Acute Oral Toxicity Evaluation of Almond Hull Powders in BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Almond Hull Powders
2.2. Assessment of Almond Hull Powders
2.3. Animals and Study Procedures
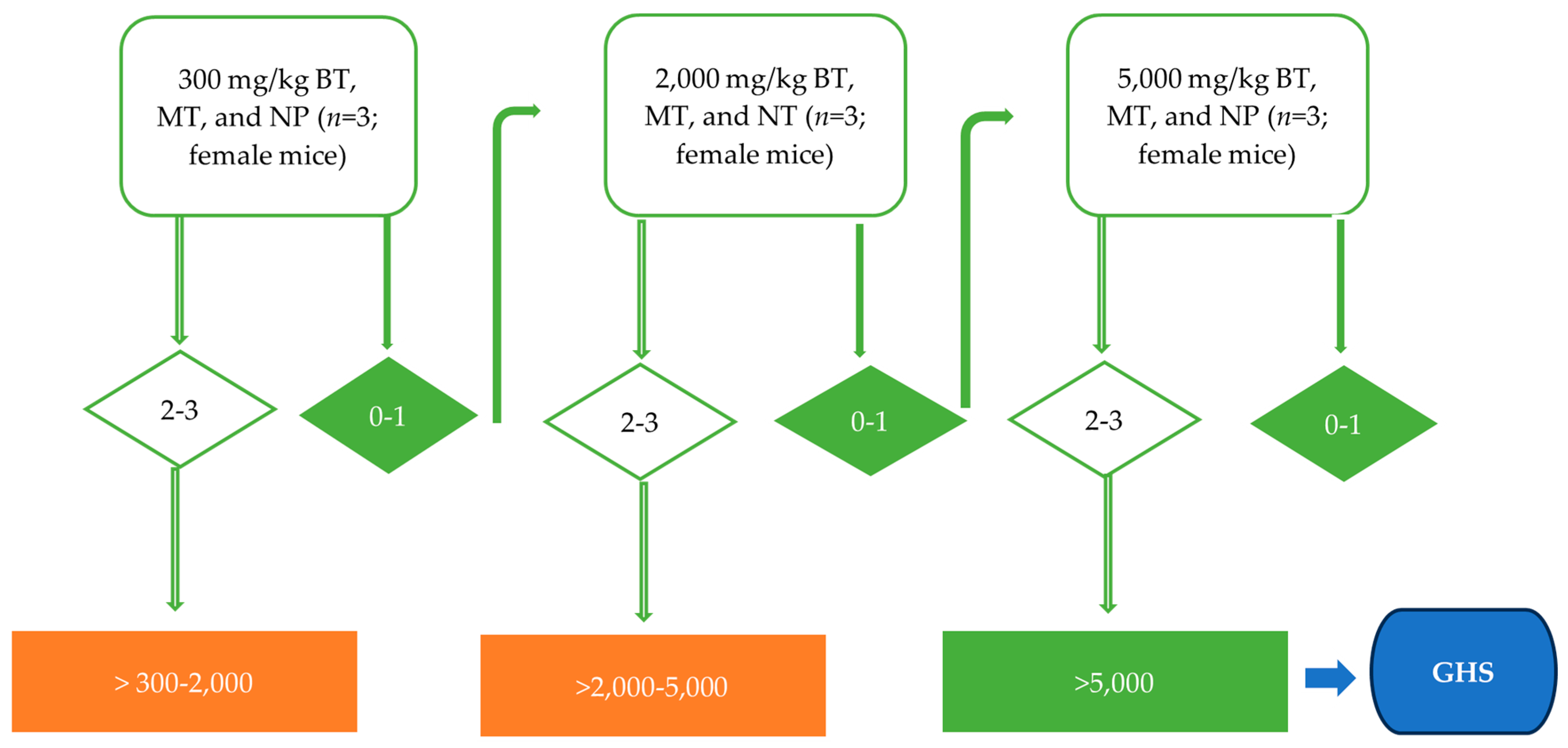
2.4. Acute Toxicity Assay
2.5. Biochemical Analysis
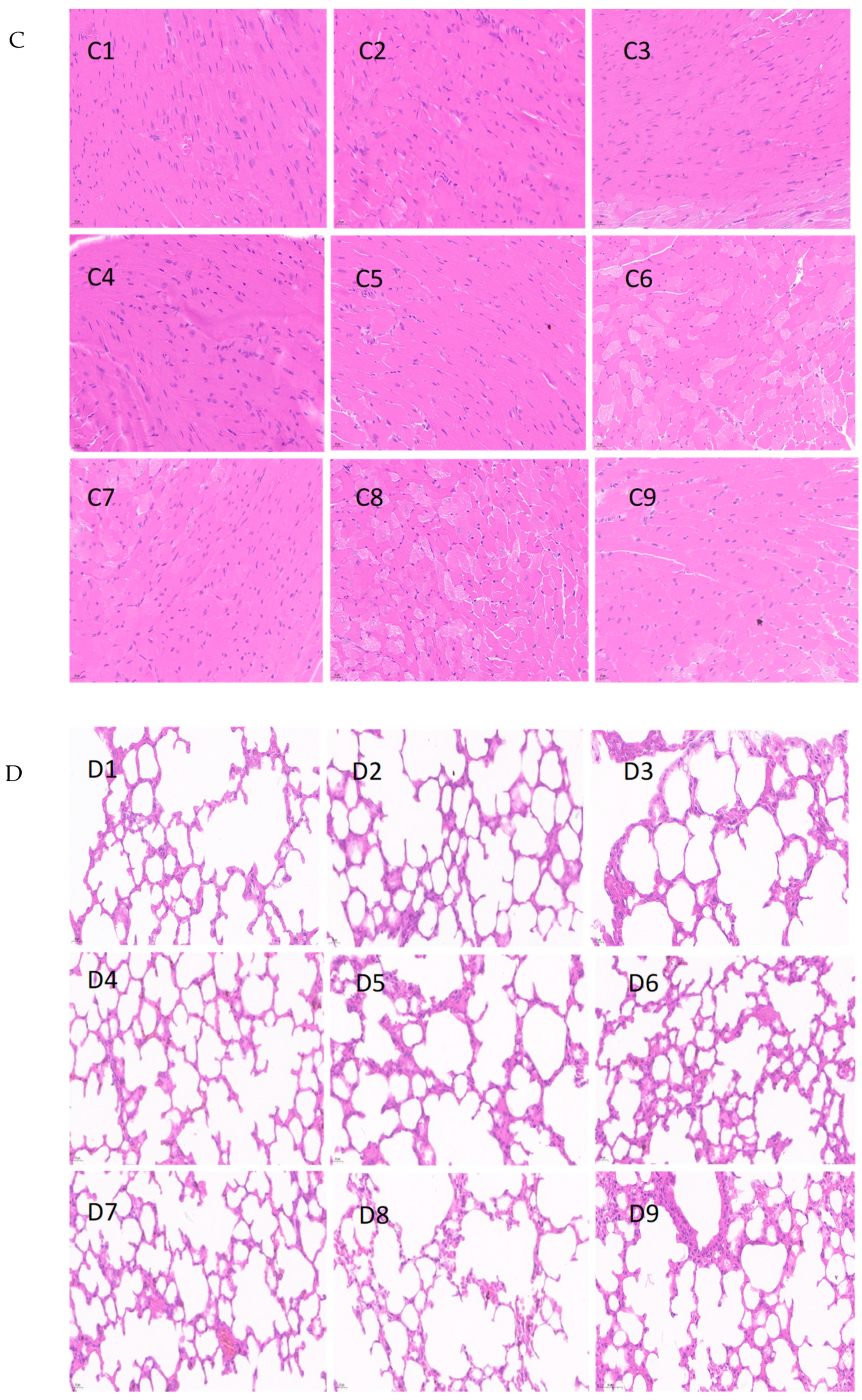
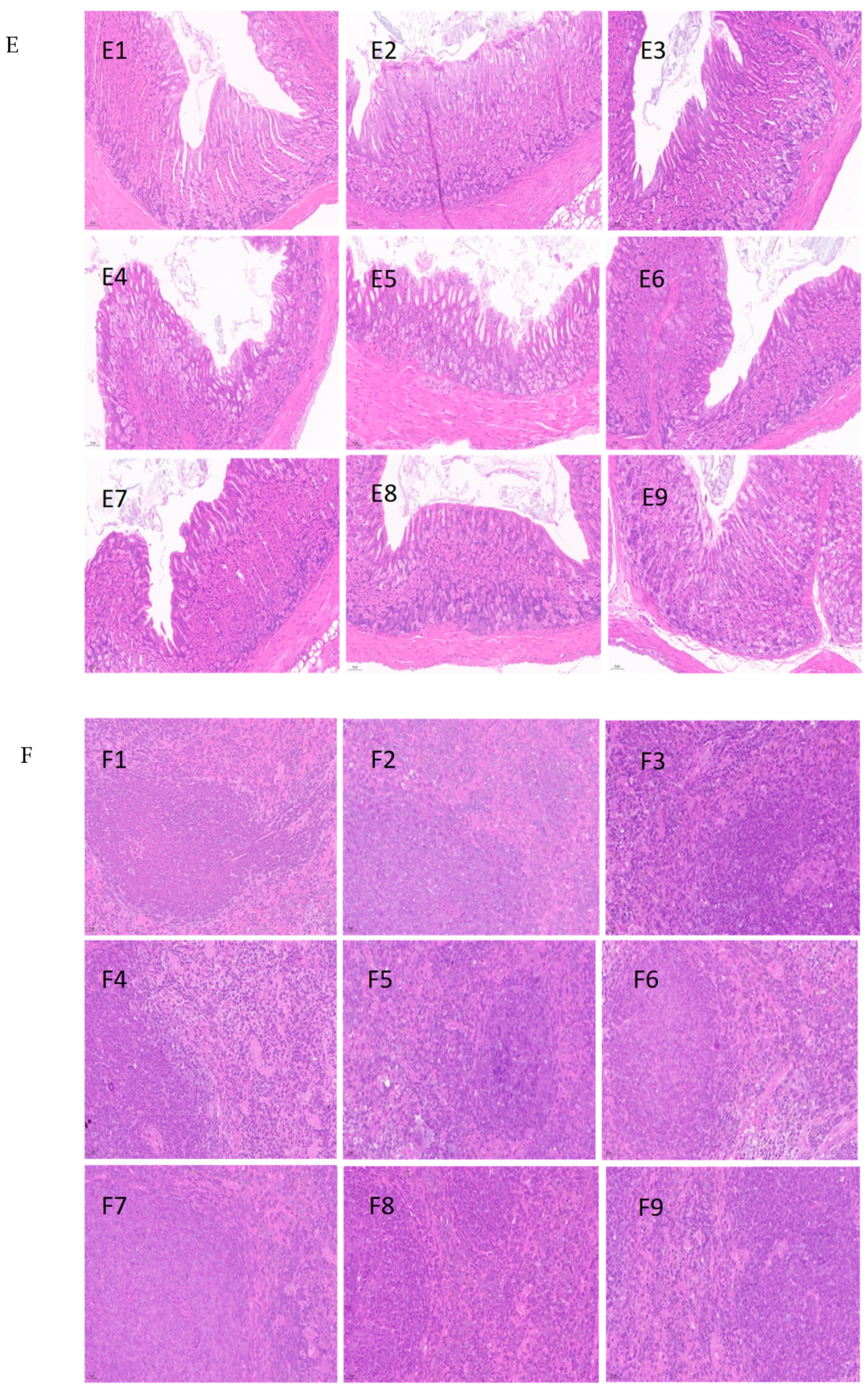
2.6. Histopathologic Study
2.7. Further Assessment of the Temporal Variation in Hepatic and Renal Function
2.8. Statistical Analysis
3. Results
3.1. Observations of Behavior Pattern
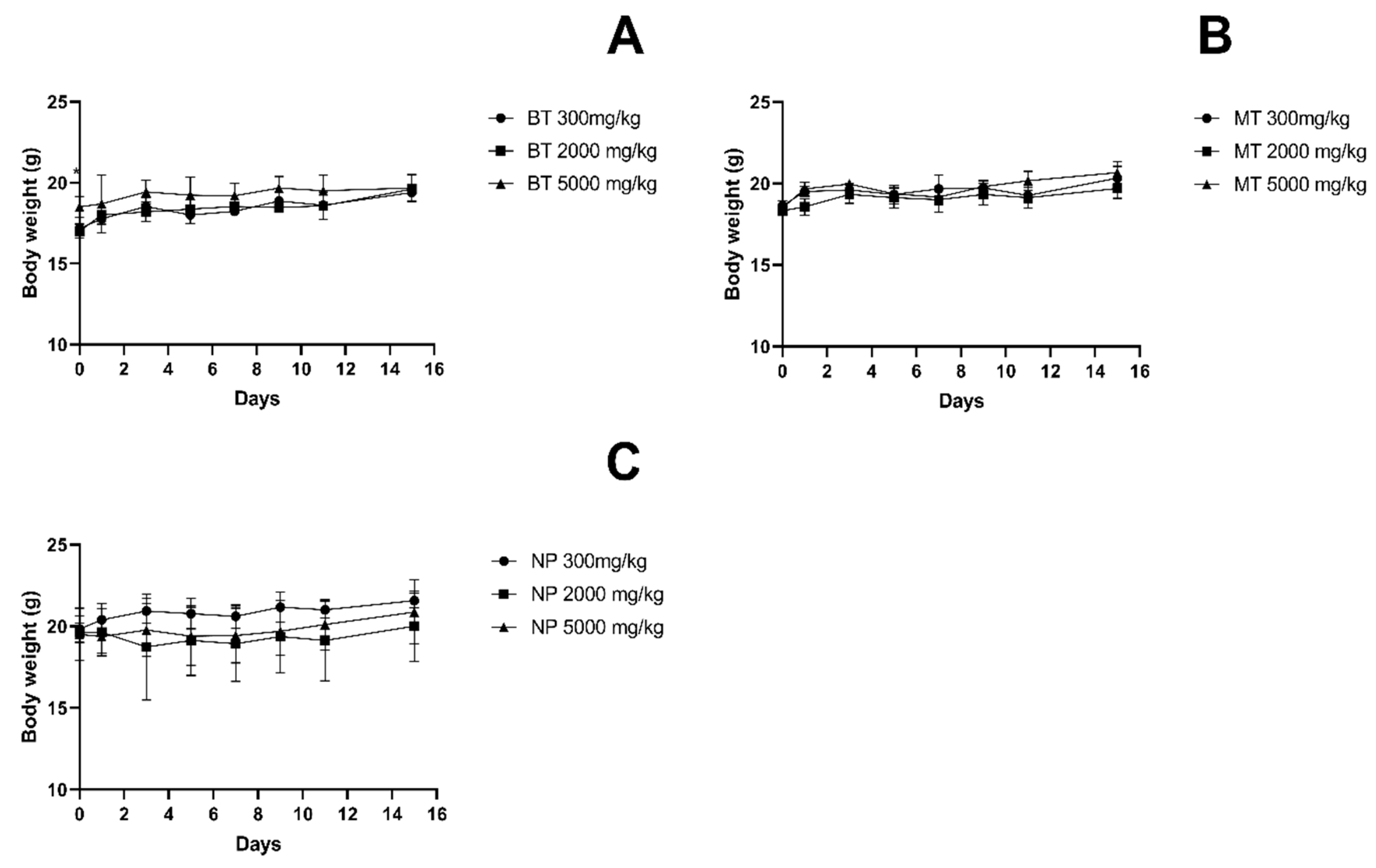
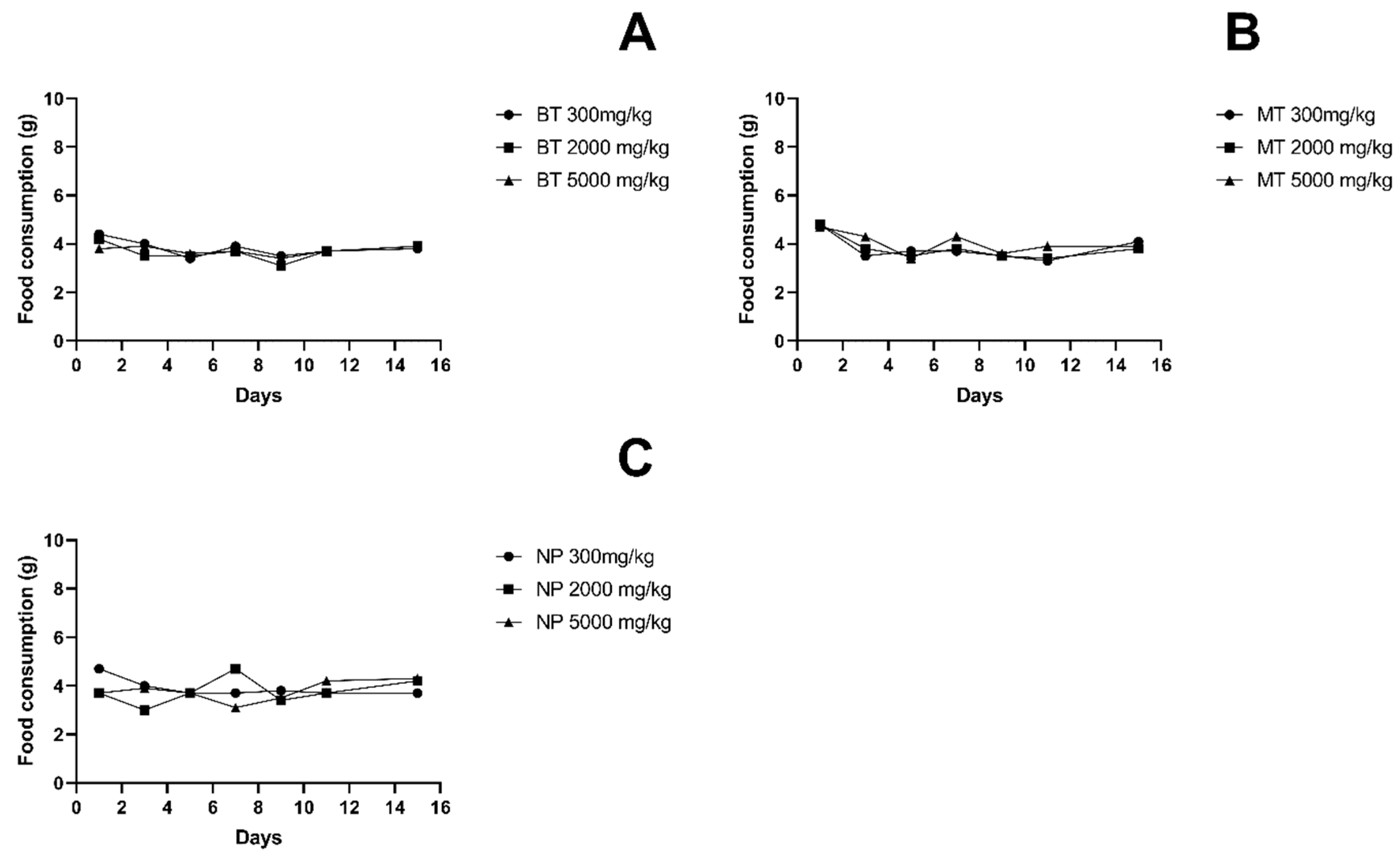
3.2. Body Weight and Food Intake
3.3. Organ to Body Weight Index
3.4. Biochemical Analysis
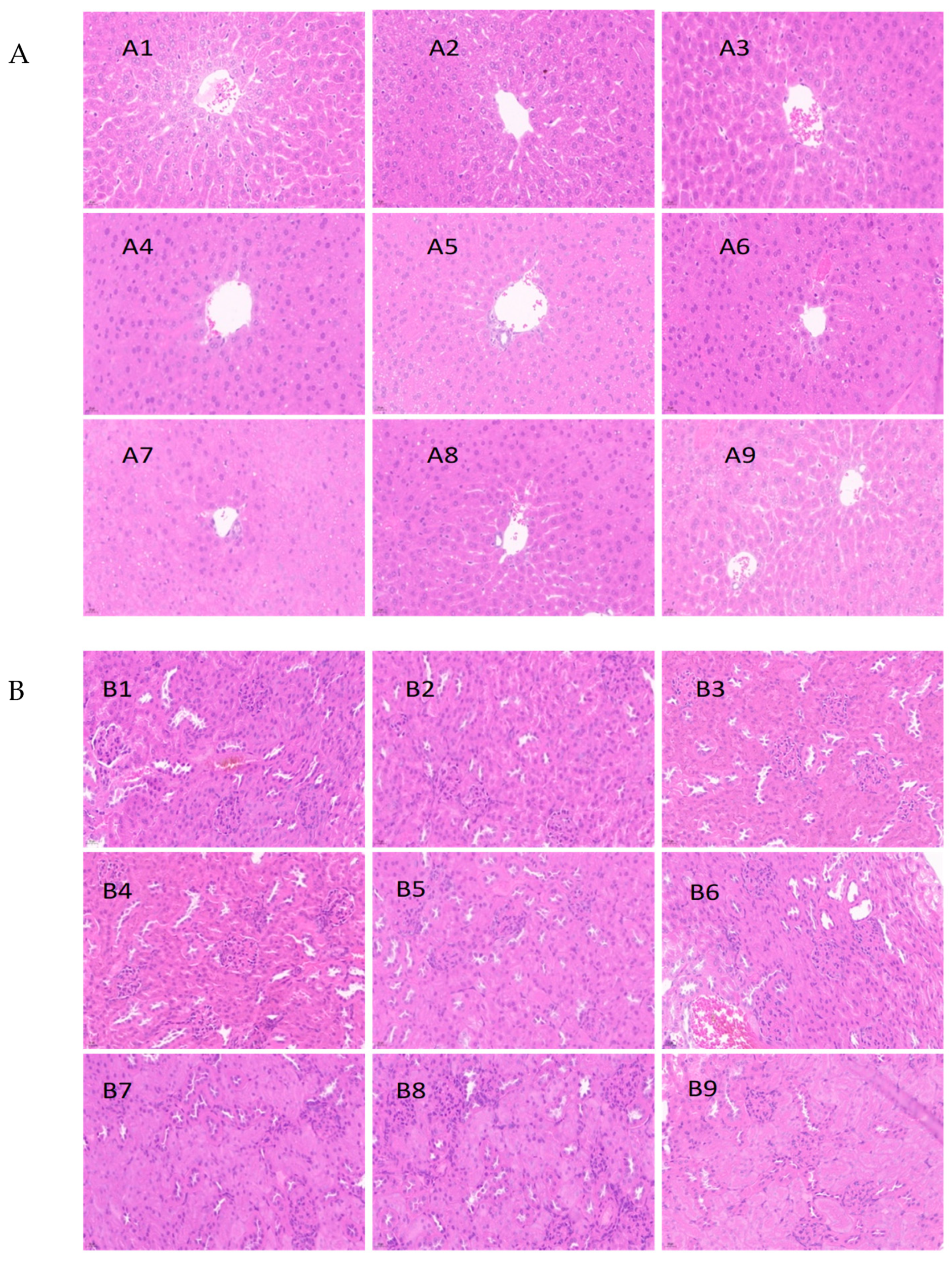
3.5. Histopathological Analysis
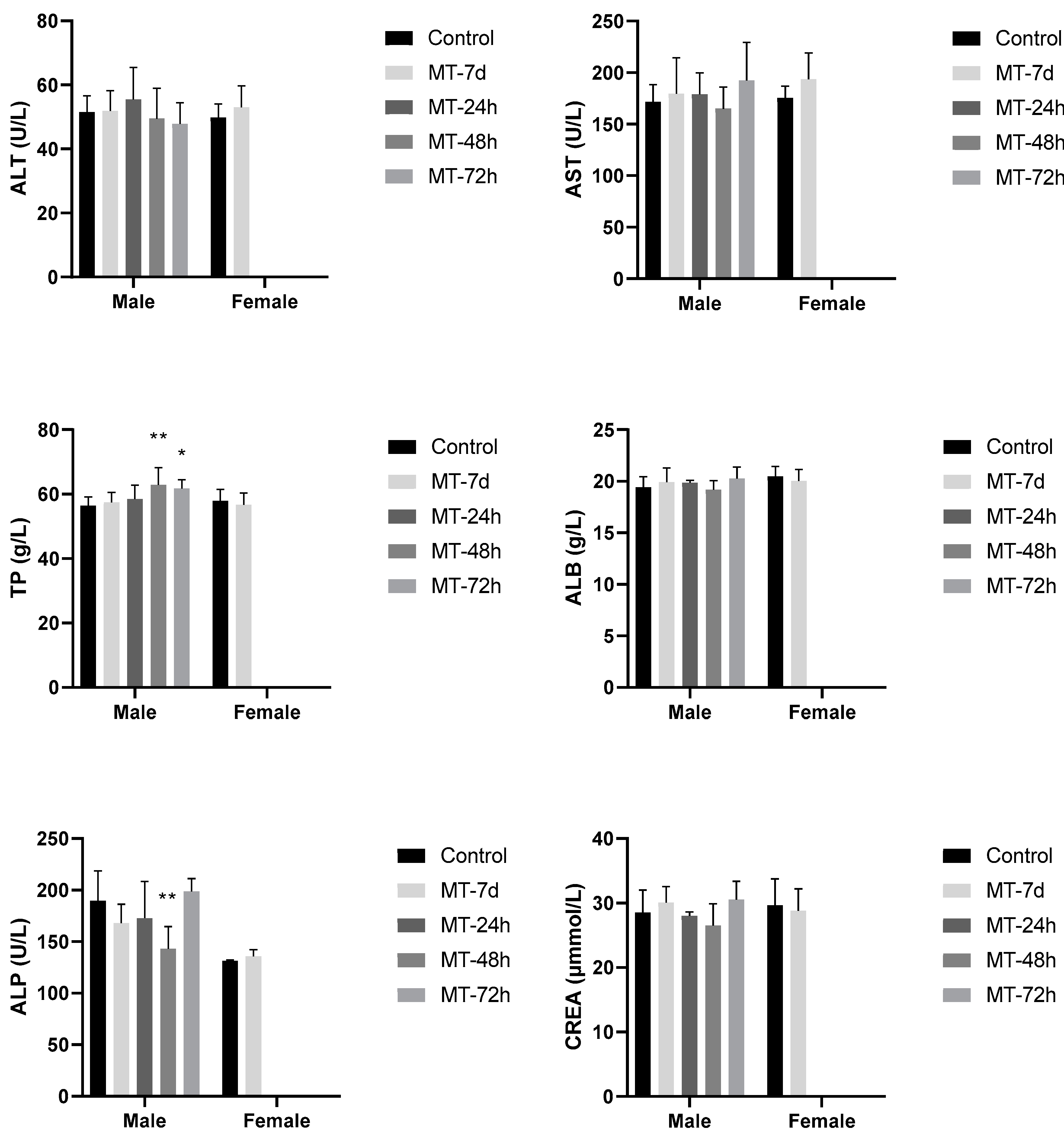
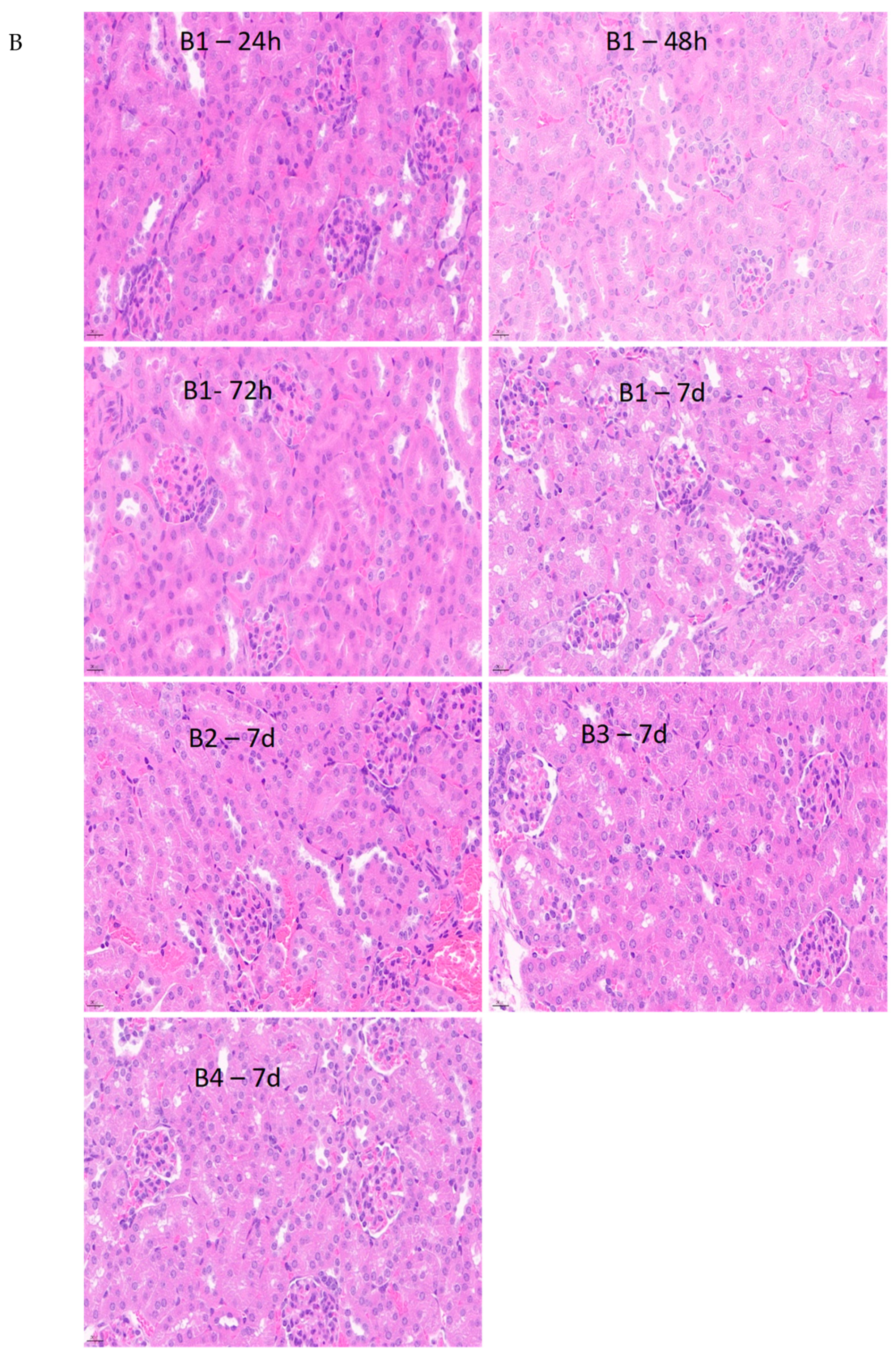
3.6. Further Assessment of the Temporal Variation in Hepatic and Renal Function
3.6.1. Hepatic and Renal Function Biomarkers
3.6.2. Histological Evaluation of Liver and Kidney Tissue Sections
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Almond Board of California. Almond Almanac. Modesto, California. 2022. Retrieved November 10, 2023. Available online: https://www.almonds.com/tools-and-resources/crop-reports/almond-almanac?field_year_value=2022 (accessed on 8 November 2023).
- Sang, S.M.; Cheng, X.F.; Fu, H.Y.; Shieh, D.E.; Bai, N.S.; Lapsley, K.; Stark, R.E.; Rosen, R.T. New type sesquiterpene lactone from almond hulls (Prunus amygdalus Batsch). Tetrahedron Lett. 2002, 43, 2547–2549. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G.W. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Prgomet, I.; Goncalves, B.; Dominguez-Perles, R.; Pascual-Seva, N.; Barros, A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Karen, L. Chapter 15—Almonds. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Steven, Z., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 373–390. [Google Scholar]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef] [PubMed]
- Offeman, R.D.; Holtman, K.M.; Covello, K.M.; Orts, W.J. Almond hulls as a biofuels feedstock: Variations in carbohydrates by variety and location in California. Ind. Crops Prod. 2014, 54, 109–114. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T. Antioxidant constituents of almond Prunus dulis (Mill.) D.A. Webb hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- An, J.; Liu, J.; Liang, Y.Y.; Ma, Y.W.; Chen, C.; Cheng, Y.L.; Peng, P.; Zhou, N.; Zhang, R.C.; Addy, M.; et al. Characterization, bioavailability and protective effects of phenolic-rich extracts from almond hulls against pro-oxidant induced toxicity in Caco-2 cells. Food Chem. 2020, 322, 126742. [Google Scholar] [CrossRef]
- Najari, Z.; Khodaiyan, F.; Yarmand, M.S.; Hosseini, S.S. Almond hulls waste valorization towards sustainable agricultural development: Production of pectin, phenolics, pullulan, and single cell protein. Waste Manag. 2022, 141, 208–219. [Google Scholar] [CrossRef]
- Liu, J.R.; Huang, L.; An, J.; Ma, Y.W.; Cheng, Y.L.; Zhang, R.C.; Peng, P.; Wang, Y.P.; Addy, M.; Chen, P.L.; et al. Application of high-pressure homogenization to improve physicochemical and antioxidant properties of almond hulls. J. Food Process Eng. 2023, 46, e14235. [Google Scholar] [CrossRef]
- Swanson, K.L.; Bill, H.M.; Asmus, J.; Heguy, J.M.; DePeters, E.J. Feeding high amounts of almond hulls to lactating cows. J. Dairy Sci. 2021, 104, 8846–8856. [Google Scholar] [CrossRef]
- Reed, B.A.; Brown, D.L. Almond hulls in diets for lactating goats—Effects on yield and composition of milk, feed-intake, and digestibility. J. Dairy Sci. 1988, 71, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Singh, A.K.; Kong, F.; Kim, W.K. Effect of almond hulls as an alternative ingredient on broiler performance, nutrient digestibility, and cecal microbiota diversity. Poult. Sci. 2021, 100, 100853. [Google Scholar] [CrossRef]
- Homedes, J.; Roura, E.; Keim, N.; Brown, D. Almond hulls in swine diet reduce body fat. Calif. Agric. 1993, 47, 27–28. [Google Scholar] [CrossRef]
- Safarian, S.; Azarmi, Y.; Jahanban-Esfahlan, A.; Jahanban-Esfahlan, H. The beneficial effects of almond (Prunus amygdalus Batsch) hull on serum lipid profile and antioxidant capacity in male rats. Turk. J. Med. Sci. 2016, 46, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Kahlaoui, M.; Bertolino, M.; Barbosa-Pereira, L.; Kbaier, H.B.; Bouzouita, N.; Zeppa, G. Almond Hull as a Functional Ingredient of Bread: Effects on Physico-Chemical, Nutritional, and Consumer Acceptability Properties. Foods 2022, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Title 21--Food and Drugs, Chapter I--Food and Drug Administration Department of Health and Human Services, Subchapter B--Food for Human Consumption, Part 172--Food Additives Permitted for Direct Addition to Food for Human Consumption, Subpart I--Multipurpose Additives, Section 172.898--Bakers yeast glycan. 2023; Volume 3. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.898#:~:text=Sec.,of%20the%20yeast%2C%20Saccharomyces%20cerevisiae (accessed on 8 November 2023).
- OECD. Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure; OECD: Paris, France, 2002. [Google Scholar]
- OECD. Test No. 423: Acute Oral toxicity—Acute Toxic Class Method; OECD: Paris, France, 2002. [Google Scholar]
- Adefegha, S.A.; Oboh, G.; Oyeleye, S.I.; Ejakpovi, I. Erectogenic, Antihypertensive, Antidiabetic, Anti-Oxidative Properties and Phenolic Compositions of Almond Fruit (Terminalia catappa L.) Parts (Hull and Drupe)—In vitro. J. Food Biochem. 2017, 41, e12309. [Google Scholar] [CrossRef]
- Song, Y.; Wang, W.; Cui, W.; Zhang, X.; Zhang, W.; Xiang, Q.; Liu, Z.; Li, N.; Jia, X. A subchronic oral toxicity study of almond skins in rats. Food Chem. Toxicol. 2010, 48, 373–376. [Google Scholar] [CrossRef]
- Clutter, S.H.; Rodiek, A.V. Feeding value of diets containing almond hulls. J. Equine Vet. Sci. 1992, 12, 99–102. [Google Scholar] [CrossRef]
- Pfeiffer, C.J. A mathematical evaluation of the thymic weight parameter. Toxicol. Appl. Pharmacol. 1968, 13, 220–227. [Google Scholar] [CrossRef]
- Jasper, R.; Locatelli, G.O.; Pilati, C.; Locatelli, C. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup®. Interdiscip. Toxicol. 2013, 5, 133–140. [Google Scholar] [CrossRef]
- Yang, X.; Schnackenberg, L.K.; Shi, Q.; Salminen, W.F. Hepatic toxicity biomarkers. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014; pp. 241–259. ISBN 9780124046306. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.; Silva, C.B.; de Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical hematological and biochemical parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Anim. Models Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.E. Special considerations in interpreting liver function tests. Am. Fam. Physician 1999, 59, 2223–2230. [Google Scholar]
- Banace, M.; Mirvagefei, A.R.; Rafei, G.R.; Amiri, B.M. Effect of sub-lethal diazinon concentrations on blood plasma biochemistry. Int. J. Environ. Res. 2008, 2, 189–198. [Google Scholar]
- Chattopadhyay, A.; Podder, S.; Agarwal, S.; Bhattacharya, S. Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice. Arch. Toxicol. 2011, 85, 327–335. [Google Scholar] [CrossRef]

| Result | MRL | Units | Method Reference | |
|---|---|---|---|---|
| Microbial | ||||
| Mold (48 h) | <10 | CFU/gram | AOAC RI PTM 051702 | |
| Yeast (48 h) | <10 | CFU/gram | AOAC RI PTM 051702 | |
| E. coli | <10 | CFU/gram | FDA BAM, Ch 4 (Plate) | |
| Total Coliform | <10 | CFU/gram | FDA BAM, Ch 4 (Plate) | |
| Salmonella | Negative | /100 g | AOAC RI PTM #100701 | |
| Metals | ||||
| Mercury (DMA) | <4.00 | ppb | EPA 7473 | |
| Arsenic (ICP-MS) | 195 | ppb | AOAC 993.14 | |
| Cadmium (ICP-MS) | 16.2 | ppb | AOAC 2015.06 | |
| Lead (ICP-MS) | 271 | ppb | AOAC 2015.06 | |
| Verified Residue | ||||
| Azoxystrobin | 0.015 | 4 | mg/kg | AOAC 2007.01 |
| Methoxyfenozide | 2.042 | 25 | mg/kg | AOAC 2007.01 |
| Chlorantraniliprole | 1.484 | 5 | mg/kg | AOAC 2007.01 |
| Pyraclostrobin | 0.334 | 7 | mg/kg | AOAC 2007.01 |
| Etoxazole | 0.074 | 2 | mg/kg | AOAC 2007.01 |
| Spinetoram | Approaching LOD (0.010) | 19 | mg/kg | AOAC 2007.01 |
| Fenpyroximate | 0.028 | 3 | mg/kg | AOAC 2007.01 |
| Spirodiclofen | 0.183 | 20 | mg/kg | AOAC 2007.01 |
| Hexythiazox | 0.729 | 10 | mg/kg | AOAC 2007.01 |
| Bifenthrin | 0.483 | 2 | mg/kg | AOAC 2007.01 |
| Cyhalothrin Lambda | Detected < LOQ (0.200) | 1.5 | mg/kg | AOAC 2007.01 |
| Oxyfluorfen | 0.081 | 0.1 | mg/kg | AOAC 2007.01 |
| Pendimethalin | 0.694 | 6 | mg/kg | AOAC 2007.01 |
| Permethrin | Detected < LOQ (0.400) | 20 | mg/kg | AOAC 2007.01 |
| Propargite | Approaching LOD (0.040) | 55 | mg/kg | AOAC 2007.01 |
| Fenpropathrin | Detected < LOQ (0.100) | 4.5 | mg/kg | AOAC 2007.01 |
| Tebuconazole | Approaching LOD (0.010) | 6 | mg/kg | AOAC 2007.01 |
| Mycotoxins | ||||
| Aflatoxin B1 | Not Detected | ppb | ||
| Aflatoxin B2 | Not Detected | ppb | ||
| Aflatoxin G1 | Not Detected | ppb | ||
| Aflatoxin G1 | Not Detected | ppb | ||
| Total Aflatoxins | Not Detected | ppb |
| Organs | BT (mg/kg bw) | MT (mg/kg bw) | NP (mg/kg bw) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 300 | 2000 | 5000 | 300 | 2000 | 5000 | 300 | 2000 | 5000 | |
| Heart | 0.443 ± 0.1 | 0.509 ± 0.058 | 0.513 ± 0.004 | 0.555 ± 0.023 | 0.518 ± 0.039 | 0.476 ± 0.055 | 0.549 ± 0.085 | 0.523 ± 0.019 | 0.525 ± 0.037 |
| Liver | 4.075 ± 0.164 | 3.878 ± 0.157 | 3.652 ± 0.126 | 3.77 ± 0.126 | 3.732 ± 0.143 | 3.632 ± 0.188 | 3.722 ± 0.078 | 3.995 ± 0.170 | 3.734 ± 0.045 |
| Spleen | 0.417 ± 0.009 | 0.424 ± 0.007 | 0.415 ± 0.037 | 0.429 ± 0.081 | 0.401 ± 0.026 | 0.409 ± 0.024 | 0.416 ± 0.021 | 0.424 ± 0.011 | 0.396 ± 0.036 |
| Stomach | 0.760 ± 0.11 | 1.109 ± 0.326 | 1.107 ± 0.208 | 0.896 ± 0.201 | 1.101 ± 0.201 | 0.866 ± 0.119 | 0.852 ± 0.022 | 1.044 ± 0.23 | 0.845 ± 0.064 |
| Kidney | 1.371 ± 0.043 | 1.356 ± 0.133 | 1.350 ± 0.107 | 1.414 ± 0.044 | 1.362 ± 0.056 | 1.276 ± 0.131 | 1.371 ± 0.086 | 1.411 ± 0.032 | 1.376 ± 0.009 |
| Lung | 0.729 ± 0.037 | 0.652 ± 0.037 | 0.637 ± 0.024 | 0.652 ± 0.061 | 0.629 ± 0.030 | 0.666 ± 0.052 | 0.623 ± 0.023 | 0.743 ± 0.137 | 0.604 ± 0.093 |
| Thymus Gland | 0.306 ± 0.071 * | 0.261 ± 0.027 | 0.244 ± 0.03 | 0.203 ± 0.061 | 0.203 ± 0.009 | 0.210 ± 0.089 | 0.115 ± 0.062 * | 0.172 ± 0.088 | 0.179 ± 0.064 |
| Parameters | Unit | BT (mg/kg bw) | MT (mg/kg bw) | NP (mg/kg bw) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 300 | 2000 | 5000 | 300 | 2000 | 5000 | 300 | 2000 | 5000 | ||
| CREA | µmol/L | 33 ± 1.73 | 31.33 ± 2.52 | 32.67 ± 0.58 | 33.67 ± 2.08 | 32.67 ± 3.79 | 33.33 ± 4.73 | 35 ± 1 | 32.33 ± 1.15 | 36.5 ± 2.12 |
| ALT | U/L | 52.67 ± 7.51 | 63 ± 3 | 51.67 ± 6.11 | 61.67 ± 11.55 | 58 ± 2.65 | 60.67 ± 11.93 | 69.67 ± 6.11 * | 50 ± 8.54 | 47 ± 1.41 * |
| AST | U/L | 276.67 ± 117.01 | 191.33 ± 12.9 | 188 ± 37.47 | 209.33 ± 30.44 | 187 ± 12.29 | 236 ± 15.72 | 211.33 ± 10.97 | 181.67 ± 32.93 | 191.5 ± 6.36 |
| ALB | g/L | 21.03 ± 0.71 | 20.2 ± 0.4 | 21.43 ± 0.38 | 21.17 ± 0.5 | 21.53 ± 0.25 | 22.07 ± 0.45 | 21.63 ± 0.45 | 21.17 ± 1.19 | 21.75 ± 0.21 |
| TP | g/L | 59.33 ± 2.38 | 56.73 ± 0.5 | 60.53 ± 1.86 | 60.27 ± 2.65 | 60.8 ± 0.85 | 62.17 ± 1.64 | 61.7 ± 1.91 | 60.27 ± 3.01 | 61.1 ± 0.28 |
| ALP | U/L | 143.67 ± 23.35 | 144.33 ± 21.59 | 170.33 ± 5.77 | 150 ± 7.07 | 159.33 ± 4.04 | 183.67 ± 10.26 | 176 ± 12.53 | 158 ± 44.03 | 166 ± 19.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yao, Y.; Cheng, Y.; Hua, W.; Zhu, X.; Miao, Q.; Huang, G.; Mi, S.; Ruan, R. Acute Oral Toxicity Evaluation of Almond Hull Powders in BALB/c Mice. Foods 2023, 12, 4111. https://doi.org/10.3390/foods12224111
Liu J, Yao Y, Cheng Y, Hua W, Zhu X, Miao Q, Huang G, Mi S, Ruan R. Acute Oral Toxicity Evaluation of Almond Hull Powders in BALB/c Mice. Foods. 2023; 12(22):4111. https://doi.org/10.3390/foods12224111
Chicago/Turabian StyleLiu, Juer, Yuyang Yao, Yanling Cheng, Wei Hua, Xinyue Zhu, Qiming Miao, Guangwei Huang, Shengquan Mi, and Roger Ruan. 2023. "Acute Oral Toxicity Evaluation of Almond Hull Powders in BALB/c Mice" Foods 12, no. 22: 4111. https://doi.org/10.3390/foods12224111





