Varietal Authenticity Assessment of QTMJ Tea Using Non-Targeted Metabolomics and Multi-Elemental Analysis with Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Sample Collection and Pretreatment
2.3. LC-MS Based Metabolomic Analysis
2.4. Multielement Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Difference of Metabolomic Fingerprints among QTMJ Tea Cultivars
3.2. Discriminating Cultivars of QTMJ Tea Based on Candidate Differential Metabolites
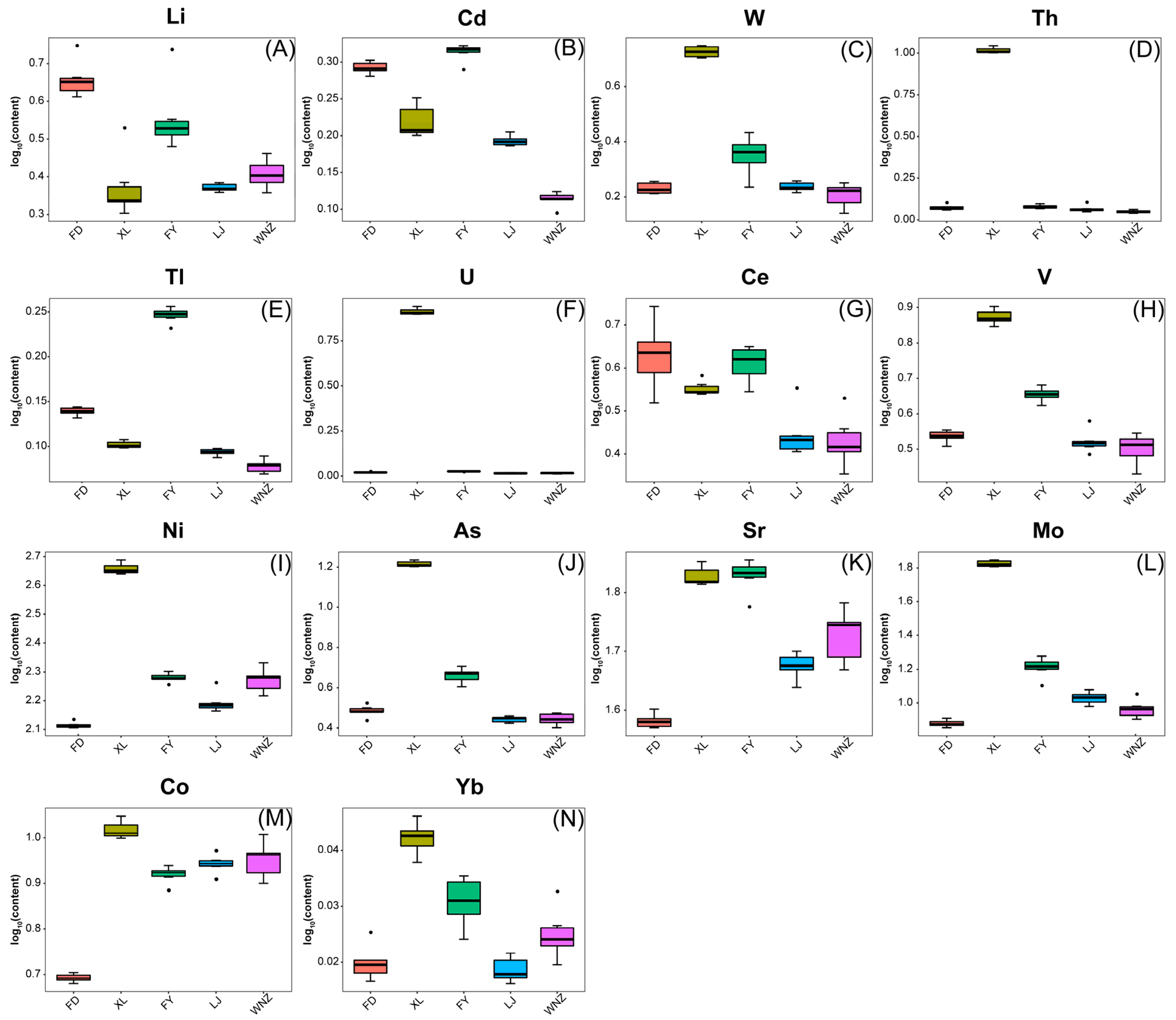
3.3. Discriminating Cultivars of QTMJ Tea Using Elemental Fingerprinting
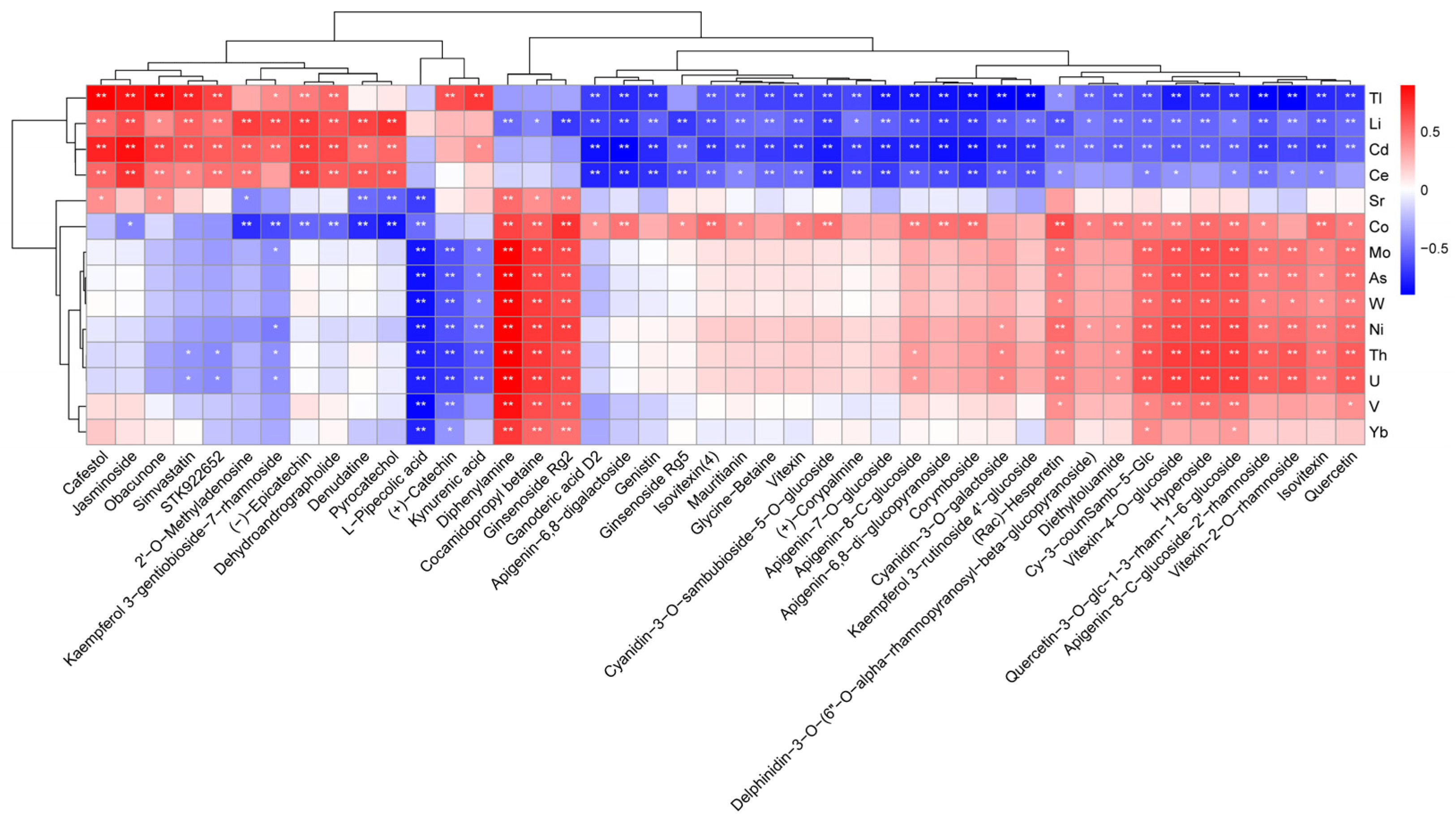
3.4. Correlation Analysis of Differential Metabolites and Mineral Elements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Engelhardt, U.H. Tea chemistry—What do and what don’t we know?—A micro review. Food Res. Int. 2020, 132, 109120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, W.; Liu, X.-H.; Yang, Z.; Gaur, R.; Wang, J.-J.; Ke, J.-P.; Bao, G.-H. Detection and quantification of flavoalkaloids in different tea cultivars and during tea processing using UPLC-TOF-MS/MS. Food Chem. 2021, 339, 127864. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Miao, N.; Zhang, X.; Li, Q.; Wang, Z.; Li, C.; Sun, X.; Lan, Y. Employment of an electronic tongue combined with deep learning and transfer learning for discriminating the storage time of Pu-erh tea. Food Control 2021, 121, 107608. [Google Scholar] [CrossRef]
- Ren, Y.-F.; Feng, C.; Ye, Z.-H.; Zhu, H.-Y.; Hou, R.-Y.; Granato, D.; Cai, H.-M.; Peng, C.-Y. Keemun black tea: Tracing its narrow-geographic origins using comprehensive elemental fingerprinting and chemometrics. Food Control 2022, 133, 108614. [Google Scholar] [CrossRef]
- Fraser, K.; Lane, G.A.; Otter, D.E.; Hemar, Y.; Quek, S.-Y.; Harrison, S.J.; Rasmussen, S. Analysis of metabolic markers of tea origin by UHPLC and high resolution mass spectrometry. Food Res. Int. 2013, 53, 827–835. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Pan, W.; Ouyang, Q.; Li, H.; Urmila, K.; Zhao, J. Recent developments of green analytical techniques in analysis of tea’s quality and nutrition. Trends Food Sci. Technol. 2015, 43, 63–82. [Google Scholar] [CrossRef]
- Esteki, M.; Memarbashi, N.; Simal-Gandara, J. Classification and authentication of tea according to their geographical origin based on FT-IR fingerprinting using pattern recognition methods. J. Food Compos. Anal. 2022, 106, 104321. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, X.; Li, Y.; Wang, M.; Liu, Z.; Dong, C. Establishment of a rapid detection model for the sensory quality and components of Yuezhou Longjing tea using near-infrared spectroscopy. LWT 2022, 164, 113625. [Google Scholar] [CrossRef]
- Lin, X.; Sun, D.-W. Recent developments in vibrational spectroscopic techniques for tea quality and safety analyses. Trends Food Sci. Technol. 2020, 104, 163–176. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, M.; Chen, Y.; Cui, Q.; Lu, C.; Wang, Y.; Li, L.; Xu, Z.; Zhong, Y.; et al. Rapid identification of the green tea geographical origin and processing month based on near-infrared hyperspectral imaging combined with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120537. [Google Scholar] [CrossRef]
- Shuai, M.; Peng, C.; Niu, H.; Shao, D.; Hou, R.; Cai, H. Recent techniques for the authentication of the geographical origin of tea leaves from camellia sinensis: A review. Food Chem. 2022, 374, 131713. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, X.; Lei, W. Identifying the Producer and Grade of Matcha Tea through Three-Dimensional Fluorescence Spectroscopy Analysis and Distance Discrimination. Foods 2023, 12, 3614. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wen, M.; Zhang, H.; Zhang, L.; Wan, X.; Ho, C.-T. LC-MS based metabolomics and sensory evaluation reveal the critical compounds of different grades of Huangshan Maofeng green tea. Food Chem. 2022, 374, 131796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, W.; Chen, Y.; Zhang, X.; Wang, X.; Wang, F.; Qian, Y.; Qiu, J. Identification of markers for tea authenticity assessment: Non-targeted metabolomics of highly similar oolong tea cultivars (Camellia sinensis var. sinensis). Food Control 2022, 374, 131796. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Zheng, G.; Wang, T.; Lin, J.; Wang, S.; Wang, X.; Chao, Q.; Cao, S.; Yang, Z.; et al. Metabolite profiling of 14 Wuyi Rock tea cultivars using UPLC-QTOF MS and UPLC-QqQ MS combined with chemometrics. Molecules 2018, 23, 104. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Yuan, Z.; Guo, G. Untargeted metabolomics coupled with chemometrics approach for Xinyang Maojian green tea with cultivar, elevation and processing variations. Food Chem. 2021, 352, 129359. [Google Scholar] [CrossRef]
- Li, P.; Dai, W.; Lu, M.; Xie, D.; Tan, J.; Yang, C.; Zhu, Y.; Lv, H.; Peng, Q.; Zhang, Y.; et al. Metabolomic analysis reveals the composition differences in 13 Chinese tea cultivars of different manufacturing suitabilities. J. Sci. Food Agric. 2018, 98, 1153–1161. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, Y.C.; Wen, X.; Peng, Y.; Ni, X. Use of mineral multi-elemental analysis to authenticate geographical origin of different cultivars of tea in Guizhou, China. J. Sci. Food Agric. 2020, 100, 3046–3055. [Google Scholar] [CrossRef]
- Long, W.; Bai, X.; Wang, S.; Chen, H.; Yin, X.-L.; Gu, H.-W.; Yang, J.; Fu, H. UHPLC-QTOF-MS-based untargeted metabolomics and mineral element analysis insight into the geographical differences of Chrysanthemum morifolium Ramat cv. “Hangbaiju” from different origins. Food Res. Int. 2023, 163, 112186. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Mi, S.; Jin, Q.; Sang, Y.; Wang, X. Characterization and discrimination of two varieties of eggplants using multi-element and metabolomics profiles coupled with chemometrics analysis. Food Res. Int. 2022, 162, 111976. [Google Scholar] [CrossRef] [PubMed]
- Karak, T.; Bhagat, R. Trace elements in tea leaves, made tea and tea infusion: A review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics combined with proteomics provides a novel interpretation of the compound differences among Chinese tea cultivars (Camellia sinensis var. sinensis) with different manufacturing suitabilities. Food Chem. 2022, 377, 131976. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, anti-inflammatory, and anticancer effects of purified flavonol glycosides and aglycones in green tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, M.; Liu, J.; Cai, J. Metabolomic and transcriptomic analyses reveal the characteristics of tea flavonoids and caffeine accumulation and regulation between Chinese varieties (Camellia sinensis var. sinensis) and Assam varieties (C. sinensis var. assamica). Genes 2022, 13, 1994. [Google Scholar] [CrossRef]
- Fang, Z.-T.; Yang, W.-T.; Li, C.-Y.; Li, D.; Dong, J.-J.; Zhao, D.; Xu, H.-R.; Ye, J.-H.; Zheng, X.-Q.; Liang, Y.-R.; et al. Accumulation pattern of catechins and flavonol glycosides in different varieties and cultivars of tea plant in China. J. Food Compos. Anal. 2021, 97, 103772. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Peng, Y.; Wang, S.; Zhang, J.; Liu, X.; Liu, J.; Wen, B.; Li, M. Biosynthesis and the Transcriptional Regulation of Terpenoids in Tea Plants (Camellia sinensis). Int. J. Mol. Sci. 2023, 24, 6937. [Google Scholar] [CrossRef]
- Panyatip, P.; Padumanonda, T.; Yongram, C.; Kasikorn, T.; Sungthong, B.; Puthongking, P. Impact of tea processing on tryptophan, melatonin, phenolic and flavonoid contents in mulberry (Morus alba L.) leaves: Quantitative analysis by LC-MS/MS. Molecules 2022, 27, 4979. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, Y.; Matsuzawa, H. Recent progress in production of amino acid-derived chemicals using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2021, 37, 49. [Google Scholar] [CrossRef] [PubMed]
- Ariyama, K.; Nishida, T.; Noda, T.; Kadokura, M.; Yasui, A. Effects of fertilization, crop year, variety, and provenance factors on mineral concentrations in onions. J. Agric. Food Chem. 2006, 54, 3341–3350. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Liao, R.; Zhao, J.; Yang, H.; Wang, F. Authentication of the geographical origin of Guizhou green tea using stable isotope and mineral element signatures combined with chemometric analysis. Food Control 2021, 125, 107954. [Google Scholar] [CrossRef]
- Guo, W.; Wang, R.; Wang, W.; Peng, Y. Rapid determination of toxic and rare-earth elements in teas by particle nebulization-ICPMS. J. Food Compos. Anal. 2020, 91, 103517. [Google Scholar] [CrossRef]
- Falandysz, J.; Fernandes, A.R.; Zhang, J. Critical review of rare earth elements (REE) in cultivated macrofungi. Food Control 2023, 155, 110085. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, K.; Wang, F.; Zhao, X.; Bai, R.; Liu, B. Effects of rare earth elements on growth and determination of secondary metabolites under in vitro conditions in Salvia miltiorrhiza. HortScience 2020, 55, 310–316. [Google Scholar] [CrossRef]
- Zhang, H.; Mo, X.; Tang, D.; Ma, Y.; Xie, Y.; Yang, H.; Shi, M.; Li, L.; Li, W.; Yan, F.; et al. Comparative analysis of volatile and carotenoid metabolites and mineral elements in the flesh of 17 kiwifruit. J. Food Sci. 2021, 86, 3023–3032. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
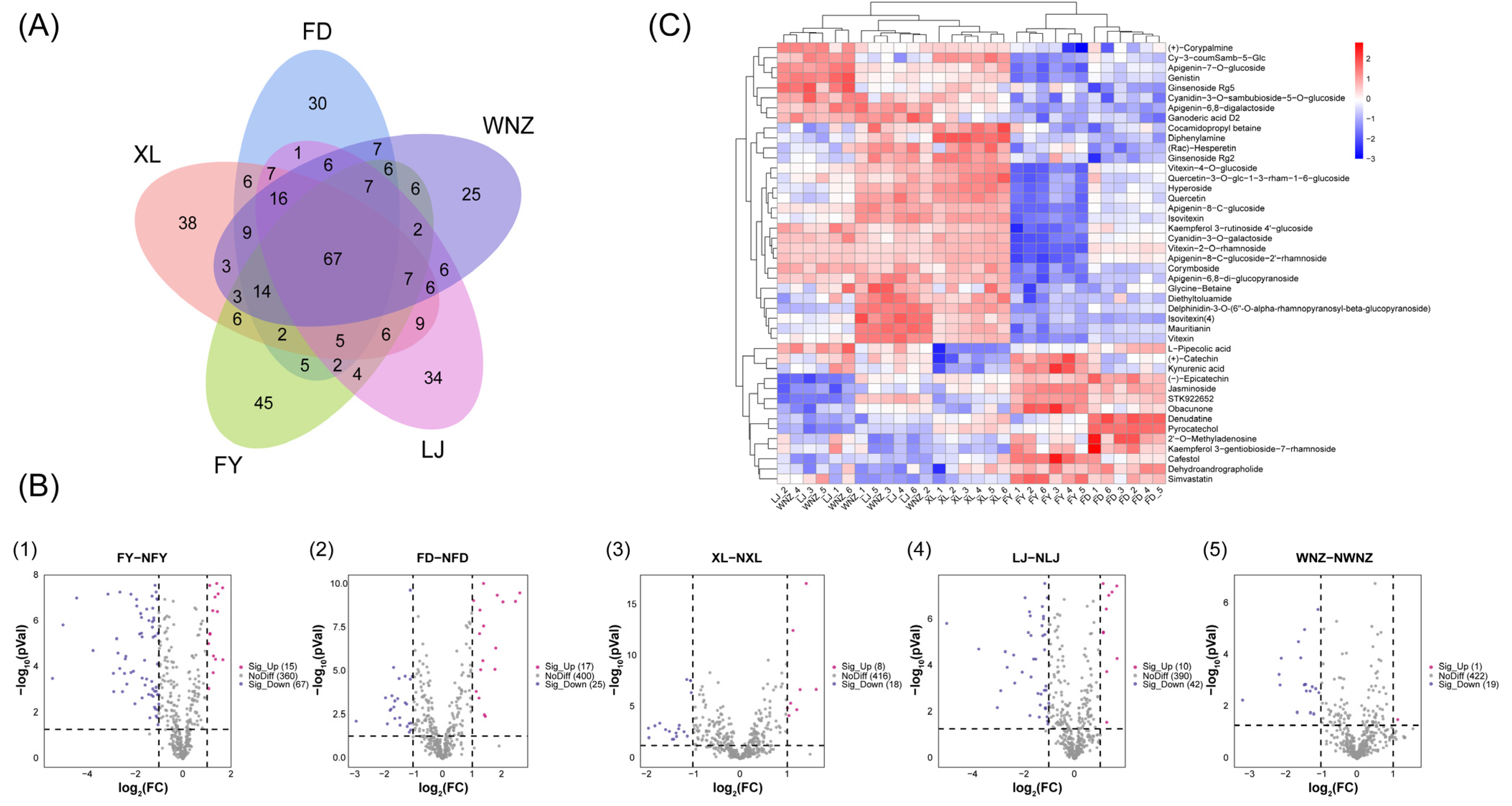
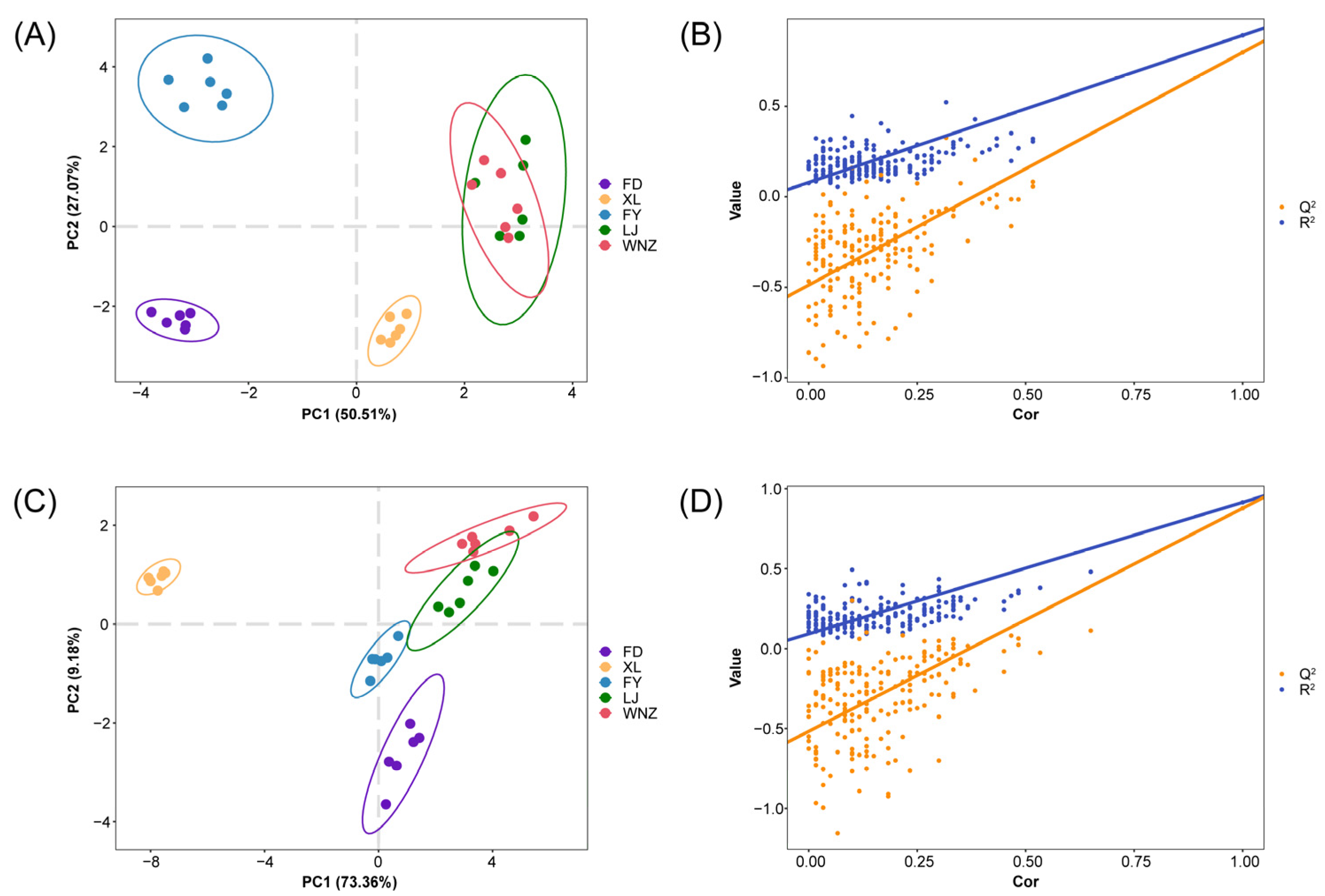
| Metabolite Name | Classification | Adduct Type | Rt (Min) | M/Z | VIP | Significance |
|---|---|---|---|---|---|---|
| (+)-Catechin | Flavanole | [M+H]+ | 7.394 | 291.08478 | 1.60 | ** |
| (−)-Epicatechin | Flavanole | [M+H]+ | 8.956 | 291.0864 | 1.67 | ** |
| Isovitexin | Flavone | [M+H]+ | 8.637 | 433.10745 | 2.09 | ** |
| Isovitexin (4) | Flavone | [M+H]+ | 9.571 | 433.1131 | 2.14 | ** |
| Corymboside | Flavone | [M+H]+ | 8.881 | 565.1485 | 2.29 | ** |
| Vitexin | Flavone | [M+H]+ | 8.768 | 433.11374 | 2.05 | ** |
| Vitexin-4-O-glucoside | Flavone | [M+H]+ | 9.199 | 595.16187 | 2.05 | ** |
| Vitexin-2-O-rhamnoside | Flavone | [M+H]+ | 9.401 | 579.16632 | 1.58 | ** |
| Apigenin-6,8-digalactoside | Flavone | [M+H]+ | 7.865 | 595.16357 | 2.06 | ** |
| Apigenin-7-O-glucoside | Flavone | [M-H]− | 10.399 | 431.09393 | 1.86 | ** |
| Apigenin-8-C-glucoside | Flavone | [M-H]− | 9.262 | 431.09247 | 2.04 | ** |
| Apigenin-8-C-glucoside-2′-rhamnoside | Flavone | [M-H]− | 9.212 | 577.15796 | 1.79 | ** |
| Apigenin-6,8-di-glucopyranoside | Flavone | [M-H]− | 8.968 | 593.14679 | 2.24 | ** |
| STK922652 | Flavone | [M+H]+ | 8.448 | 425.1402 | 1.87 | ** |
| Kaempferol 3-rutinoside 4′-glucoside | Flavonole | [M+H]+ | 9.138 | 741.21985 | 1.78 | ** |
| Kaempferol 3-gentiobioside-7-rhamnoside | Flavonole | [M-H]− | 9.444 | 755.20581 | 2.01 | ** |
| Hyperoside | Flavonole | [M+H]+ | 9.199 | 465.09711 | 2.21 | ** |
| Quercetin | Flavonole | [M-H]− | 11.887 | 301.03671 | 1.97 | ** |
| Quercetin-3-O-glc-1-3-rham-1-6-glucoside | Flavonole | [M+H]+ | 9.199 | 773.20691 | 2.23 | ** |
| Mauritianin | Flavonole | [M+Na]+ | 9.664 | 763.19934 | 1.81 | ** |
| (Rac)-Hesperetin | Flavanone | [M+H]+ | 10.928 | 303.08292 | 2.17 | ** |
| Genistin | Isoflavone | [M+H]+ | 10.419 | 433.11099 | 1.83 | ** |
| Cy-3-coumSamb-5-Glc | Anthocyanidin | [M]+ | 9.627 | 449.10898 | 2.21 | ** |
| Cyanidin-3-O-galactoside | Anthocyanidin | [M]+ | 9.442 | 889.25623 | 1.89 | ** |
| Cyanidin-3-O-sambubioside-5-O-glucoside | Anthocyanidin | [M-2H]− | 7.177 | 741.18036 | 2.13 | ** |
| Delphinidin-3-O-(6″-O-alpha-rhamnopyranosyl-beta-glucopyranoside) | Anthocyanidin | [M]+ | 9.213 | 611.13898 | 1.72 | ** |
| (+)-Corypalmine | Alkaloid | [M+H]+ | 3.429 | 342.17606 | 1.64 | ** |
| 2′-O-Methyladenosine | Alkaloid | [M+H]+ | 5.986 | 282.11865 | 2.13 | ** |
| Denudatine | Alkaloid | [M+H]+ | 19.91 | 344.25394 | 1.57 | ** |
| Kynurenic acid | Alkaloid | [M+H]+ | 7.699 | 190.04939 | 1.52 | ** |
| Cocamidopropyl Betaine | Alkaloid | [M+H]+ | 14.884 | 343.29813 | 2.13 | ** |
| Glycine Betaine | Alkaloid | [M+H]+ | 0.954 | 118.08591 | 1.62 | ** |
| Cafestol | Terpenoid | [M+H]+ | 19.939 | 317.20554 | 1.54 | ** |
| Dehydroandrographolide | Terpenoid | [M+H]+ | 16.4 | 333.20135 | 1.70 | ** |
| Jasminoside | Terpenoid | [M+H]+ | 12.07 | 303.0484 | 1.76 | ** |
| Ganoderic acid D2 | Terpenoid | [M+Na]+ | 18.257 | 553.27069 | 1.53 | ** |
| Ginsenoside Rg2 | Terpenoid | [M+H]+ | 24.279 | 785.49689 | 2.45 | ** |
| Ginsenoside Rg5 | Terpenoid | [M-H]− | 24.26 | 765.48004 | 1.94 | ** |
| Obacunone | Terpenoid | [M+Na]+ | 8.16 | 477.18555 | 1.80 | ** |
| L-Pipecolic acid | Organic acid | [M+H]+ | 1.076 | 130.08755 | 1.63 | ** |
| Diphenylamine | Other types | [M+H]+ | 17.737 | 170.09619 | 2.60 | ** |
| Pyrocatechol | Other types | [M+Na]+ | 15.327 | 553.29712 | 1.60 | ** |
| Simvastatin | Other types | [M+Na]+ | 18.648 | 441.25537 | 1.97 | ** |
| Diethyltoluamide | Other types | [M+H]+ | 14.488 | 192.13638 | 1.93 | ** |
| (carbon number 11) | Unknow | [M+H]+ | 2.211 | 229.15523 | 2.04 | ** |
| 2-hydroxy-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}propyl(9Z,12Z,15Z)-octadeca-9,12,15-trienoate | Unknow | [M+H]+ | 20.007 | 515.3147 | 1.67 | ** |
| LPC 16:0 | Unknow | [M+H]+ | 18.84 | 496.3378 | 1.59 | ** |
| LPC 18:3 | Unknow | [M+H]+ | 17.294 | 518.32202 | 1.63 | ** |
| N-((S)-10-(((2S,3R)-1-(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)-3-methyl-1-oxopentan-2-yl)amino)-1,2,3-trimethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl)acetamide | Unknow | [M+Na]+ | 9.816 | 763.25299 | 1.99 | ** |
| NCGC00180744-03 | Unknow | [M-H2O+H]+ | 9.975 | 331.15503 | 2.78 | ** |
| NCGC00380867-01 | Unknow | [M+NH4]+ | 18.254 | 532.34662 | 1.54 | ** |
| NCGC00384602-01 | Unknow | [M+Na]+ | 12.147 | 471.21854 | 2.09 | ** |
| NP-000062(6) | Unknow | [M-H]− | 8.538 | 563.13953 | 1.93 | ** |
| (2S,3R,4S,5S,6R)-2-[(2E)-3,7-dimethylocta-2,6-dienoxy]-6-[[(2S,3R,4S,5S)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxane-3,4,5-triol | Unknow | [M+FA-H]− | 12.143 | 493.22742 | 2.31 | ** |
| Elemental | FD | XL | FY | LJ | WNZ | VIP | Significance |
|---|---|---|---|---|---|---|---|
| Li [mg kg−1] | 0.18 ± 0.03 a | 0.07 ± 0.03 c | 0.13 ± 0.04 b | 0.07 ± 0.00 c | 0.08 ± 0.01 c | 1.78 | ** |
| V [mg kg−1] | 0.12 ± 0.01 c | 0.32 ± 0.02 a | 0.18 ± 0.01 b | 0.12 ± 0.01 c | 0.11 ± 0.02 c | 1.11 | ** |
| Mn [mg kg−1] | 228.28 ± 6.97 d | 217.49 ± 8.56 d | 824.42 ± 39.9 a | 486.69 ± 21.94 c | 657.17 ± 65.73 b | 0.10 | ** |
| Co [mg kg−1] | 0.20 ± 0.01 d | 0.47 ± 0.02 a | 0.37 ± 0.02 c | 0.39 ± 0.02 bc | 0.40 ± 0.04 b | 1.88 | ** |
| Ni [mg kg−1] | 6.58 ± 0.16 d | 22.95 ± 1.02 a | 9.61 ± 0.35 b | 7.89 ± 0.68 c | 9.46 ± 0.91 b | 1.75 | ** |
| Cu [mg kg−1] | 17.54 ± 0.49 a | 17.09 ± 0.64 ab | 18.06 ± 1.01 a | 16.92 ± 0.68 ab | 16.2 ± 1.51 b | 0.79 | * |
| Zn [mg kg−1] | 58.42 ± 1.63 b | 65.66 ± 2.62 a | 50.28 ± 3.12 c | 53.34 ± 2.14 c | 52.64 ± 5.07 c | 0.67 | ** |
| Ga [mg kg−1] | 0.19 ± 0.00 e | 0.26 ± 0.01 c | 0.38 ± 0.01 a | 0.23 ± 0.02 d | 0.28 ± 0.02 b | 0.45 | ** |
| As [mg kg−1] | 0.10 ± 0.01 c | 0.77 ± 0.03 a | 0.18 ± 0.02 b | 0.09 ± 0.00 c | 0.09 ± 0.01 c | 1.24 | ** |
| Zr [mg kg−1] | 0.09 ± 0.10 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 0.62 | |
| Mo [mg kg−1] | 0.33 ± 0.02 d | 3.29 ± 0.14 a | 0.77 ± 0.11 b | 0.49 ± 0.05 c | 0.41 ± 0.06 cd | 1.49 | ** |
| Cd [mg kg−1] | 0.05 ± 0.00 a | 0.03 ± 0.00 b | 0.05 ± 0.00 a | 0.03 ± 0.00 b | 0.01 ± 0.00 c | 1.29 | ** |
| Sn [mg kg−1] | 0.30 ± 0.01 ab | 0.33 ± 0.03 a | 0.31 ± 0.01 ab | 0.3 ± 0.03 b | 0.29 ± 0.04 b | 0.25 | * |
| Sb [mg kg−1] | 0.05 ± 0.01 ab | 0.03 ± 0.00 c | 0.06 ± 0.01 a | 0.03 ± 0.02 c | 0.04 ± 0.01 bc | 0.93 | ** |
| Ba [mg kg−1] | 7.15 ± 0.24 d | 7.94 ± 0.33 c | 13.46 ± 0.75 a | 7.54 ± 0.51 cd | 9.16 ± 0.95 b | 0.13 | ** |
| La [mg kg−1] | 0.10 ± 0.02 ab | 0.08 ± 0.03 abc | 0.10 ± 0.02 as | 0.07 ± 0.01 bc | 0.06 ± 0.03 c | 0.90 | * |
| Ce [mg kg−1] | 0.17 ± 0.04 a | 0.13 ± 0.01 b | 0.15 ± 0.02 ab | 0.09 ± 0.02 c | 0.09 ± 0.02 c | 1.10 | ** |
| Nd [mg kg−1] | 0.04 ± 0.01 c | 0.04 ± 0.00 b | 0.05 ± 0.01 a | 0.03 ± 0.01 cd | 0.02 ± 0.00 d | 0.37 | ** |
| Gd [mg kg−1] | 0.03 ± 0.01 bc | 0.02 ± 0.00 c | 0.04 ± 0.01 b | 0.02 ± 0.02 c | 0.06 ± 0.02 a | 0.15 | ** |
| W [mg kg−1] | 0.04 ± 0.00 c | 0.22 ± 0.01 a | 0.06 ± 0.02 b | 0.04 ± 0.00 c | 0.03 ± 0.01 c | 1.23 | ** |
| Pb [mg kg−1] | 0.29 ± 0.03 a | 0.26 ± 0.02 a | 0.26 ± 0.04 a | 0.16 ± 0.02 b | 0.13 ± 0.02 b | 0.82 | ** |
| Sr [μg kg−1] | 1.86 ± 0.05 d | 3.31 ± 0.13 a | 3.32 ± 0.21 a | 2.32 ± 0.12 c | 2.63 ± 0.28 b | 1.28 | ** |
| Be [μg kg−1] | 2.11 ± 0.56 cd | 3.02 ± 0.33 b | 6.80 ± 0.59 a | 1.85 ± 0.19 d | 2.48 ± 0.58 bc | 0.07 | ** |
| Pr [μg kg−1] | 8.93 ± 1.96 bc | 10.03 ± 0.93 b | 13.37 ± 1.42 a | 7.21 ± 1.96 cd | 6.08 ± 0.71 d | 0.53 | ** |
| Sm [μg kg−1] | 6.53 ± 1.13 b | 10.43 ± 0.41 a | 10.23 ± 1.06 a | 5.62 ± 1.12 bc | 5.23 ± 0.99 c | 0.26 | ** |
| Eu [μg kg−1] | 3.43 ± 0.41 c | 4.00 ± 0.19 b | 6.41 ± 0.35 a | 3.51 ± 0.37 c | 4.04 ± 0.56 b | 0.12 | ** |
| Tb [μg kg−1] | 0.72 ± 0.15 c | 1.64 ± 0.10 a | 1.12 ± 0.09 b | 0.62 ± 0.13 cd | 0.57 ± 0.10 d | 0.69 | ** |
| Dy [μg kg−1] | 4.40 ± 0.59 c | 11.35 ± 0.65 a | 6.56 ± 0.63 b | 3.63 ± 0.50 cd | 3.57 ± 0.85 d | 0.86 | ** |
| Ho [μg kg−1] | 0.68 ± 0.07 c | 1.92 ± 0.11 a | 1.12 ± 0.12 b | 0.92 ± 0.72 bc | 0.62 ± 0.12 c | 0.98 | ** |
| Er [μg kg−1] | 2.10 ± 0.15 c | 5.98 ± 0.40 a | 3.29 ± 0.38 b | 1.78 ± 0.35 c | 1.91 ± 0.52 c | 0.96 | ** |
| Tm [μg kg−1] | 0.22 ± 0.04 c | 0.69 ± 0.12 a | 0.34 ± 0.02 b | 0.16 ± 0.08 c | 0.21 ± 0.10 c | 0.73 | ** |
| Yb [μg kg−1] | 2.34 ± 0.37 d | 5.10 ± 0.36 a | 3.68 ± 0.54 b | 2.19 ± 0.27 d | 2.96 ± 0.54 c | 1.25 | ** |
| Lu [μg kg−1] | 0.19 ± 0.05 c | 0.58 ± 0.07 a | 0.33 ± 0.07 b | 0.16 ± 0.04 c | 0.19 ± 0.08 c | 0.90 | ** |
| Hf [μg kg−1] | 2.05 ± 1.75 a | 1.49 ± 0.11 ab | 1.36 ± 0.17 ab | 1.01 ± 0.16 b | 0.97 ± 0.28 b | 0.61 | |
| Ta [μg kg−1] | 0.80 ± 0.24 a | 0.48 ± 0.05 bc | 0.45 ± 0.03 c | 0.60 ± 0.14 bc | 0.63 ± 0.09 b | 0.90 | ** |
| Tl [μg kg−1] | 18.93 ± 0.71 b | 13.32 ± 0.57 c | 38.13 ± 1.68 a | 12.12 ± 0.53 d | 9.93 ± 1.01 e | 1.22 | ** |
| Bi [μg kg−1] | 9.60 ± 0.63 c | 20.58 ± 0.97 a | 10.82 ± 0.77 b | 4.50 ± 0.37 e | 5.78 ± 0.81 d | 0.49 | ** |
| Th [μg kg−1] | 9.30 ± 2.20 b | 469.52 ± 20.97 a | 9.88 ± 1.54 b | 8.18 ± 2.77 b | 6.00 ± 1.00 b | 1.33 | ** |
| U [μg kg−1] | 2.57 ± 0.23 b | 357.96 ± 16.47 a | 3.23 ± 0.21 b | 1.96 ± 0.32 b | 1.92 ± 0.31 b | 1.39 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wu, Y.; Zhao, Z.; Liu, Z.; Liu, R.; Pang, Y.; Yang, C.; Zhang, Y.; Nie, J. Varietal Authenticity Assessment of QTMJ Tea Using Non-Targeted Metabolomics and Multi-Elemental Analysis with Chemometrics. Foods 2023, 12, 4114. https://doi.org/10.3390/foods12224114
Liu H, Wu Y, Zhao Z, Liu Z, Liu R, Pang Y, Yang C, Zhang Y, Nie J. Varietal Authenticity Assessment of QTMJ Tea Using Non-Targeted Metabolomics and Multi-Elemental Analysis with Chemometrics. Foods. 2023; 12(22):4114. https://doi.org/10.3390/foods12224114
Chicago/Turabian StyleLiu, Huahong, Yuxin Wu, Ziwei Zhao, Zhi Liu, Renjun Liu, Yuelan Pang, Chun Yang, Yun Zhang, and Jinfang Nie. 2023. "Varietal Authenticity Assessment of QTMJ Tea Using Non-Targeted Metabolomics and Multi-Elemental Analysis with Chemometrics" Foods 12, no. 22: 4114. https://doi.org/10.3390/foods12224114





