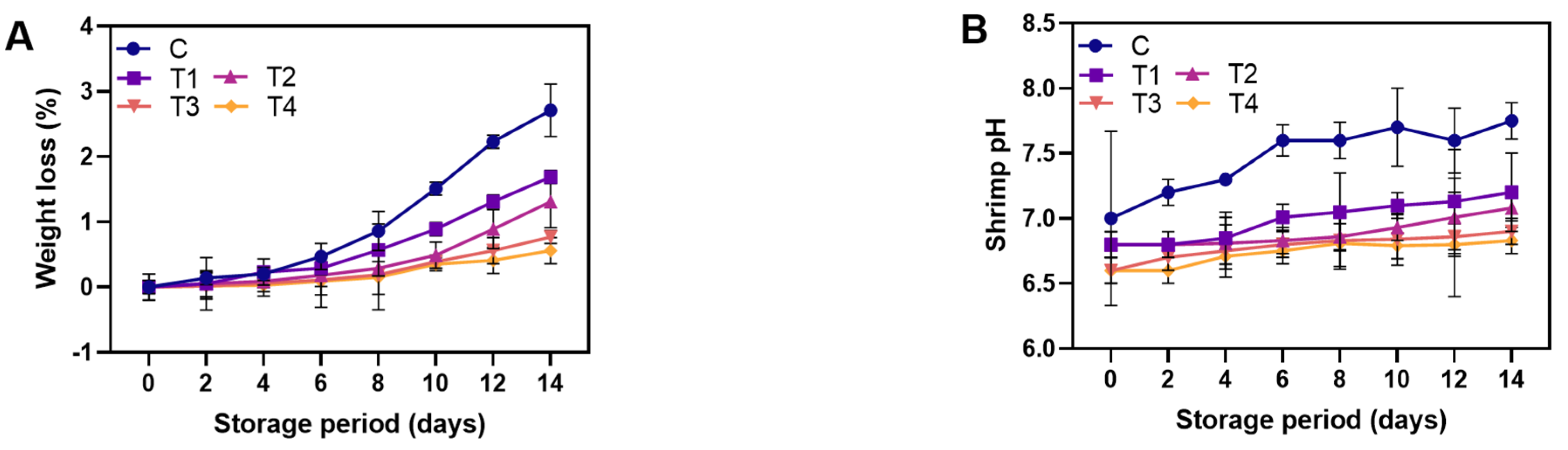
2.6.1. Weight Loss
2.6.2. pH
2.6.3. Extraction of Polyphenol Oxidase
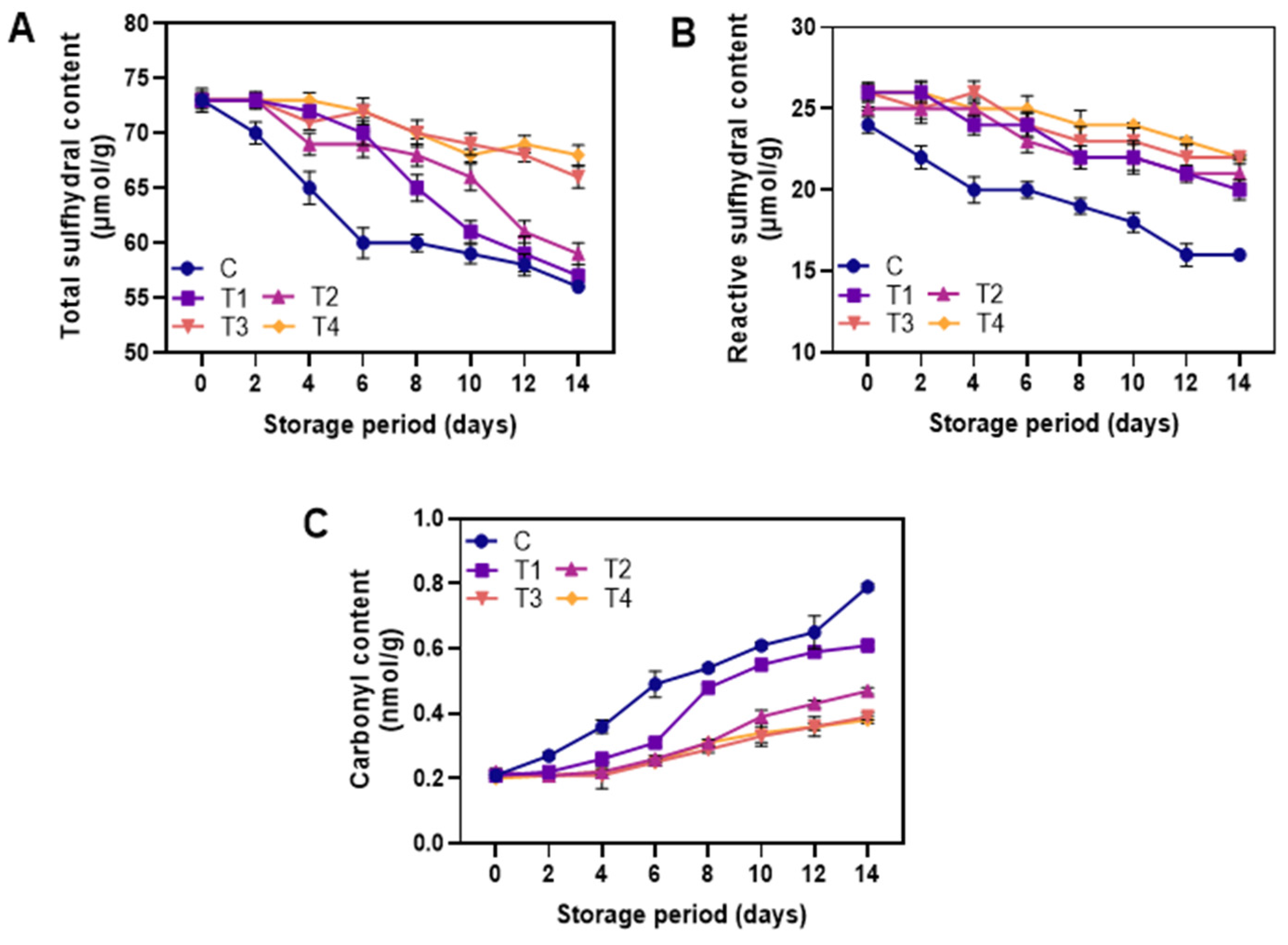
2.6.4. Total Sulfhydryl Content
Reactive Sulfhydryl Content
2.6.5. Carbonyl Content
2.6.6. Peroxide Value (PV)
2.6.7. Thiobarbituric Acid Reactive Substance (TBARS)
2.6.8. Anisidine Value (AnV)
2.6.9. Total Oxidation Value
2.7. Total Volatile Basic Nitrogen (TVB-N)
2.8. Microbiological Analyses
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Different Coating Solutions
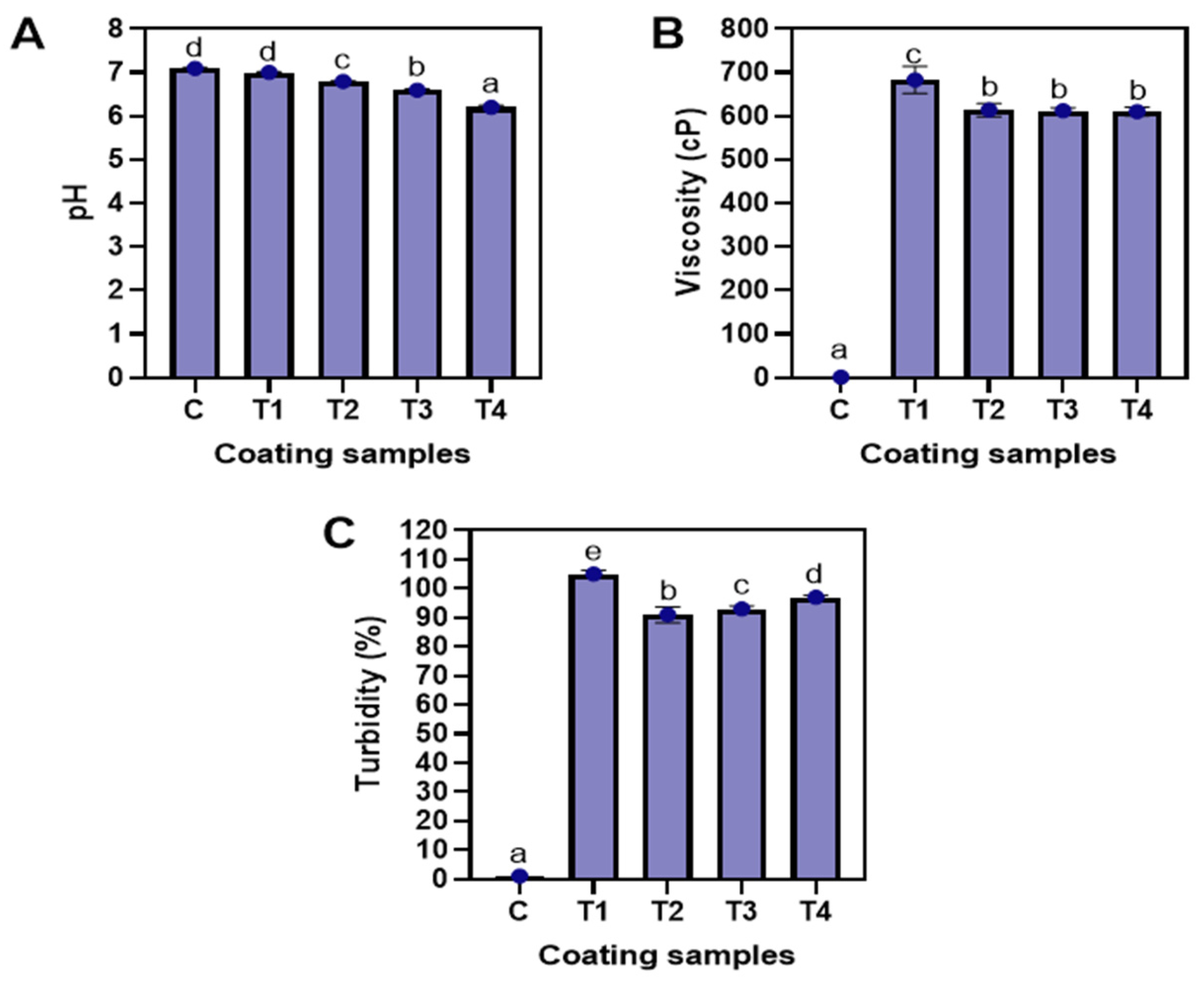
3.1.1. pH, Viscosity, and Turbidity
3.1.2. Whiteness Index, Particle Size, and Polydispersity Index
3.2. Shelf-Life Analysis of Refrigerated Shrimp Using Different ALG-Based Coatings
3.2.1. Physicochemical Properties
3.2.2. Brown Pigment Enzyme Activities
3.2.3. Protein Oxidation
3.2.4. Lipid Oxidation
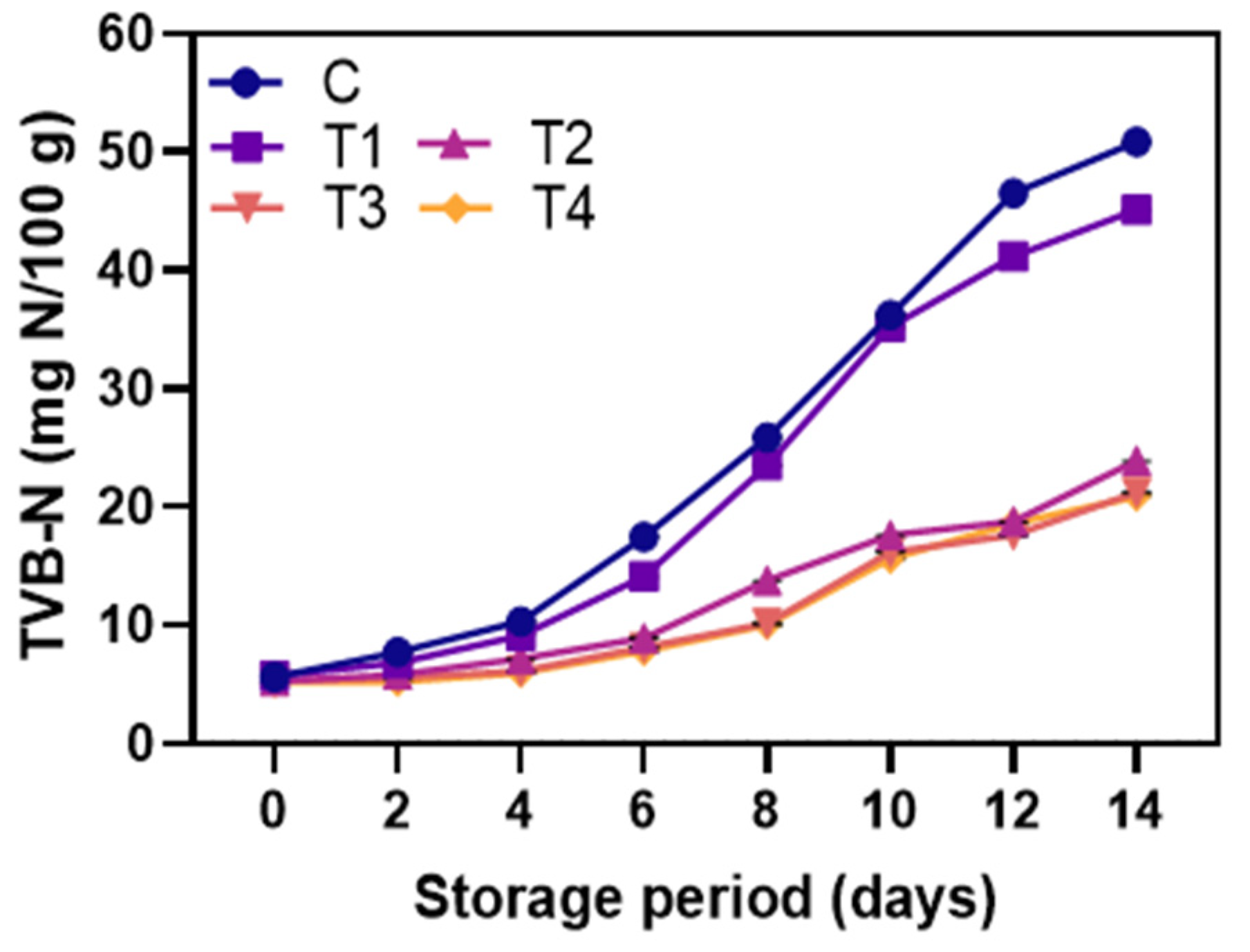
3.2.5. TVB-N
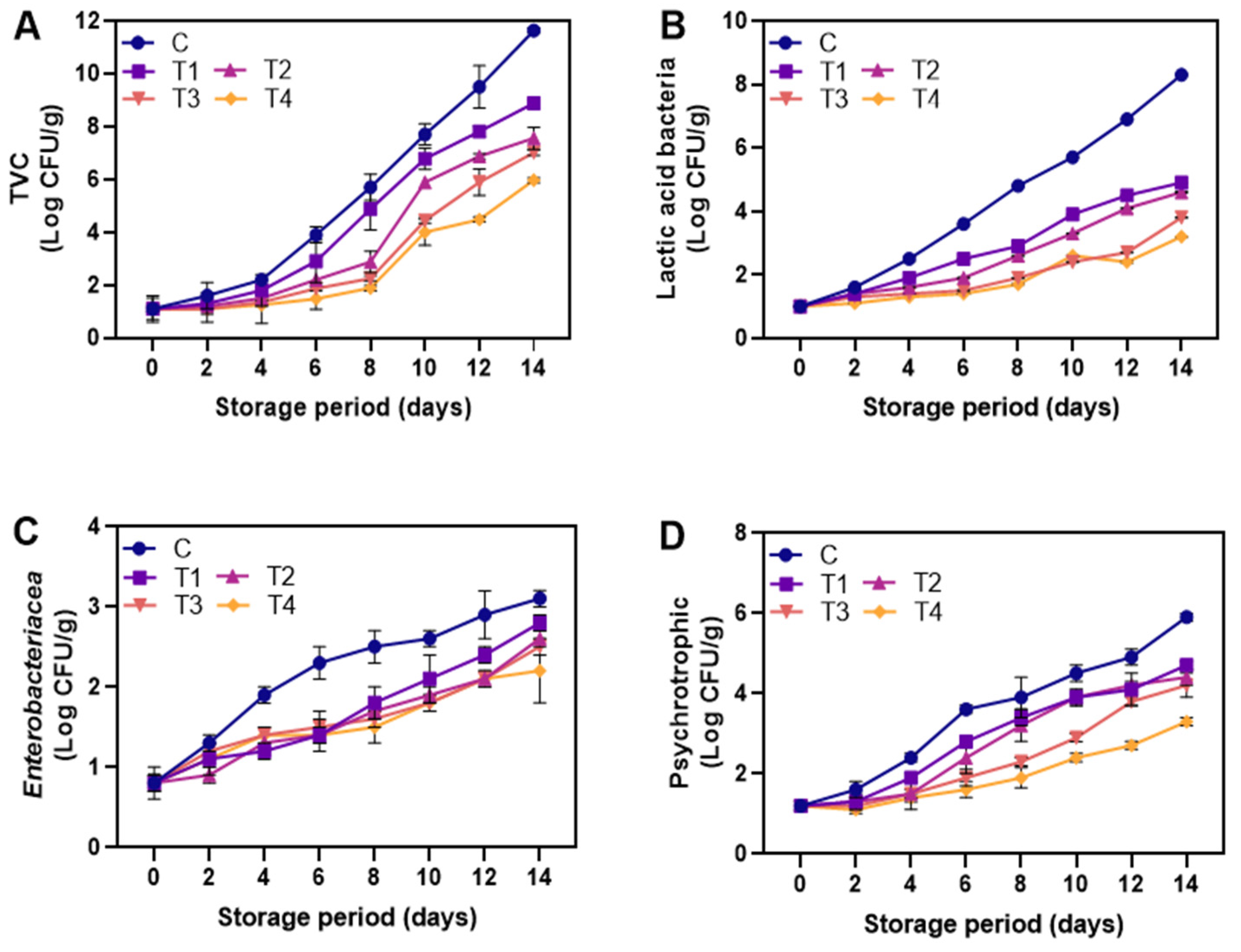
3.2.6. Microbial Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabu, S.; Xavier, K.M.; Sasidharan, A. Efficacy of pomegranate phenolic extract and chitosan as an edible coating for shelf life extension of Indian white shrimp during refrigerated storage. J. Packag. Technol. Res. 2021, 5, 59–67. [Google Scholar] [CrossRef]
- Licciardello, F.; Kharchoufi, S.; Muratore, G.; Restuccia, C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life 2018, 17, 114–119. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef]
- Nagarajan, M.; Rajasekaran, B.; Benjakul, S.; Venkatachalam, K. Influence of chitosan-gelatin edible coating incorporated with longkong pericarp extract on refrigerated black tiger Shrimp (Penaeus monodon). Curr. Res. Food Sci. 2021, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Sharifimehr, S.; Soltanizadeh, N.; Hossein Goli, S.A. Effects of edible coating containing nano-emulsion of Aloe vera and eugenol on the physicochemical properties of shrimp during cold storage. J. Sci. Food Agric. 2019, 99, 3604–3615. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hong, W.-S.; Oh, S.-W. Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef]
- Alexandre, S.; Vital, A.C.P.; Mottin, C.; do Prado, R.M.; Ornaghi, M.G.; Ramos, T.R.; Guerrero, A.; Pilau, E.J.; do Prado, I.N. Use of alginate edible coating and basil (Ocimum spp.) extracts on beef characteristics during storage. J. Food Sci. Technol. 2021, 58, 3835–3843. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Venkatachalam, K. Influence of prolonged salting on the physicochemical properties of duck egg white. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef] [Green Version]
- Khaledian, S.; Basiri, S.; Shekarforoush, S.S. Shelf-life extension of pacific white shrimp using tragacanth gum-based coatings containing Persian lime peel (Citrus latifolia) extract. LWT 2021, 141, 110937. [Google Scholar] [CrossRef]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X.; Bi, X.; Liu, X.; Shui, Y.; Lin, H. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruits and vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.H.; Lee, S.-Y.; Oh, S.-W. Enhancing safety and quality of shrimp by nanoparticles of sodium alginate-based edible coating containing grapefruit seed extract. Int. J. Biol. Macromol. 2021, 189, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Josewin, S.W.; Ghate, V.; Kim, M.-J.; Yuk, H.-G. Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon. Food Control 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Venkatachalam, K. The different concentrations of citric acid on inhibition of Longkong pericarp browning during low temperature storage. Int. J. Fruit Sci. 2015, 15, 353–368. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Emam-Djomeh, Z. Effect of active edible coatings made by basil seed gum and thymol on oil uptake and oxidation in shrimp during deep-fat frying. Carbohydr. Polym. 2016, 137, 249–254. [Google Scholar] [CrossRef]
- Basiri, S.; Shekarforoush, S.S.; Aminlari, M.; Akbari, S. The effect of pomegranate peel extract (PPE) on the polyphenol oxidase (PPO) and quality of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. LWT-Food Sci. Technol. 2015, 60, 1025–1033. [Google Scholar] [CrossRef]
- Simpson, B.K.; Marshall, M.R.; Otwell, W.S. Phenol oxidase from shrimp (Penaeus setiferus): Purification and some properties. J. Agric. Food Chem. 1987, 35, 918–921. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.L. Contribution of lipid and protein oxidation to rheological differences between chicken white and red muscle myofibrillar proteins. J. Agric. Food Chem. 1996, 44, 779–784. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Robinson, H.W.; Hogden, C.G. The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Parrilla-Taylor, D.P.; Zenteno-Savín, T.; Magallón-Barajas, F.J. Antioxidant enzyme activity in pacific whiteleg shrimp (Litopenaeus vannamei) in response to infection with white spot syndrome virus. Aquaculture 2013, 380, 41–46. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Benjakul, S.; Bauer, F. Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze–thaw cycles. Food Chem. 2001, 72, 207–217. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Choo, W.S.; Dykes, G.A. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. LWT-Food Sci. Technol. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- de Abreu, D.P.; Losada, P.P.; Maroto, J.; Cruz, J. Lipid damage during frozen storage of Atlantic halibut (Hippoglossus hippoglossus) in active packaging film containing antioxidants. Food Chem. 2011, 126, 315–320. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mehdizadeh, T.; Mojaddar Langroodi, A.; Rezaei, F. Effect of chitosan edible coating enriched with lemon verbena extract and essential oil on the shelf life of vacuum rainbow trout (Oncorhynchus mykiss). J. Food Saf. 2020, 40, e12781. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Lichanporn, I.; Srilaong, V.; Wongs-Aree, C.; Kanlayanarat, S. Postharvest physiology and browning of longkong (Aglaia dookkoo Griff.) fruit under ambient conditions. Postharvest Biol. Technol. 2009, 52, 294–299. [Google Scholar] [CrossRef]
- Farajzadeh, F.; Motamedzadegan, A.; Shahidi, S.-A.; Hamzeh, S. The effect of chitosan-gelatin coating on the quality of shrimp (Litopenaeus vannamei) under refrigerated condition. Food Control 2016, 67, 163–170. [Google Scholar] [CrossRef]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of edible coatings based on ultrasound-treated whey proteins in quality attributes of frozen Atlantic salmon (Salmo salar). Innov. Food Sci. Emerg. Technol. 2012, 14, 92–98. [Google Scholar] [CrossRef]
- Cheng, J.; Cui, L. Effects of high-intensity ultrasound on the structural, optical, mechanical and physicochemical properties of pea protein isolate-based edible film. Ultrason. Sonochemistry 2021, 80, 105809. [Google Scholar] [CrossRef]
- Amiri, A.; Sharifian, P.; Soltanizadeh, N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018, 111, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jambrak, A.R.; Herceg, Z.; Šubarić, D.; Babić, J.; Brnčić, M.; Brnčić, S.R.; Bosiljkov, T.; Čvek, D.; Tripalo, B.; Gelo, J. Ultrasound effect on physical properties of corn starch. Carbohydr. Polym. 2010, 79, 91–100. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochemistry 2017, 38, 835–842. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. Lwt 2020, 118, 108851. [Google Scholar] [CrossRef]
- Lu, F.; Liu, D.; Ye, X.; Wei, Y.; Liu, F. Alginate–calcium coating incorporating nisin and EDTA maintains the quality of fresh northern snakehead (Channa argus) fillets stored at 4 C. J. Sci. Food Agric. 2009, 89, 848–854. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Q.; Qiu, M.; Li, S. Chitosan-based edible coatings for quality preservation of postharvest whiteleg shrimp (Litopenaeus vannamei). J. Food Sci. 2012, 77, C491–C496. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Benjakul, S. Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. Int. J. Food Microbiol. 2011, 149, 247–253. [Google Scholar] [CrossRef]
- Shamshad, S.; Riaz, M.; Zuberi, R.; Qadri, R. Shelf life of shrimp (Penaeus merguiensis) stored at different temperatures. J. Food Sci. 1990, 55, 1201–1205. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Bajpai, V.; Rahman, A.; Dung, N.; Huh, M.; Kang, S. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. J. Food Sci. 2008, 73, M314–M320. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, M. Properties of phenoloxidase isolated from the cephalothorax of kuruma prawn (Penaeus japonicus). J. Food Biochem. 2005, 29, 470–485. [Google Scholar] [CrossRef]
- Jang, M.S.; Sanada, A.; Ushio, H.; Tanaka, M.; Ohshima, T. Inhibitory effect of enokitake extract on melanosis of shrimp. Fish. Sci. 2003, 69, 379–384. [Google Scholar] [CrossRef]
- Balti, R.; Mansour, M.B.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Masse, A. Active exopolysaccharides based edible coatings enriched with red seaweed (Gracilaria gracilis) extract to improve shrimp preservation during refrigerated storage. Food Biosci. 2020, 34, 100522. [Google Scholar] [CrossRef]
- Wu, G.; Farouk, M.; Clerens, S.; Rosenvold, K. Effect of beef ultimate pH and large structural protein changes with aging on meat tenderness. Meat Sci. 2014, 98, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Morachis-Valdez, A.G.; Gómez-Oliván, L.M.; García-Argueta, I.; Hernández-Navarro, M.D.; Díaz-Bandera, D.; Dublán-García, O. Effect of chitosan edible coating on the biochemical and physical characteristics of carp fillet (Cyprinus carpio) stored at−18 C. Int. J. Food Sci. 2017, 2017, 2812483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- Ansarian, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R.; Bimakr, M. Nanoemulsion-based basil seed gum edible film containing resveratrol and clove essential oil: In vitro antioxidant properties and its effect on oxidative stability and sensory characteristic of camel meat during refrigeration storage. Meat Sci. 2022, 185, 108716. [Google Scholar] [CrossRef]
- Rysman, T.; Van Hecke, T.; Van Poucke, C.; De Smet, S.; Van Royen, G. Protein oxidation and proteolysis during storage and in vitro digestion of pork and beef patties. Food Chem. 2016, 209, 177–184. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Gómez, M. Shelf life of fresh foal meat under MAP, overwrap and vacuum packaging conditions. Meat Sci. 2012, 92, 610–618. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Singh, A.; Nagarajan, M.; Benjakul, S. Effect of chitooligosaccharide and α-tocopherol on physical properties and oxidative stability of shrimp oil-in-water emulsion stabilized by bovine serum albumin-chitosan complex. Food Control 2022, 137, 108899. [Google Scholar] [CrossRef]
- Reesha, K.; Panda, S.K.; Bindu, J.; Varghese, T. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Gallardo, J.; González, M.J.; Lois, S.; Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.N.; Silva, E.M.; Loir, A.; Rees, J.-F.; Rogez, H.; Larondelle, Y. Antioxidant capacity of four polyphenol-rich Amazonian plant extracts: A correlation study using chemical and biological in vitro assays. Food Chem. 2008, 106, 331–339. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Buamard, N.; Benjakul, S. Ethanolic coconut husk extract: In vitro antioxidative activity and effect on oxidative stability of shrimp oil emulsion. Eur. J. Lipid Sci. Technol. 2017, 119, 1700131. [Google Scholar] [CrossRef]
- Xu, D.; Xue, H.; Sun, L.; Wang, Y. Retardation of melanosis development and quality degradation of Litopenaeus vannamei with starving treatment during cold storage. Food Control 2018, 92, 412–419. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P. Extraction and analysis of lipids. In Food Lipids, Chemistry Nutrition and Biotechnology, 2nd ed.; Akoh, C.C., dan Min, D.B., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 133–168. [Google Scholar]
- Chi, S.; Zivanovic, S.; Penfield, M. Application of chitosan films enriched with oregano essential oil on bologna–active compounds and sensory attributes. Food Sci. Technol. Int. 2006, 12, 111–117. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Yarnpakdee, S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J. Food Sci. Technol. 2015, 52, 6182–6193. [Google Scholar] [CrossRef] [Green Version]
- Castro, P.; Millan, R.; Pennedo, J.C.; Sanjuan, E.; Santana, A.; Caballero, M.J. Effect of storage conditions on total volatile base nitrogen determinations in fish muscle extracts. J. Aquat. Food Pro. Technol. 2012, 21, 519–523. [Google Scholar] [CrossRef]
- European Commission. The European Commission 1995–2000. Available online: https://op.europa.eu/en/publication-detail/-/publication/53418368-63b0-456a-95a5-c704de93a5f9/language-en (accessed on 31 January 2023).
- Wu, T.H.; Bechtel, P.J. Ammonia, dimethylamine, trimethylamine, and trimethylamine oxide from raw and processed Fish by-products. J. Aquat. Food Prod. Technol. 2008, 17, 27–38. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Combined effect of ethanolic eoconut husk extract and modified atmospheric packaging (MAP) in extending the shelf life of Asian sea bass slices. J. Aquat. Food Prod. Technol. 2019, 28, 689–702. [Google Scholar] [CrossRef]
- Alparslan, Y.; Metin, C.; Yapici, H.H.; Baygar, T.; Gunlu, A.; Baygar, T. Combined effect of orange peel essential oil and gelatin coating on the quality and shelf life of shrimps. J. Food. Saf. Food Qual. 2017, 68, 53–80. [Google Scholar] [CrossRef]
- Mohebi, E.; Shahbazi, Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: A novel functional wrapping design. LWT-Food Sci. Technol. 2017, 76, 108–116. [Google Scholar] [CrossRef]
- Liu, X.; Jia, Y.; Hu, Y.; Xia, X.; Li, Y.; Zhou, J.; Liu, Y. Effect of Citrus wilsonii Tanaka extract combined with alginate-calcium coating on quality maintenance of white shrimps (Litopenaeus vannamei Boone). Food Control 2016, 68, 83–91. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Labrador, P.; Rideout, J.A. Antimicrobial terpenoids from Lansium domesticum. Philipp. Agric. Sci. 2006, 89, 101. [Google Scholar]
- Munir, T.; Munawar, K.S.; Mohyuddin, A. An overview of the antibacterial implications of Lansium domesticum. J. Basic Appl. Sci. 2018, 14, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Mirhosseini, S.H.; Hosseini, S.M.H. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT-Food Sci. Technol. 2015, 64, 898–904. [Google Scholar] [CrossRef]
- Tsai, G.; Su, W.-H.; Chen, H.-C.; Pan, C.-L. Antimicrobial activity of shrimp chitin and chitosan from different treatments. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenphun, N.; Rajasekaran, B.; Palanisamy, S.; Venkatachalam, K. Impact of Longkong Pericarp Extract on the Physicochemical Properties of Alginate-Based Edible Nanoparticle Coatings and Quality Maintenance of Shrimp (Penaeus monodon) during Refrigerated Storage. Foods 2023, 12, 1103. https://doi.org/10.3390/foods12051103
Charoenphun N, Rajasekaran B, Palanisamy S, Venkatachalam K. Impact of Longkong Pericarp Extract on the Physicochemical Properties of Alginate-Based Edible Nanoparticle Coatings and Quality Maintenance of Shrimp (Penaeus monodon) during Refrigerated Storage. Foods. 2023; 12(5):1103. https://doi.org/10.3390/foods12051103
Chicago/Turabian StyleCharoenphun, Narin, Bharathipriya Rajasekaran, Suguna Palanisamy, and Karthikeyan Venkatachalam. 2023. "Impact of Longkong Pericarp Extract on the Physicochemical Properties of Alginate-Based Edible Nanoparticle Coatings and Quality Maintenance of Shrimp (Penaeus monodon) during Refrigerated Storage" Foods 12, no. 5: 1103. https://doi.org/10.3390/foods12051103





