The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apples
2.2. Chemicals
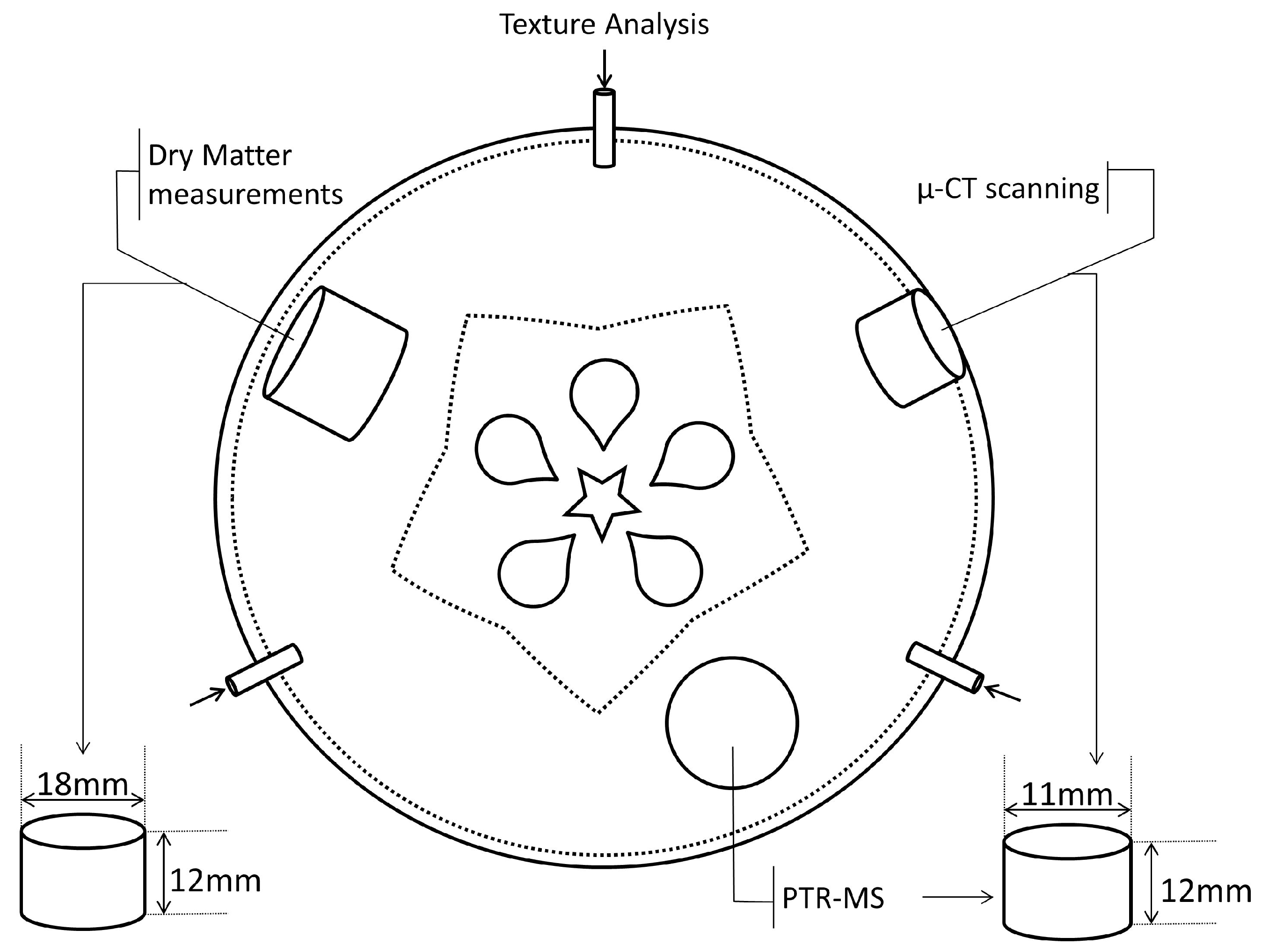
2.3. Texture Analyzer
2.4. Soluble Solids Content and Dry Matter Concentration
2.5. Headspace Volatile Organic Compound Measurement
2.6. X-ray Micro-CT Scanning and Image Analysis
2.7. Data Analysis
2.7.1. Headspace Analysis
2.7.2. Statistical Analysis
3. Results
3.1. Headspace Analysis of VOCs from Fresh Cut Cylinders
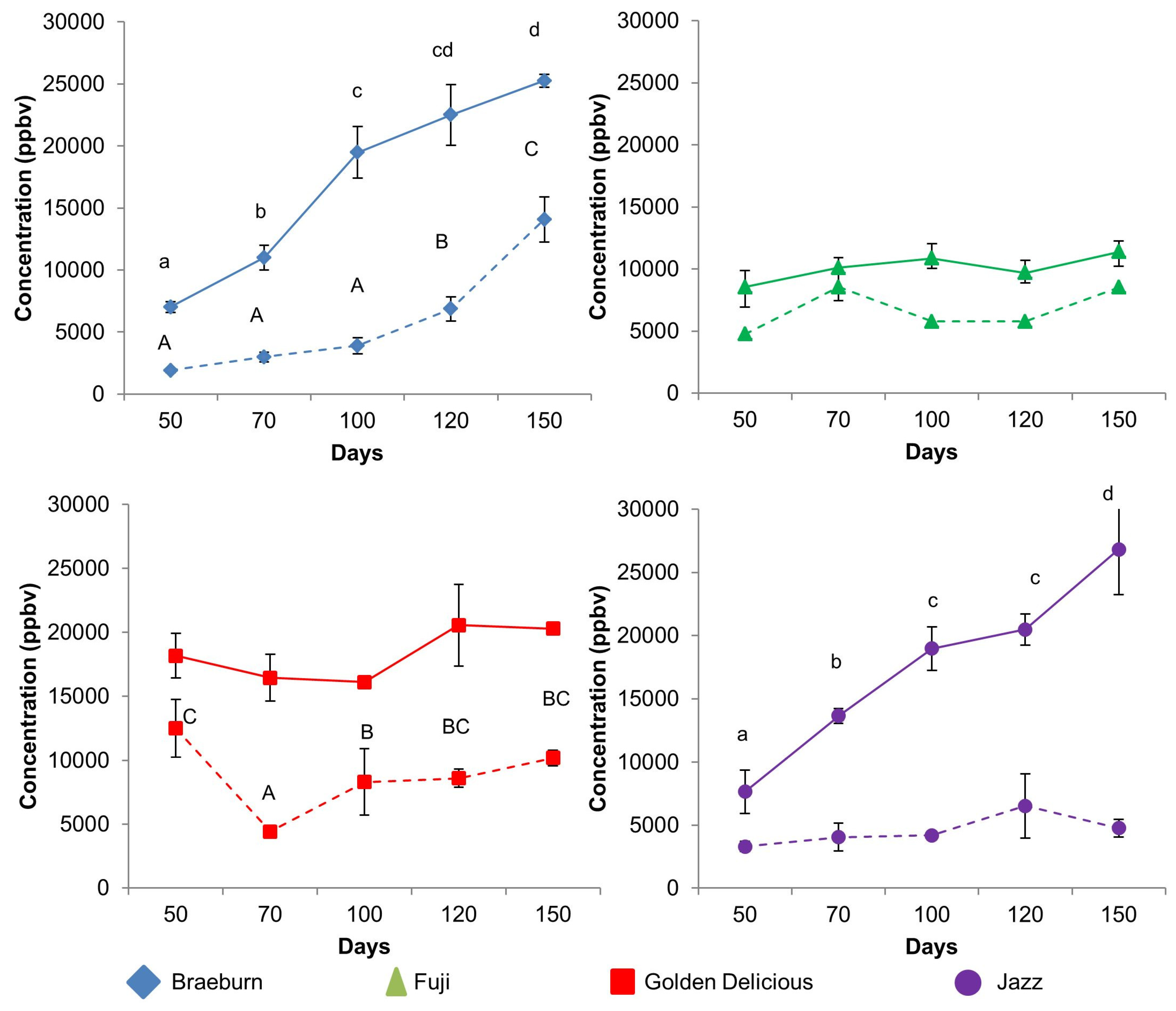
3.1.1. Comparing Untreated and SF-Treated Apples
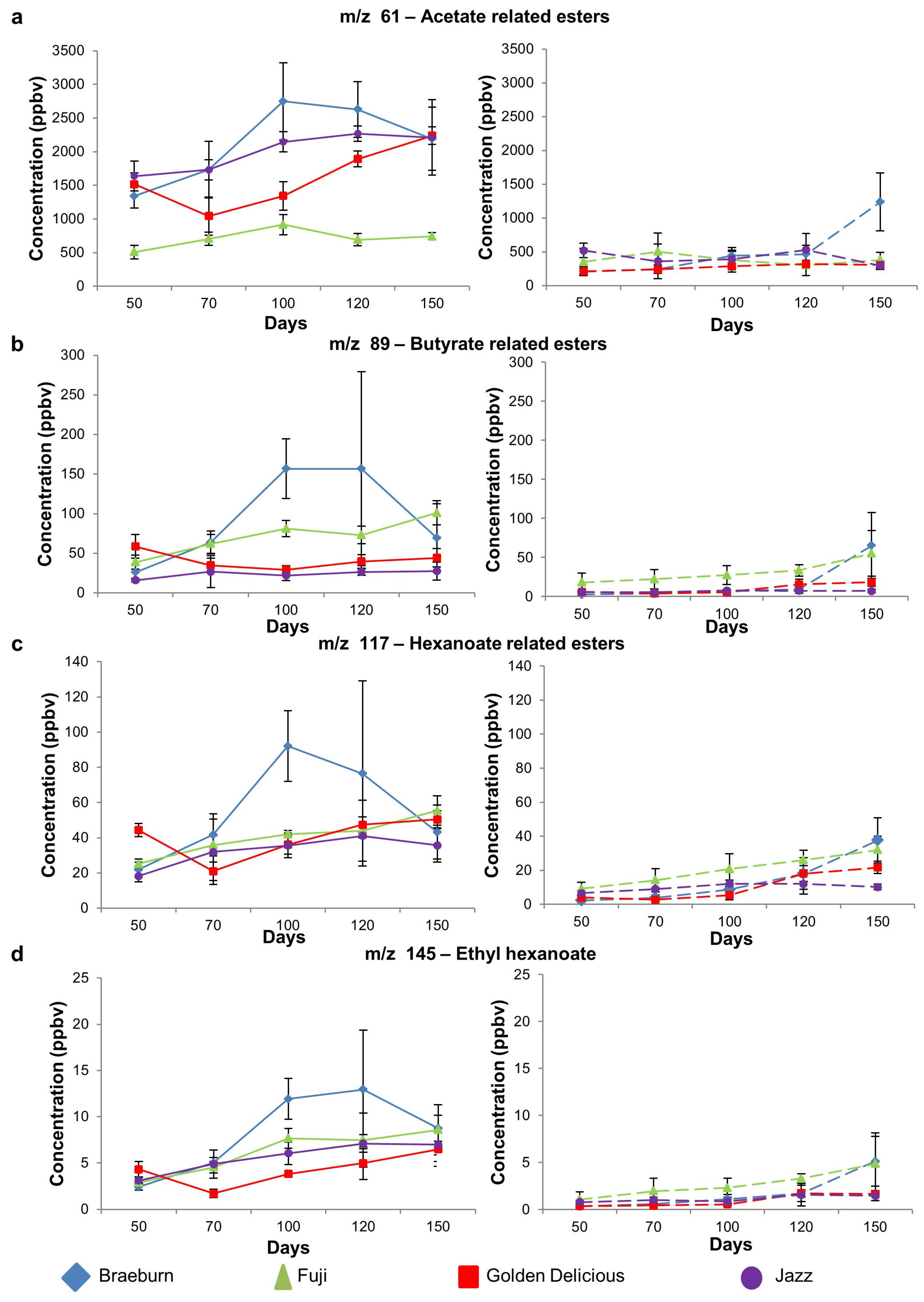
3.1.2. Ester Compounds
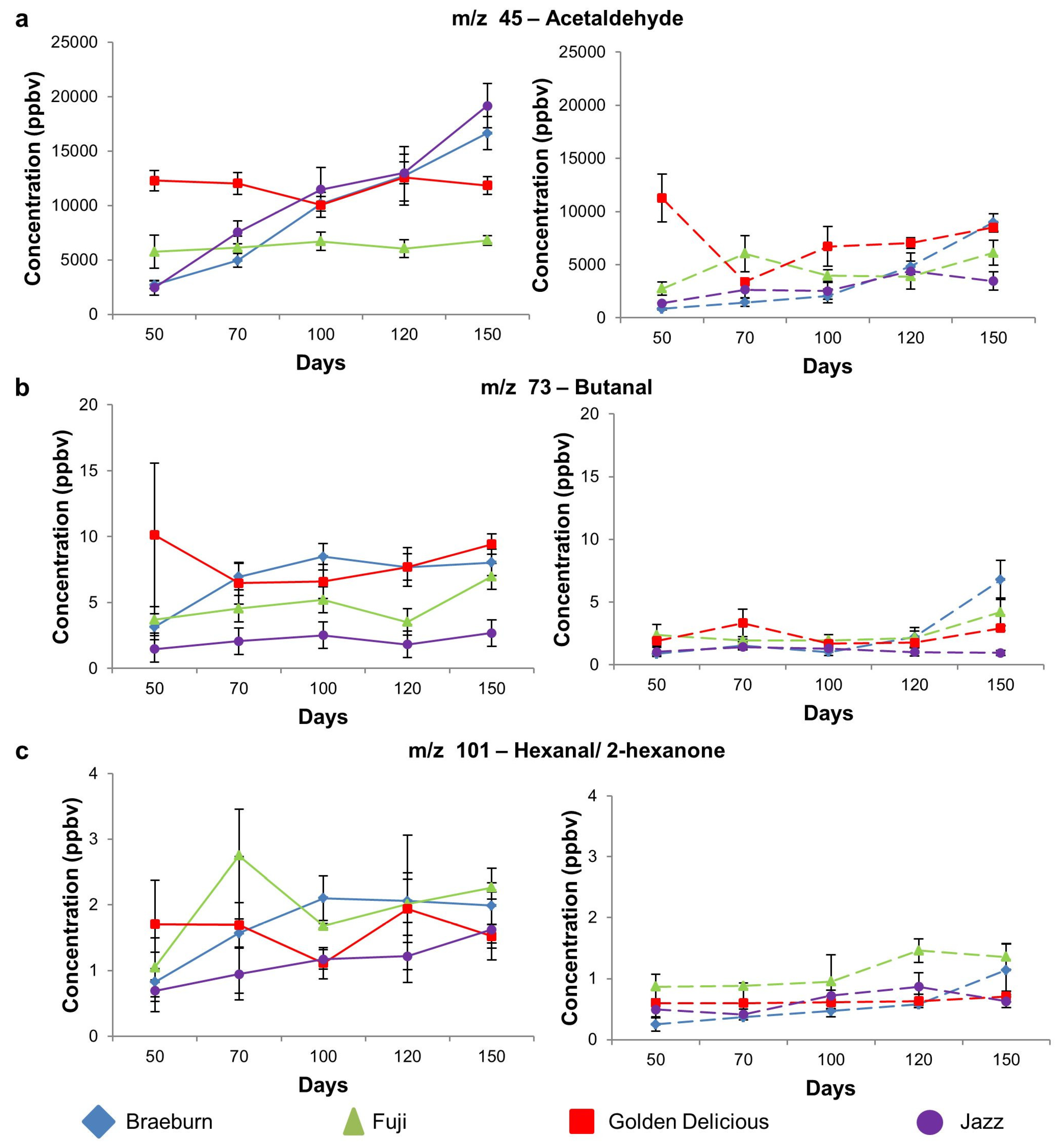
3.1.3. Aldehyde Compounds
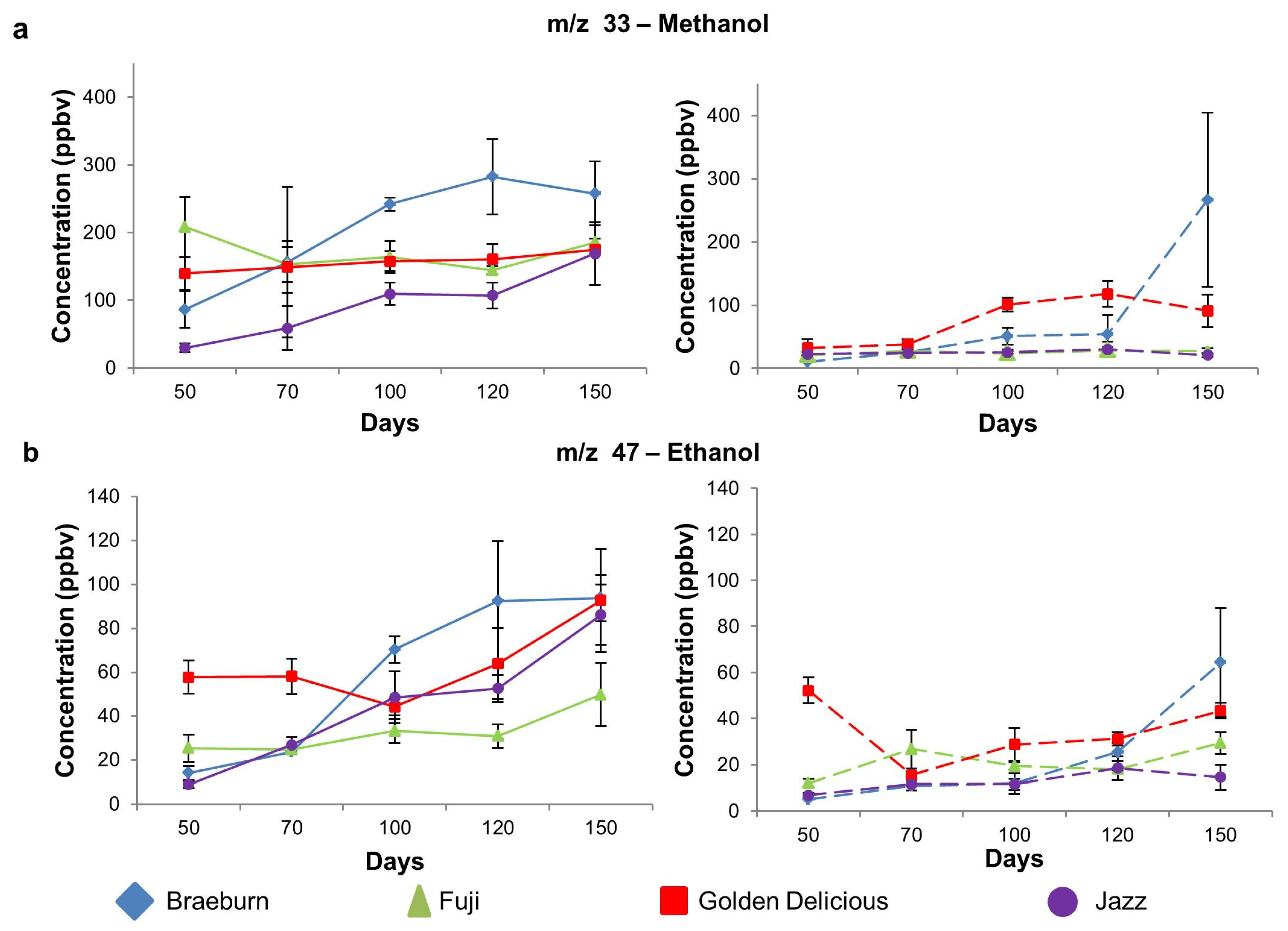
3.1.4. Alcohol Compounds
3.2. Texture, Physico-Chemical and µ-CT Results
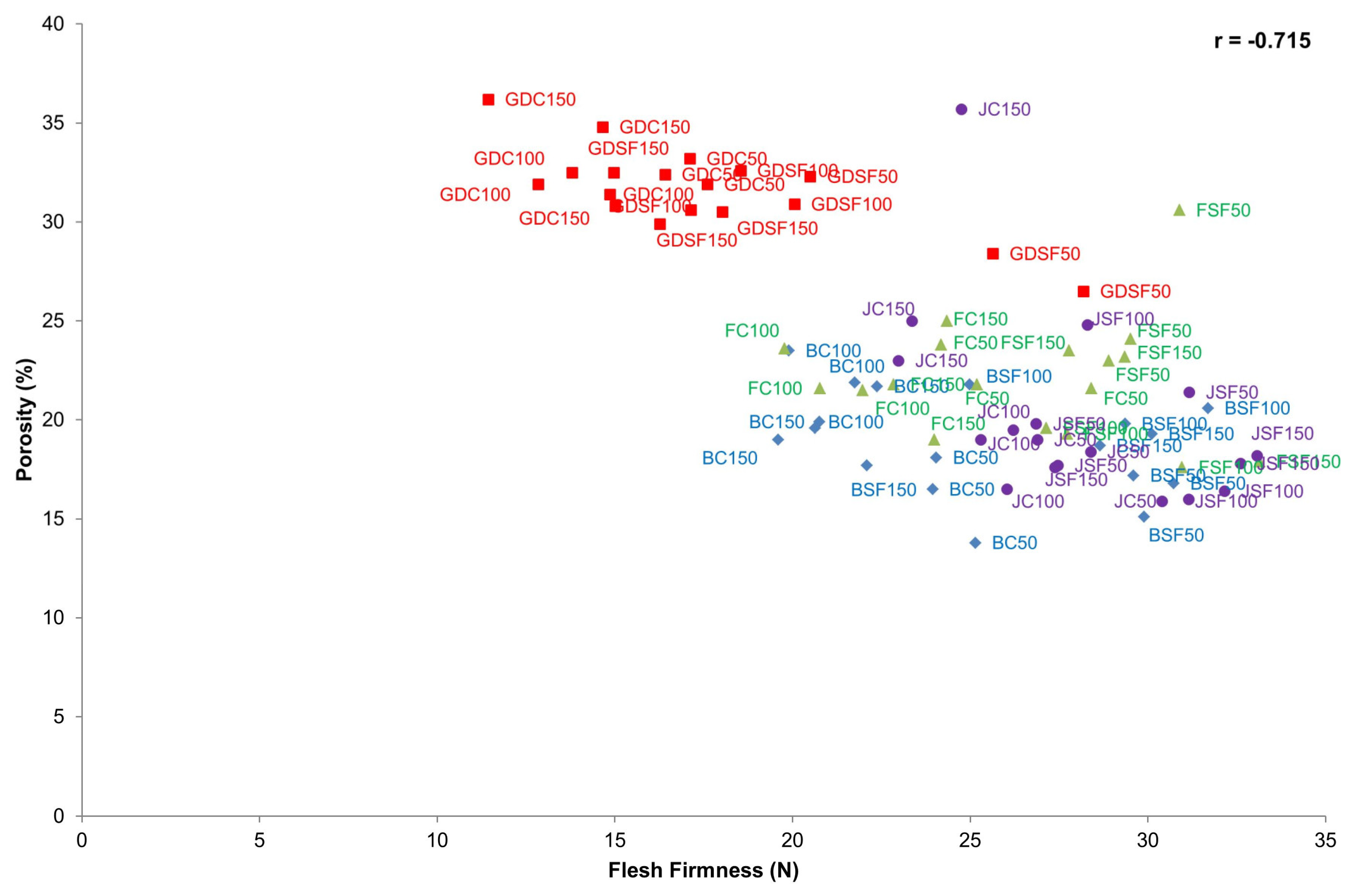
3.2.1. Relation between Porosity and Flesh Firmness
3.2.2. The Relationship between Connectivity and Anisotropy to Porosity
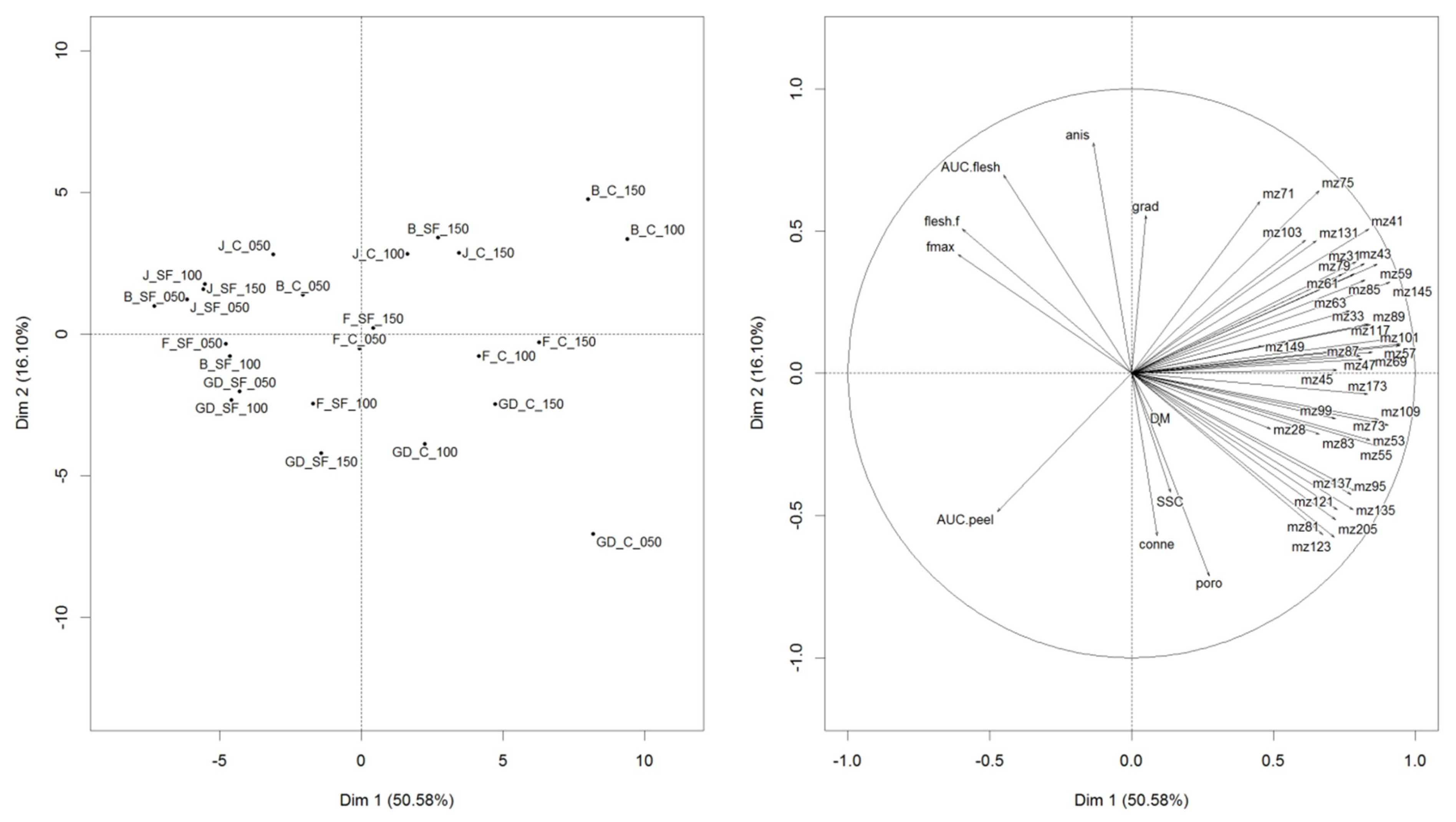
3.3. Understanding the Inter-Relationships between VOC Release, Texture, Physico-Chemical, and Morphological Properties
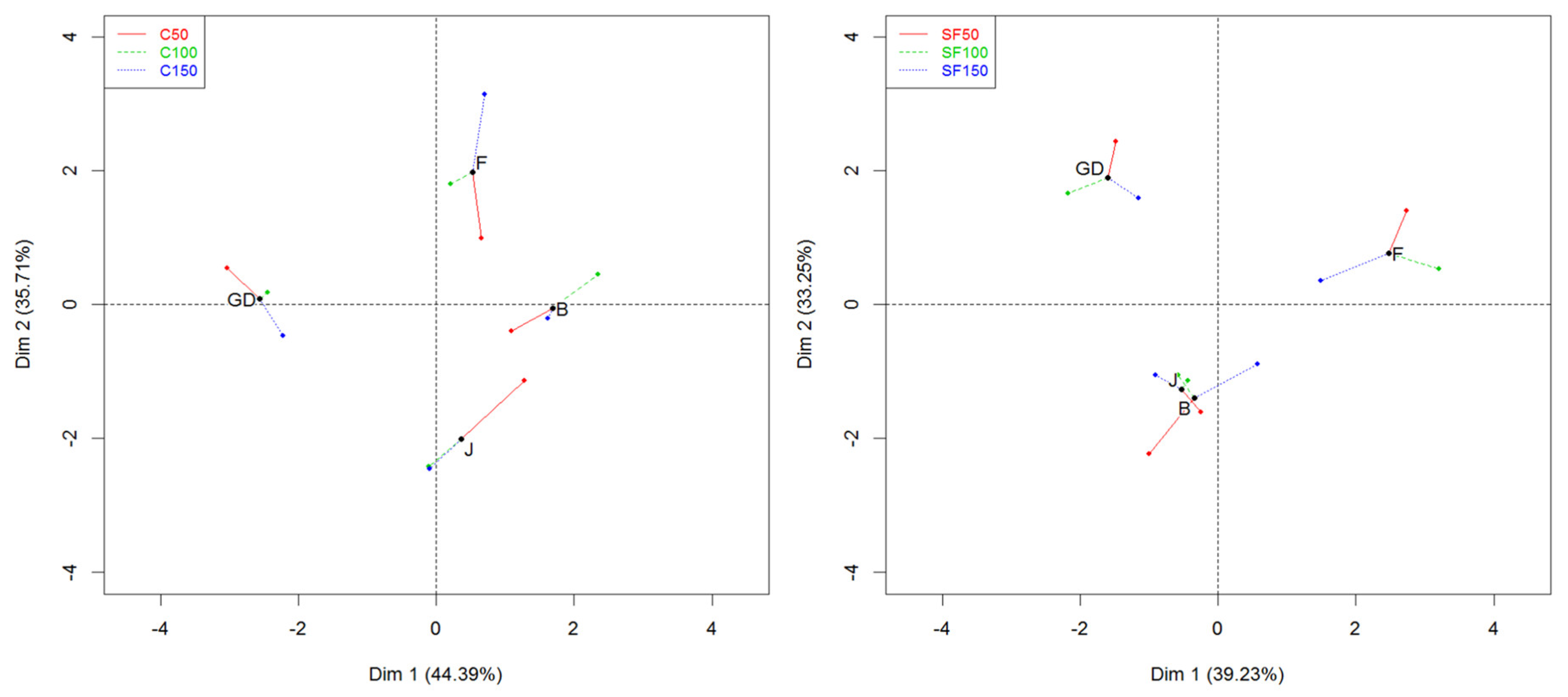
3.4. MFA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M. Postharvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Barry, C.; Giovannoni, J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- DeEll, J.R.; Khanizadeh, S.; Saad, F.; Ferree, D.C. Factors affecting apple fruit firmness—A review. J. Am. Pomol. Soc. 2001, 55, 8–27. [Google Scholar]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.L.; Morris, J.R. Temperature Effects on Produce Degradation. In Produce Degradation; CRC Press: Boca Raton, FL, USA, 2005; pp. 599–647. [Google Scholar]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Schaffer, R.J.; Cohen, D.; Gleave, A.P.; Crowhurst, R.N.; Janssen, B.J.; Yao, J.-L.; Newcomb, R.D.; Friel, E.N.; Souleyre, E.J.F.; Bolitho, K.; et al. Genomics approach reveals that aroma production in apple as controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Muche, B.M.; Jordan, M.; Forney, C.F.; Speers, R.A.; Rupasinghe, H.P.V. Effect of 1-methylcyclopropene (1-MCP) and storage atmosphere on the volatile aroma composition of cloudy and clear apple juices. Beverages 2022, 6, 59. [Google Scholar] [CrossRef]
- Fan, X.; Blankenship, S.M.; Mattheis, J.P. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, M.C.; Ku, K.H. Chemical, physical, and sensory properties of 1-MCP-treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage. Appl. Biol. Chem. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Yoo, J.; Jung, H.; Win, N.M.; Kwon, J.-G.; Cho, Y.-J.; Jung, H.-E.; Lee, D.H.; Kang, I.-K. Changes in fruit quality attributes, cell wall materials, and related hydrolases activities in 1-methylcyclopropene (1-MCP)-treated ‘Honggeum’ apples during cold storage. Sci. Technol. 2020, 38, 870–879. [Google Scholar] [CrossRef]
- Małachowska, M.; Tomala, K. Effect of preharvest and postharvest application of 1-MCP on the quality of Gala Schniga® SchniCo Red(s) apples during long-term storage. Agriculture 2022, 12, 2073. [Google Scholar] [CrossRef]
- Tomala, K.; Guzek, D.; Głabska, D.; Małachowska, M.; Widłak, Ł.; Krupa, T.; Gutkowska, K. Maintaining the quality of ‘Red Jonaprince’ apples during storage by 1-Methylcyclopropene preharvest and postharvest treatment. Agriculture 2022, 12, 1189. [Google Scholar] [CrossRef]
- Khan, A.A.; Vincent, J.F.V. Anisotropy of apple parenchyma. J. Sci. Food Agric. 1990, 52, 455–466. [Google Scholar] [CrossRef]
- Khan, A.A.; Vincent, J.F.V. Anisotropy in the fracture properties of apple flesh as investigated by crack-opening tests. J. Mat. Sci. 1993, 28, 45–51. [Google Scholar] [CrossRef]
- Harker, F.R.; Hallett, I.C. Physiological changes associated with development of mealiness of apple fruit during cool storage. HortScience 1992, 27, 1291–1294. [Google Scholar] [CrossRef]
- Ting, V.J.L.; Silcock, P.; Bremer, P.J.; Biasioli, F. X-ray micro-computer tomographic method to visualize the microstructure of different apple cultivars. J. Food Sci. 2013, 78, E1735–E1742. [Google Scholar] [CrossRef] [PubMed]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Kwon, S.I.; Watkins, C.B.; Kang, I.K. Characterization of fruit quality attributes and cell wall metabolism in 1-methylcycloprenes (1-MCP) treated “Summer King” and “Green Ball” apples during cold storage. Font. Plant Sci. 2019, 10, 1513. [Google Scholar] [CrossRef]
- International Standards for Fruit and Vegetables: Apples. 2021. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/apples_12ebba9f-en-fr (accessed on 5 November 2023).
- Dever, M.C.; Cliff, M.A.; Hall, J.W. Analysis of variation and multivariate relationships among analytical and sensory characteristics in whole apple evaluation. J. Sci. Food Agric. 1995, 69, 329–338. [Google Scholar] [CrossRef]
- Bourne, M.C. Principles of Objective Texture Measurement. In Food Texture and Viscosity, 2nd ed.; Academic Press: London, UK, 2002; pp. 107–188. [Google Scholar]
- Saei, A.; Tustin, D.S.; Zamani, Z.; Talaie, A.; Hall, A.J. Cropping effects on the loss of apple fruit firmness during storage: The relationship between texture retention and fruit dry matter concentration. Sci. Hortic. 2011, 130, 256–265. [Google Scholar] [CrossRef]
- Biasioli, F.; Gasperi, F.; Aprea, E.; Endrizzi, I.; Framondino, V.; Marini, F.; Mott, D.; Märk, T.D. Correlation of PTR-MS spectral fingerprints with sensory characterisation of flavour and odour profile of “Trentingrana” cheese. Food Qual. Prefer. 2006, 17, 63–75. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Doube, M.; Kłosowski, M.; Arganda-Carreras, I.; Cordeliéres, F.; Dougherty, R.; Jackson, J.; Schmid, B.; Hutchinson, J.; Shefelbine, S. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Cappellin, L.; Makhoul, S.; Schuhfried, E.; Romano, A.; Sanchez del Pulgar, J.; Aprea, E.; Farneti, B.; Costa, F.; Gasperi, F.; Biasioli, F. Ethylene: Absolute real-time high sensitivity detection with PTR/SRI-MS. The example of fruits, leaves and bacteria. Int. J. Mass Spectrom. 2014, 365–366, 33–41. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for Multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Soukoulis, C.; Cappellin, L.; Aprea, E.; Costa, F.; Viola, R.; Märk, T.; Gasperi, F.; Biasioli, F. PTR-ToF-MS, A novel, rapid, high sensitivity and non-invasive tool to monitor volatile compound release during fruit post-harvest storage: The case study of apple ripening. Food Bioprocess Technol. 2013, 6, 2831–2843. [Google Scholar] [CrossRef]
- Aprea, E.; Biasioli, F.; Märk, T.D.; Gasperi, F. PTR-MS study of esters in water and water/ethanol solutions: Fragmentation patterns and partition coefficients. Int. J. Mass Spectrom. 2007, 262, 114–121. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.M.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Maek, T.D.; Gasperi, F. On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Dandekar, A.M.; Teo, G.; Defilippi, B.G.; Uratsu, S.L.; Passey, A.J.; Kader, A.A.; Stow, J.R.; Colgan, R.J.; James, D.J. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004, 13, 373–384. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) fruits. J. Agric. Food Chem. 2004, 52, 5694–5701. [Google Scholar] [CrossRef] [PubMed]
- Thewes, F.R.; Both, V.; Brackmann, A.; de Freitas Ferreira, D.; Wagnee, R. 1-methylcyclopropene effects on volatile profile and quality of ‘Royal Gala’ apples produced in Southern Brazil and stored in controlled atmosphere. Cienc. Rural 2015, 45, 2259–2266. [Google Scholar] [CrossRef]
- Ferenczi, A.; Song, J.; Tian, M.; Vlachonasios, K.; Dilley, D.; Beaudry, R. Volatile Ester Suppression and Recovery following 1-Methylcyclopropene Application to Apple Fruit. J. Am. Soc. Hortic. Sci. 2006, 131, 691–701. [Google Scholar] [CrossRef]
- Costa, F.; Cappellin, L.; Longhi, S.; Guerra, W.; Magnago, P.; Porro, D.; Soukoulis, C.; Salvi, S.; Velasco, R.; Biasioli, F.; et al. Assessment of apple (Malus × domestica Borkh.) fruit texture by a combined acoustic-mechanical profiling strategy. Postharvest Biol. Technol. 2011, 61, 21–28. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Romano, A.; Biasioli, F.; Costa, G.; Costa, F. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics 2014, 11, 838–850. [Google Scholar] [CrossRef]
- Ting, V.J.L.; Soukoulis, C.; Silcock, P.; Cappellin, L.; Romano, A.; Aprea, E.; Bremer, P.J.; Maerk, T.D.; Gasperi, F.; Biasioli, F. In vitro and in vivo flavor release from intact and fresh-cut apple in relation with genetic, textural, and physicochemical parameters. J. Food Sci. 2012, 77, C1226–C1233. [Google Scholar] [PubMed]
- Ting, V.J.L.; Romano, A.; Silcock, P.; Bremer, P.J.; Corollaro, M.L.; Soukoulis, C.; Cappellin, L.F.G.; Biasioli, F. Apple flavor: Linking sensory perception to volatile release and textural properties. J. Sens. Stud. 2015, 30, 195–210. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Lurie, S.; Pre-Aymard, C.; Ravid, U.; Larkov, O.; Fallik, E. Effect of 1-Methylcyclopropene on Volatile Emission and Aroma in Cv. Anna Apples. J. Agric. Food Chem. 2002, 50, 4251–4256. [Google Scholar] [CrossRef]
- Dixon, J. Factors affecting apple aroma/flavour volatile concentration: A Review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Flath, R.A.; Black, D.R.; Guadagni, D.G.; McFadden, W.H.; Schultz, T.H. Identification and organoleptic evaluation of compounds in Delicious apple essence. J. Agric. Food Chem. 1967, 15, 29–35. [Google Scholar] [CrossRef]
- Knee, M.; Hatfield, S.G.S. The metabolism of alcohols by apple fruit tissue. J. Sci. Food Agric. 1981, 32, 593–600. [Google Scholar] [CrossRef]
- Lee, J.; Rudell, D.; Davies, P.; Watkins, C. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics 2012, 8, 742–753. [Google Scholar] [CrossRef]
- Allan-Wojtas, P.; Sanford, K.A.; McRae, K.B.; Carbyn, S. An integrated microstructural and sensory approach to describe apple texture. J. Am. Soc. Hortic. Sci. 2003, 128, 381–390. [Google Scholar] [CrossRef]
- Odgaard, A.; Gundersen, H.J.G. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 1993, 14, 173–182. [Google Scholar] [CrossRef]
- Power, F.B.; Chesnut, V.K. The odourous constituents of apples. Emanation of acetaldehyde from the ripe fruit. J. Am. Chem. Soc. 1920, 42, 1509–1526. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Buchanan, D.A.; Fellman, J.K. Change in apple fruit volatiles after storage in atmospheres inducing anaerobic metabolism. J. Agric. Food Chem. 1991, 39, 1602–1605. [Google Scholar] [CrossRef]
- McGlone, V.A.; Jordan, R.B.; Seelye, R.; Clark, C.J. Dry-matter—A better predictor of the post-storage soluble solids in apples? Postharvest Biol. Technol. 2003, 28, 431–435. [Google Scholar] [CrossRef]
- White, A. Apple Tree Named ‘Scifresh’. USPP 13888P3, 17 June 2003. [Google Scholar]
- Herremans, E.; Verboven, P.; Bongaers, E.; Estrade, P.; Verlinden, B.E.; Wevers, M.; Hertog, M.L.A.T.M.; Nicolai, B.M. Characterisation of ‘Braeburn’ browning disorder by means of X-ray micro-CT. Postharvest Biol. Technol. 2013, 75, 114–124. [Google Scholar] [CrossRef]
- Jarvis, M.C. Intercellular separation forces generated by intracellular pressure. Plant Cell Environ. 1998, 21, 1307–1310. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A. Aroma biochemistry of fruits and vegetables. In Phytochemistry of Fruit and Vegetables; Tomás-Barberán, F.A., Robins, R.J., Eds.; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Cadena, R.S.; Cruz, A.G.; Netto, R.R.; Castro, W.F.; Faria, J.dA.F.; Bolini, H.M.A. Sensory profile and physicochemical characteristics of mango nectar sweetened with high intensity sweeteners throughout storage time. Food Res. Int. 2013, 54, 1670–1679. [Google Scholar] [CrossRef]
| Mechanical Parameters | Definition |
|---|---|
| Fmax, (N) | Maximum force required to puncture the fruit skin |
| Flesh firmness, flesh.F (N) | Averaged force measured after skin rupture |
| Gradient, grad (N/mm) | Stiffness of skin measured as a slope from the start of the curve until Fmax |
| Area under the curve, AUC peel (N⋅mm) | Mechanical work needed to reach the rupture point of the skin indicated by Fmax taken as the area under the curve |
| AUC flesh (N⋅mm) | Work measured under the curve after skin rupture |
| m/z | Tentatively Identified Compounds | Untreated (C) | Treated (SF) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Braeburn | Fuji | Golden Delicious | Jazz | p-Value | Braeburn | Fuji | Golden Delicious | Jazz | p-Value | ||
| 28 | Ethylene | 464 | 321 | 775 | 850 | 0.36 | 288 | 525 | 157 | 262 | 0.49 |
| (191) | (118) | (399) | (564) | (204) | (267) | (115) | (229) | ||||
| 31 | CH3O+ | 0.5 a | 1.3 ab | 0.8 ab | 1.4 b | 0.04 | 0.2 | 0.4 | 0.1 | 0.2 | 0.62 |
| (0.3) | (0.5) | (0.3) | (0.1) | (0.3) | (0.5) | (0.2) | (0.1) | ||||
| 33 | Methanol | 87 ab | 208 c | 140 bc | 30 a | 0.00 | 11 a | 21 ab | 33 b | 23 ab | 0.03 |
| (27) | (44) | (24) | (6.1) | (0.1) | (0.4) | (13.1) | (3.7) | ||||
| 41 | Alcohol and ester frag. | 343 | 358 | 353 | 393 | 0.67 | 119 | 248 | 138 | 214 | 0.04 |
| (28) | (91) | (47) | (30) | (28) | (81) | (20) | (49) | ||||
| 43 | Alcohol and ester frag. | 1526 b | 755 a | 1938 b | 1840 b | 0.00 | 286 | 508 | 373 | 639 | 0.86 |
| (192) | (141) | (199) | (264) | (80) | (248) | (54) | (143) | ||||
| 45 | Acetaldehyde | 2730 a | 5755 b | 12298 b | 2468 a | 0.00 | 837 a | 2732 a | 11271 b | 1388 a | 0.00 |
| (365) | (1524) | (947) | (688) | (82) | (621) | (2246) | (41) | ||||
| 47 | Ethanol | 14 ab | 25 b | 58 c | 9.0 a | 0.00 | 5.1 a | 12 a | 52 b | 6.8 a | 0.00 |
| (3.2) | (6.1) | (7.5) | (1.5) | (0.2) | (1.8) | (5.6) | (0.9) | ||||
| 53 | C4H5+ | 2.6 a | 3.8 ab | 5.4 b | 2.0 a | 0.13 | 1.0 a | 2.0 b | 1.2 a | 1.1 a | 0.01 |
| (0.5) | (0.2) | (1.9) | (0.3) | (0.1) | (0.2) | (0.5) | (0.2) | ||||
| 55 | C4H7+ | 69 a | 88 ab | 148 b | 42 a | 0.10 | 25 a | 43 b | 38 ab | 28 ab | 0.03 |
| (8.2) | (10) | (55) | (1.9) | (1.2) | (5.4) | (8.1) | (8.3) | ||||
| 57 | Alcohol and ester frag. | 207 a | 221 a | 487 b | 219 a | 0.00 | 47 a | 150 b | 116 ab | 101 ab | 0.03 |
| (28) | (35) | (77) | (13) | (11) | (53) | (30) | (27) | ||||
| 59 | Acetone | 23 a | 29 ab | 32 b | 28 ab | 0.67 | 12 | 14 | 19 | 21 | 0.07 |
| (2) | (3.9) | (4.7) | (2.8) | (0.8) | (2.2) | (1.6) | (6.9) | ||||
| 61 | Frag. of acetate esters/acetic acid | 1339 b | 509 a | 1518 b | 1638 b | 0.00 | 211 | 350 | 211 | 519 | 0.04 |
| (176) | (102) | (165) | (221) | (65) | (199) | (42) | (108) | ||||
| 63 | Ethylene glycol | 7.7 a | 6.6 a | 25 b | 9.0 a | 0.00 | 1.9 a | 4.0 a | 19 b | 4.1 a | 0.00 |
| (0.8) | (1.1) | (2.1) | (1.1) | (0.3) | (2.1) | (3.4) | (0.5) | ||||
| 69 | Isoprene | 4.1 a | 4.3 a | 8.2 b | 2.8 a | 0.00 | 1.8 a | 1.8 a | 4.1 b | 2.3 a | 0.00 |
| (0.7) | (0.8) | (1.9) | (0.3) | (0.5) | (0.2) | (0.5) | (0.4) | ||||
| 71 | Alcohol and ester frag. | 53 b | 55 b | 24 a | 42 ab | 0.00 | 27 | 41 | 25 | 36 | 0.15 |
| (3.9) | (14) | (4.1) | (0.9) | (7.3) | (11) | (1.7) | (10) | ||||
| 73 | Butanal | 3.2 ab | 3.7 ab | 10 b | 1.5 a | 0.02 | 0.9 a | 2.4 b | 1.9 ab | 1.0 a | 0.15 |
| (0.8) | (0.4) | (5.4) | (0.3) | (0.2) | (0.9) | (0.2) | (0.4) | ||||
| 75 | Butyl propanoate | 22 a | 24 a | 14 a | 49 b | 0.00 | 1.5 | 19 | 2 | 11 | 0.09 |
| (6.6) | (2.2) | (1.1) | (8.7) | (0.9) | (16) | (1.2) | (5.2) | ||||
| 79 | C2H7O3+ | 1.3 a | 0.6 a | 3.0 b | 1.5 a | 0.00 | 0.3 | 0.4 | 0.4 | 0.6 | 0.56 |
| (0.6) | (0.3) | (0.1) | (0.5) | (0.1) | (0.3) | (0.2) | (0.4) | ||||
| 81 | Terpene-related frag., aldehydes (trans-2-hexenal) | 9.8 a | 15 a | 44 b | 4.9 a | 0.00 | 5.5 ab | 11 b | 10 ab | 3.5 a | 0.02 |
| (0.8) | (2) | (11) | (1.6) | (0.9) | (2) | (4.4) | (1.9) | ||||
| 83 | Alcohols (hexanal, trans-2-hexenol, cis-2-hexenol) | 18 ab | 26 ab | 31 b | 13 a | 0.02 | 8.2 | 15 | 12 | 10 | 0.18 |
| (2.9) | (2.9) | (11) | (0.8) | (0.3) | (3) | (4.6) | (3.2) | ||||
| 85 | Alcohols (1-Hexanol, nonanol), ester frag. | 10 a | 9.7 a | 16 b | 15 b | 0.00 | 1.4 a | 5.1 b | 5.6 b | 3.5 ab | 0.00 |
| (1.5) | (1.9) | (1.7) | (1.1) | (0.7) | (0.9) | (0.6) | (1.1) | ||||
| 87 | Frag. (pentanal, 2-pentanone) | 1.1 ab | 2.4 c | 1.5 b | 0.8 a | 0.00 | 0.3 a | 0.8 b | 0.8 b | 0.5 a | 0.00 |
| (0.2) | (0.3) | (0.4) | (0.1) | (0.1) | (0.2) | (0.1) | (0.1) | ||||
| 89 | Butyrate related esters (ethyl butanoate, propyl butanoate, butyl butanoate) | 26 a | 39 ab | 59 b | 16 a | 0.00 | 2.6 | 18 | 6.1 | 5.8 | 0.05 |
| (9.5) | (9) | (15) | (2.1) | (0.7) | (12) | (1.4) | (0.7) | ||||
| 95 | Farnesene frag. | 0.9 a | 1.3 ab | 4.6 b | 0.3 a | 0.01 | 0.1 | 0.3 | 0.8 | 0.3 | 0.10 |
| (0.7) | (0.6) | (2) | (0.5) | (0.2) | (0.3) | (0.3) | (0.2) | ||||
| 99 | Aldehydes (trans-2-hexanal), esters (ethyl hexanoate, hexyl acetate) | 5.4 a | 11 b | 11 b | 3.4 a | 0.00 | 3.3 a | 7.0 b | 4.1 ab | 2.3 a | 0.00 |
| (1.1) | (1.9) | (2.6) | (0.9) | (0.8) | (0.8) | (1.8) | (0.6) | ||||
| 101 | Aldehydes(2-hexanone, hexanal) | 0.8 | 1 | 1.7 | 0.7 | 0.12 | 0.3 a | 0.9 | 0.6 ab | 0.5 ab | 0.01 |
| (1.5) | (0.4) | (0.7) | (0.2) | (0.1) | (0.2) | (0.2) | (0.1) | ||||
| 103 | Esters (isoamyl esters, propyl acetate, ethyl 2-methyl butanoate, methyl butanoate) | 18 a | 29 b | 9.7 a | 20 ab | 0.00 | 3.2 a | 17 b | 6.6 a | 8.4 a | 0.00 |
| (1.1) | (6.1) | (2.1) | (4.3) | (1) | (3.8) | (3.6) | (2.9) | ||||
| 109 | Unidentified | 1.4 a | 1.5 a | 4.1 b | 0.7 a | 0.00 | 0.7 | 0.6 | 0.8 | 0.4 | 0.34 |
| (0.3) | (0.5) | (1.1) | (0.2) | (0.1) | (0.2) | (0.4) | (0.1) | ||||
| 117 | Esters (hexanoates, ethyl 2-methyl butanoate, Isobutyl acetate, butyl acetate) | 22 a | 25 a | 44 b | 18 a | 0.00 | 2.3 a | 9.2 b | 4.0 ab | 6.6 ab | 0.02 |
| (4.3) | (2.6) | (3.7) | (3.3) | (1.1) | (3.7) | (1.3) | (1.6) | ||||
| 121 | Acetophenone | 0.7 a | 0.9 a | 3.7 b | 0.4 a | 0.00 | 0.1 | 0.5 | 0.4 | 0.3 | 0.35 |
| (0.3) | (1) | (1.2) | (0.2) | (0.1) | (0.5) | (0.2) | (0.1) | ||||
| 123 | Farnesene frag. | 0.4 a | 1.1 a | 2.3 b | 0.3 a | 0.00 | 0.3 | 0.4 | 0.5 | 0.3 | 0.58 |
| (0.3) | (0.7) | (1.4) | (0.5) | (0.3) | (0.3) | (0.2) | (0.2) | ||||
| 131 | Esters (heptanoates, methyl hexyl-esters) | 1.4 b | 3.1 c | 0.6 a | 1.2 ab | 0.00 | 0.4 | 0.9 | 0.5 | 0.6 | 0.35 |
| (0.4) | (0.1) | (0.2) | (0.3) | (0.2) | (0.6) | (0.2) | (0.1) | ||||
| 135 | farnesene frag., P-cymene | 0.5 a | 0.7 a | 3.4 b | 0.2 a | 0.00 | 0.3 | 0.4 | 0.3 | 0.1 | 0.80 |
| (0.4) | (0.4) | (0.3) | (0.4) | (0.2) | (0.6) | (0.5) | (0.1) | ||||
| 137 | Monoterpenes, farnesene frag. | 0.7 a | 0.3 a | 3.2 b | 0 a | 0.00 | 0.1 | 0 | 0.3 | 0.1 | 0.28 |
| (0.7) | (0.5) | (0.4) | (0.1) | (0.1) | (0.1) | (0.3) | (0.2) | ||||
| 145 | Esters (ethyl hexanoate, butyl butanoate) | 2.4 a | 2.9 a | 4.3 b | 3.1 ab | 0.01 | 0.3 | 1.1 | 0.4 | 0.8 | 0.20 |
| (0.3) | (0.1) | (0.9) | (0.4) | (0.3) | (0.8) | (0.2 | (0.2) | ||||
| 149 | Farnesene frag., Phenyls (estragole, anethol) | 1.9 a | 0.6 a | 5.3 b | 4.0 a | 0.01 | 0.3 | 0.7 | 0.2 | 0.3 | 0.57 |
| (0.1) | (0.4) | (1.4) | (2.1) | (0.3) | (0.6) | (0.2) | (0.4) | ||||
| 173 | Decanoates | 1.0 ab | 1.4 ab | 2.2 b | 0.8 a | 0.03 | 0.2 | 0.2 | 0.3 | 0.4 | 0.46 |
| (0.4) | (0.1) | (0.8) | (1.4) | (0.1) | (0.2) | (0.1) | (0.1) | ||||
| 205 | Alpha-farnesene | 1.4 a | 1.8 a | 7.3 b | 0.4 a | 0.00 | 0 | 0.6 | 0.3 | 0.1 | 0.02 |
| (0.3) | (1.1) | (2.3) | (0.4) | (0) | (0.1) | (0.3) | (0.1) | ||||
| Cultivar | Untreated (days) | Treated (SF) (days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 100 | 120 | 150 | 50 | 70 | 100 | 120 | 150 | ||||
| Ester | m/z 61 | Acetate-related esters | Braeburn | A | ab | ab | B | b | A | A | A | A | B |
| Fuji | A | ab | b | ab | ab | - | - | - | - | - | |||
| Golden Delicious | Ab | ab | ab | bc | c | - | - | - | - | - | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 89 | Butyrate-related esters | Braeburn | - | - | - | - | - | A | A | A | A | B | |
| Fuji | a | ab | bc | bc | c | AB | A | AB | BC | C | |||
| Golden Delicious | b | ab | ab | ab | ab | - | - | - | - | - | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 117 | Hexanoate-related esters | Braeburn | - | - | - | - | - | A | A | A | A | B | |
| Fuji | a | ab | ab | ab | b | A | AB | AB | AB | B | |||
| Golden Delicious | bc | ab | b | bc | c | A | A | A | B | B | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 145 | Ethyl hexanoate/butyl butanoate | Braeburn | a | ab | ab | b | b | A | A | A | A | B | |
| Fuji | a | ab | ab | ab | b | - | - | - | - | - | |||
| Golden Delicious | ab | a | ab | ab | b | A | A | A | B | B | |||
| Jazz | a | ab | ab | b | b | - | - | - | - | - | |||
| Aldehyde | m/z 45 | Acetaldehyde | Braeburn | a | a | b | b | c | A | A | A | B | C |
| Fuji | - | - | - | - | - | A | B | AB | AB | B | |||
| Golden Delicious | - | - | - | - | - | C | A | AB | B | BC | |||
| Jazz | a | b | bc | c | d | A | AB | AB | B | AB | |||
| m/z 73 | Butanal | Braeburn | a | ab | b | b | b | A | A | A | A | B | |
| Fuji | - | - | - | - | - | A | A | A | AB | B | |||
| Golden Delicious | - | - | - | - | - | A | B | A | A | AB | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 101 | Hexanal/2-hexanone | Braeburn | a | ab | b | b | b | A | A | A | A | B | |
| Fuji | a | ab | ab | b | b | - | - | - | - | - | |||
| Golden Delicious | - | - | - | - | - | - | - | - | - | - | |||
| Jazz | a | ab | ab | ab | b | A | A | AB | B | AB | |||
| Alcohol | m/z 33 | Methanol | Braeburn | a | ab | ab | b | b | A | A | A | A | B |
| Fuji | - | - | - | - | - | A | AB | AB | B | B | |||
| Golden Delicious | - | - | - | - | - | A | A | B | B | B | |||
| Jazz | a | ab | bc | bc | c | - | - | - | - | - | |||
| m/z 47 | Ethanol | Braeburn | a | a | b | b | b | A | A | A | A | B | |
| Fuji | a | a | ab | ab | b | - | - | - | - | - | |||
| Golden Delicious | a | ab | ab | ab | b | C | A | B | B | C | |||
| Jazz | a | ab | bc | c | d | A | AB | AB | B | AB | |||
| DM | SSC | Fmax | Flesh.f | Gradient | |
|---|---|---|---|---|---|
| DM | - | ||||
| SSC | 0.789 | - | |||
| Fmax | 0.244 | 0.132 | - | ||
| Flesh.f | 0.116 | 0.029 | 0.900 | ||
| Gradient | −0.006 | 0.001 | 0.468 | 0.520 | - |
| AUC.peel | 0.316 | 0.297 | 0.189 | 0.014 | −0.713 |
| AUC.flesh | −0.123 | −0.276 | 0.773 | 0.861 | 0.497 |
| Porosity | 0.005 | 0.187 | −0.691 | −0.715 | −0.484 |
| Anisotropy | −0.156 | −0.382 | 0.435 | 0.468 | 0.284 |
| Connectivity | −0.082 | 0.170 | −0.319 | −0.257 | −0.066 |
| AUC.peel | AUC.flesh | Porosity | Anisotropy | ||
| DM | |||||
| SSC | |||||
| Fmax | |||||
| Flesh.f | |||||
| Grad | |||||
| AUC.peel | - | ||||
| AUC.flesh | −0.188 | - | |||
| Porosity | 0.183 | −0.811 | - | ||
| Anisotropy | −0.191 | 0.654 | −0.619 | - | |
| Connectivity | −0.057 | −0.356 | 0.493 | −0.745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, V.J.L.; Silcock, P.; Biasioli, F.; Bremer, P. The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage. Foods 2023, 12, 4050. https://doi.org/10.3390/foods12224050
Ting VJL, Silcock P, Biasioli F, Bremer P. The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage. Foods. 2023; 12(22):4050. https://doi.org/10.3390/foods12224050
Chicago/Turabian StyleTing, Valentina J. L., Pat Silcock, Franco Biasioli, and Phil Bremer. 2023. "The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage" Foods 12, no. 22: 4050. https://doi.org/10.3390/foods12224050
APA StyleTing, V. J. L., Silcock, P., Biasioli, F., & Bremer, P. (2023). The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage. Foods, 12(22), 4050. https://doi.org/10.3390/foods12224050








