Migration Degree of Selected Mycotoxins in the Distillation Process and Their Determination in Distilled Spirits from Pilot-Scale Continuous Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
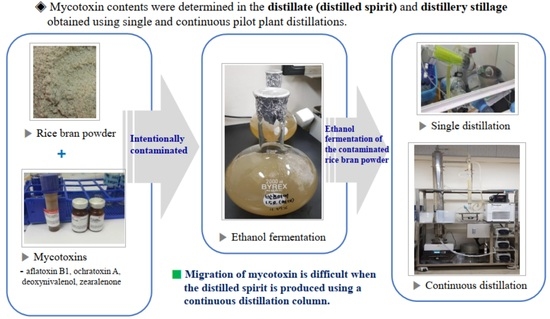
2.2. Sample Preparation of Mycotoxin Contamination for Ethanol Fermentation
2.3. Preparation of Distillate and Distillery Stillage from Single-Stage Distillation
2.4. Preparation of Distillate Fractions for Determining Aflatoxin B1 and Ochratoxin A from Pilot-Plant Scale Continuous Distillation Column
2.5. Preparation of Distilled Spirits and Distillery Stillage for Determining Aflatoxin B1 and Ochratoxin A Contents from Pilot-Plant Scale Distillation Column
2.6. Analysis of Ochratoxin A
2.7. Analysis of Aflatoxin B1
2.8. Analysis of Deoxynivalenol
2.9. Analysis of Zearalenone
3. Results and Discussion
3.1. Determination of Ochratoxin A, Aflatoxin B1, Deoxynivalenol, and Zearalenone Contents after Single-Stage Distillation
3.2. Determination of Ochratoxin A and Aflatoxin B1 after Repeated Single-Stage Distillation
3.3. Determination of Ochratoxin A and Aflatoxin B1 Contents in the Distillate Fractions Using Pilot-Plant Scale Distillation Column
3.4. Determination of Ochratoxin A and Aflatoxin B1 in the Distilled Spirit from Pilot-Plant Scale Distillation Column
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spaho, N. Distillation techniques in the fruit spirits production. In Distillation—Innovative Application and Modeling; Mendes, M.F., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Qin, Y.; Shin, J.A.; Lee, K.T. Determination of acetaldehyde, methanol and fusel oils in distilled liquors and sake’s by headspace gas chromatography. Food Sci. Biotechnol. 2020, 29, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Fanelli, F.; Zucca, P.; Liuzzi, V.C.; Quintieri, L.; Cimmarusti, M.T.; Monaci, L.; Haidukowski, M.; Logrieco, A.F.; Sanjust, E.; et al. Aflatoxin B1 and M1 degradation by Lac2 from Pleurotus pulmonarius and redox mediators. Toxins 2016, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Baydar, T.; Erkekoglu, P.; Sipahi, H.; Sahin, G. Aflatoxin B1, M1 and ochratoxin A levels in infant formulae and baby foods marketed in Ankara, Turkey. J. Food Drug Anal. 2007, 15, 89–92. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Baek, S.G.; Kim, S.; Paek, J.S.; Park, J.J.; Choi, J.; Choi, J.H.; Jang, J.Y.; Kim, J. Trends in mycotoxin contamination of cereals and cereal products in Korea. Res. Plant Dis. 2022, 28, 179–194. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; de Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Zaki, M.; El-Midany, S.A.; Shaheen, H.M.; Rizzi, L. Mycotoxins in Animals: Occurrence, Effects, Prevention and Management. J. Toxicol. Environ. Health Sci. 2012, 4, 13–28. Available online: https://academicjournals.org/article/article1379602600_Zaki%20et%20al.pdf (accessed on 18 September 2023). [CrossRef]
- National Library of Medicine. Chemical and Physical Characteristics of the Principal Mycotoxins. IARC Sci. Publ. 2012, 158, 31–38. Available online: https://pubmed.ncbi.nlm.nih.gov/23477194/ (accessed on 18 September 2023).
- Mishra, S.; Dixit, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Influence of temperature and pH on the degradation of deoxynivalenol (DON) in aqueous medium: Comparative cytotoxicity of DON and degraded product. Food Addit. Contam. 2014, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Hanna, M.A.; Eskridge, K.M.; Bullerman, L.B. Heat stability of zearalenone in an aqueous buffered model system. J. Agric. Food Chem. 2003, 51, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Degradation of aflatoxin B1 during the fermentation of alcoholic beverages. Toxins 2013, 5, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Cho, H.; Seo, D.W.; Jeong, H.G.; Kim, S.C.; Lee, J.H.; Hong, S.T.; Lee, K.T. Approach study for mass balance of pesticide residues in distillers’ stillage along with distillate and absence verification of pesticides in distilled spirits from pilot-scale of distillation column. Molecules 2019, 24, 2572. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, Y.; Inoue, T.; Uyama, A.; Mochizuki, N. The fate of mycotoxins during the distillation process of barley shochu, a distilled alcoholic beverage. Biosci. Biotechnol. Biochem. 2012, 76, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Food Code. 9.2.6 Ochratoxin A. 2023. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 18 September 2023).
- Ministry of Food and Drug Safety. Food Code. 9.2.2 Aflatoxin B1. 2023. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 18 September 2023).
- Ministry of Food and Drug Safety. Food Code. 9.2.7 Deoxynivalenol. 2023. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 18 September 2023).
- Ministry of Food and Drug Safety. Food Code. 9.2.8 Zearalenone. 2023. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 18 September 2023).
- Ministry of Food and Drug Safety. Food Code. 3. Food Standards and Specification. 2023. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 18 September 2023).
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagatomo, Y.; Uyama, A.; Mochizuki, N. Fate of mycotoxins during beer brewing and fermentation. Biosci. Biotechnol. Biochem. 2013, 77, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Belincanta, J.; Kakuta Ravagnani, T.M.; Pereira, J.A.F. Hydrodynamic and tray efficiency behavior in parastillation column. Braz. J. Chem. Eng. 2006, 23, 135–146. [Google Scholar] [CrossRef]


| Mycotoxin | Solubility in Ethanol | Molecular Weight (g/mol) | Vapor Pressure | Log P |
|---|---|---|---|---|
| Aflatoxin B1 | Soluble in absolute ethanol (Sigma) | 312.3 | 2.65 × 10−10 mmHg at 25 °C | 1.23 (est) |
| Ochratoxin A | Soluble in ethanol (10–50 mg/mL) | 403.8 | 1.81 × 10−15 mm Hg at 25 °C | 5.19 |
| Deoxynivalenol | Soluble to 10 mg/mL in 100% ethanol (Sigma) | 296.3 | 6.8 × 10−11 mm Hg at 25 °C | −0.71 (est) |
| Zearalenone | Soluble to 20 mg/mL in ethanol | 318.4 | 0 mm Hg at 20 °C | 3–4 (predicted) |
| Mycotoxin | ||||
|---|---|---|---|---|
| Aflatoxin B1 | Ochratoxin A | Deoxynivalenol | Zearalenone | |
| Unit | ppb | ppb | ppm | ppb |
| Spiking amount | 40 | 20 | 4 | 800 |
| Raw material | ND 1 | 0.32 | ND | 17.43 |
| Distillate | ND | 0.19 | ND | ND |
| DMR (%) 2 | 0 | 0.95 | 0 | 0 |
| Distillery stillage | 21.60 | 14.50 | 3.06 | 769.34 |
| DSRR rate (%) 3 | 54.0 | 72.5 | 76.5 | 96.2 |
| 1st Distillation 1 | 2nd Distillation 2 | 3rd Distillation 2 | |
|---|---|---|---|
| Aflatoxin B1 | ND 3 | ND | ND |
| Ochratoxin A | 0.19 | Trace (0.11) 4 | 0.22 |
| Mycotoxin | ||||
|---|---|---|---|---|
| Aflatoxin B1 | Ochratoxin A | Deoxynivalenol | Zearalenone | |
| MRL 1 (ppb) | 10 | 5 | 1000 | 200 |
| LOD | 0.18 (μg/kg) | 0.05 (μg/kg) | 0.07 (mg/kg) | 2 (μg/kg) |
| LOQ | 0.55 (μg/kg) | 0.15 (μg/kg) | 0.23 (mg/kg) | 6 (μg/kg) |
| Calibration curves | Y = 1.9765 × 10−5 X (R2 = 0.999) | Y = 6.6425 × 10−5 X (R2 = 0.999) | Y = 9.6468 × 10−6 X (R2 = 0.999) | Y = 4.6 × 10−4 X (R2 = 0.999) |
| Standard concentration range | 0.1–2 (μg/mL) | 1–25 (ng/mL) | 0.1–2 (μg/mL) | 50–500 (μg/L) |
| Alcohol (%) | Distillate Volume (mL) | Amount of Ochratoxin A | DMR (%) 1 | |
|---|---|---|---|---|
| Contaminated content | - | - | 3.3 ppb (5 μg) | - |
| Distillate fraction 1 | 87.3 | 38 | ND 2 | 0 |
| Distillate fraction 2 | 74.8 | 28 | ND | 0 |
| Distillate fraction 3 | 33.7 | 110 | ND | 0 |
| Alcohol (%) | Distillate Volume (mL) | Amount of Aflatoxin B1 | DMR (%) 1 | |
|---|---|---|---|---|
| Contaminated content | - | - | 2 ppb (3 μg) | - |
| Distillate fraction 1 | 87.1 | 60 | ND 2 | 0 |
| Distillate fraction 2 | 47.6 | 40 | ND | 0 |
| Distillate fraction 3 | 15.4 | 65 | ND | 0 |
| Distillate fraction 4 | 5.5 | 40 | ND | 0 |
| Distillate fraction 5 | 3.3 | 130 | 2.35 ppb (0.3 μg) | 10 |
| Ochratoxin A | Aflatoxin B1 | ||
|---|---|---|---|
| Contaminated content | - | 3.84 ppb (7.5 μg) | 7.69 ppb (15 μg) |
| Fermented liquid | Content of mycotoxin | 4.05 ppb (7.9 μg) | 9.64 ppb (18.8 μg) |
| Recovery (%) 1 | 105.5 | 125.3 | |
| Distilled spirit | Obtained volume (mL) | 155 | 155 |
| Alcohol (%) | 93.9 | 95.4 | |
| Content of mycotoxin | ND 4 | ND | |
| DMR (%) 2 | 0 | 0 | |
| Distillery stillage | Content of mycotoxin | 2.70 ppb (5.26 μg) | 3.65 ppb (7.12 μg) |
| Alcohol (%) | 0 | 0 | |
| DSRR (%) 3 | 70.3 | 47.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-A.; Lee, K.-T. Migration Degree of Selected Mycotoxins in the Distillation Process and Their Determination in Distilled Spirits from Pilot-Scale Continuous Distillation. Foods 2023, 12, 4189. https://doi.org/10.3390/foods12234189
Shin J-A, Lee K-T. Migration Degree of Selected Mycotoxins in the Distillation Process and Their Determination in Distilled Spirits from Pilot-Scale Continuous Distillation. Foods. 2023; 12(23):4189. https://doi.org/10.3390/foods12234189
Chicago/Turabian StyleShin, Jung-Ah, and Ki-Teak Lee. 2023. "Migration Degree of Selected Mycotoxins in the Distillation Process and Their Determination in Distilled Spirits from Pilot-Scale Continuous Distillation" Foods 12, no. 23: 4189. https://doi.org/10.3390/foods12234189