Development and Verification of a Poultry Management Tool to Quantify Salmonella from Live to Final Product Utilizing RT-PCR
Abstract
:1. Introduction
2. Materials and Methods
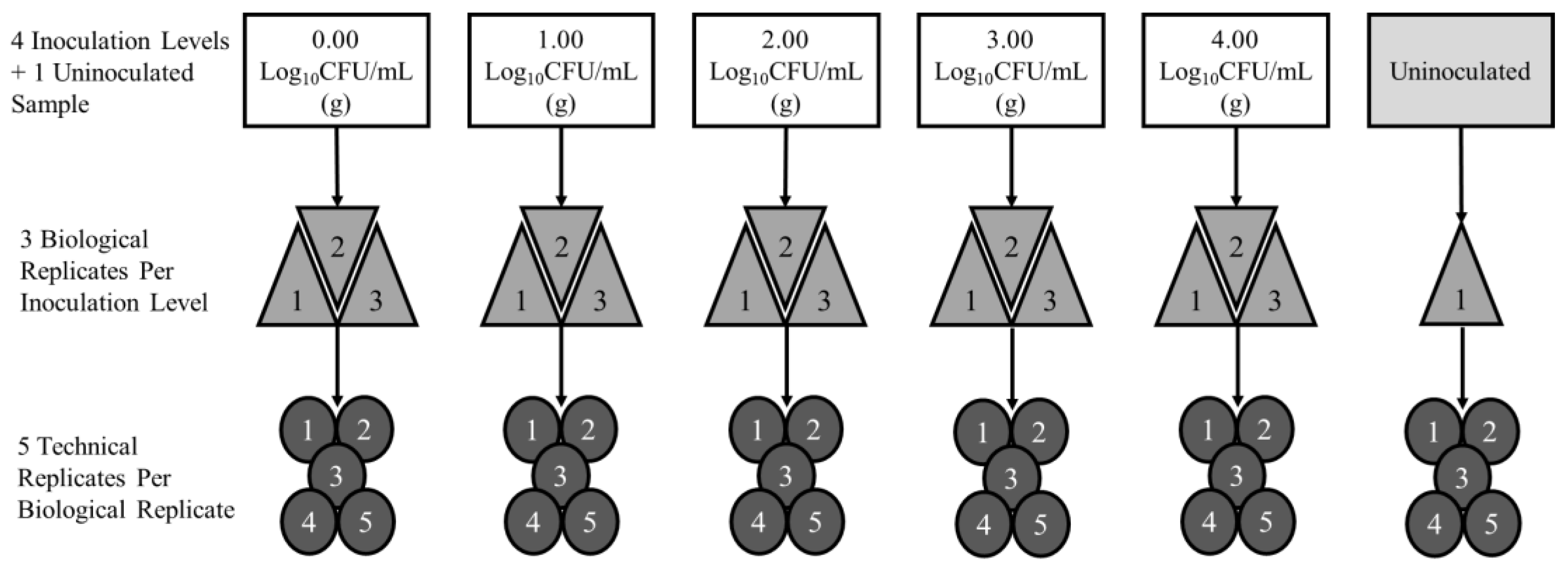
2.1. Experimental Design
2.2. Sample Collection
2.2.1. Boot Swabs
2.2.2. Processing Rinses
2.2.3. Ground Chicken and Turkey
2.3. Inoculation Procedures
2.4. Sample Processing and Curve Development
2.4.1. Boot Swabs
2.4.2. Processing Rinsates
2.4.3. Ground Chicken and Turkey
2.5. Curve Verification
3. Results
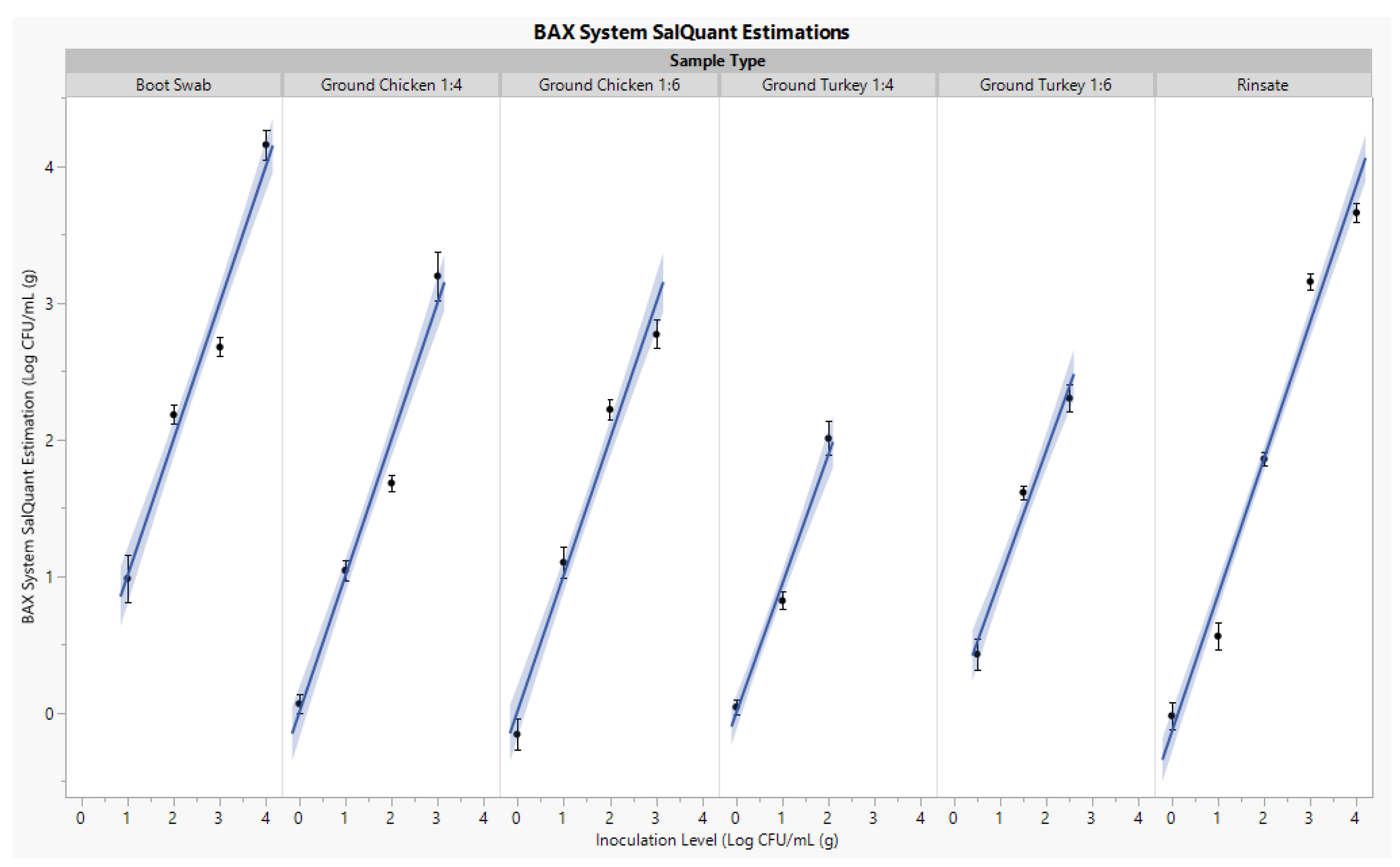
3.1. Curve Development
3.2. Curve Verification
4. Discussion
4.1. Enumerative Methods
4.2. Primary Production
4.3. Process Controls
4.4. Final Product and Consumer Risk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FloorSeattle, 1012 First Avenue Fifth; Washington 98104-1008. Most Consumers Don’t Use Food Thermometers Despite Importance to Food Safety. Food Safety News. Available online: https://www.foodsafetynews.com/2015/02/thermometers-crucial-for-food-safety/(accessed on 9 August 2022).
- Young, I.; Waddell, L. Barriers and Facilitators to Safe Food Handling among Consumers: A Systematic Review and Thematic Synthesis of Qualitative Research Studies. PLoS ONE 2016, 11, e0167695. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the Disease Burden of 14 Pathogens in Food Sources in the United States using Attribution Data from Outbreak Investigations and Expert Elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemical Disinfectants|Disinfection & Sterilization Guidelines|Guidelines Library|Infection Control|CDC. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html (accessed on 10 August 2022).
- National Advisory Committee on Microbiological Criteria for Foods. Response to Questions Posed by the Food Safety and Inspection Service Regarding Salmonella Control Strategies in Poultry. J. Food Prot. 2019, 82, 645–668. [Google Scholar] [CrossRef]
- USDA ERS—Sector at a Glance. Available online: https://www.ers.usda.gov/topics/animal-products/poultry-eggs/sector-at-a-glance/ (accessed on 9 August 2022).
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Salmonella Homepage|CDC. Available online: https://www.cdc.gov/salmonella/index.html (accessed on 10 August 2022).
- Berghaus, R.D.; Thayer, S.G.; Law, B.F.; Mild, R.M.; Hofacre, C.L.; Singer, R.S. Enumeration of Salmonella and Campylobacter Spp. in Environmental Farm Samples and Processing Plant Carcass Rinses from Commercial Broiler Chicken Flocks. Appl. Environ. Microbiol. 2013, 79, 4106–4114. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.P.; Evans, R.D.; Regalado, J.; Sullivan, J.F.; Dutta, V.; Elvinger, F.; Pierson, F.W. Preharvest Salmonella Detection for Evaluation of Fresh Ground Poultry Product Contamination. J. Food Prot. 2015, 78, 1266–1271. [Google Scholar] [CrossRef]
- Rimet, C.-S.; Maurer, J.J.; Pickler, L.; Stabler, L.; Johnson, K.K.; Berghaus, R.D.; Villegas, A.M.; Lee, M.; França, M. Salmonella Harborage Sites in Infected Poultry That May Contribute to Contamination of Ground Meat. Front. Sustain. Food Syst. 2019, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Rahman, U.U. Postharvest Intervention Technologies for Safety Enhancement of Meat and Meat based Products; a Critical Review. J. Food Sci. Technol. 2016, 53, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Ricke, S.C.; Kundinger, M.M.; Miller, D.R.; Keeton, J.T. Alternatives to Antibiotics: Chemical and Physical Antimicrobial Interventions and Foodborne Pathogen Response. Poult. Sci. 2005, 84, 667–675. [Google Scholar] [CrossRef]
- Intervention Technologies for Ensuring Microbiological Safety of Meat: Current and Future Trends—Chen—2012—Comprehensive Reviews in Food Science and Food Safety—Wiley Online Library. Available online: https://ift.onlinelibrary.wiley.com/doi/abs/10.1111/j.1541-4337.2011.00177.x (accessed on 10 August 2022).
- Roll, V.F.B.; Dai Prá, M.A.; Roll, A.P. Research on Salmonella in Broiler Litter Reused for up to 14 Consecutive Flocks. Poult. Sci. 2011, 90, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Vandeplas, S.; Dauphin, R.D.; Beckers, Y.; Thonart, P.; Théwis, A. Salmonella in Chicken: Current and Developing Strategies to Reduce Contamination at Farm Level. J. Food Prot. 2010, 73, 774. [Google Scholar] [CrossRef] [PubMed]
- USDA—APHIS. Procedures for Collection, Isolation, and Identification of Salmonella from House Environmental Samples, Cloacal Swabs, and Hatchery Samples; USDA—APHIS: Riverdale Park, MD, USA.
- USDA—FSIS. Pathogen Reduction—Salmonella and Campylobacter Performance Standards Verification Testing; USDA—FSIS: Washington, DC, USA, 2019. [Google Scholar]
- McEntire, J.; Acheson, D.; Siemens, A.; Eilert, S.; Robach, M. The Public Health Value of Reducing Salmonella Levels in Raw Meat and Poultry. Food Prot. Trends 2014, 34, 386–391. [Google Scholar]
- Microbiology Laboratory Guidebook|Food Safety and Inspection Service. Available online: http://www.fsis.usda.gov/news-events/publications/microbiology-laboratory-guidebook (accessed on 9 August 2022).
- Malorny, B.; Löfström, C.; Wagner, M.; Krämer, N.; Hoorfar, J. Enumeration of Salmonella Bacteria in Food and Feed Samples by Real-Time PCR for Quantitative Microbial Risk Assessment. Appl. Environ. Microbiol. 2008, 74, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Russek, E.; Colwell, R.R. Computation of Most Probable Numbers. Appl. Environ. Microbiol. 1983, 45, 1646–1650. [Google Scholar] [CrossRef] [Green Version]
- Wohlsen, T.; Bates, J.; Vesey, G.; Robinson, W.A.; Katouli, M. Evaluation of the Methods for Enumerating Coliform Bacteria from Water Samples using Precise Reference Standards. Lett. Appl. Microbiol. 2006, 42, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Indicator Organism Assays: Chaos, Confusion and Criteria|Food Safety. Available online: https://www.food-safety.com/articles/3850-indicator-organism-assays-chaos-confusion-and-criteria (accessed on 10 August 2022).
- Frontiers|Microbiome Profiles of Commercial Broilers Through Evisceration and Immersion Chilling during Poultry Slaughter and the Identification of Potential Indicator Microorganisms. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00345/full (accessed on 10 August 2022).
- Hervert, C.J.; Alles, A.S.; Martin, N.H.; Boor, K.J.; Wiedmann, M. Evaluation of Different Methods to Detect Microbial Hygiene Indicators Relevant in the Dairy Industry. J. Dairy Sci. 2016, 99, 7033–7042. [Google Scholar] [CrossRef] [Green Version]
- Kornacki, J.; Johnson, J. Enterobacteriaceae, Coliforms, and Escherichia coli as Quality and Safety Indicators. In Compendium of Methods for The Microbiological Examination of Foods; American Journal of Public Health: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- De Villena, J.F.; Vargas, D.A.; Bueno López, R.; Chávez-Velado, D.R.; Casas, D.E.; Jiménez, R.L.; Sanchez-Plata, M.X. Bio-Mapping Indicators and Pathogen Loads in a Commercial Broiler Processing Facility Operating with High and Low Antimicrobial Intervention Levels. Foods 2022, 11, 775. [Google Scholar] [CrossRef]
- McKee, S. Salmonella Control in Poultry Processing. 4. American Meat Science Association. In Proceedings of the 65th Annual Reciprocal Meat Conference, Fargo, ND, USA, 17–20 June 2012; Available online: https://meatscience.org/docs/default-source/publications-resources/rmc/2012/12_mckee_r2.pdf?sfvrsn=0 (accessed on 10 January 2023).
- Santos, F.B.O.; Li, X.; Payne, J.B.; Sheldon, B.W. Estimation of Most Probable Number Salmonella Populations on Commercial North Carolina Turkey Farms. J. Appl. Poult. Res. 2005, 14, 700–708. [Google Scholar] [CrossRef]
- Peng, Y.; Deng, X.Y.; Harrison, M.A.; Alali, W.Q. Salmonella Levels Associated with Skin of Turkey Parts. J. Food Prot. 2016, 79, 801–805. [Google Scholar] [CrossRef]
- USDA—FSIS. Refrigeration & Food Safety|Food Safety and Inspection Service. Available online: http://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/refrigeration (accessed on 9 August 2022).
- Kwon, J.A.; Yim, D.-G.; Kim, H.-J.; Ismail, A.; Kim, S.-S.; Lee, H.J.; Jo, C. Effect of Temperature Abuse on Quality and Metabolites of Frozen/Thawed Beef Loins. Food Sci. Anim. Resour. 2022, 42, 341–349. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Timepoint (h) | R2 | Log RMSE * | Enumerable Range s |
|---|---|---|---|---|
| Boot Swab | 10 | 0.87 | 0.43 | 1.00–4.00 Log CFU/mL |
| Processing Rinsate | 6 | 0.90 | 0.35 | 0.00–4.00 Log CFU/mL |
| Ground Chicken 1:4 | 8 | 0.87 | 0.43 | 0.00–3.00 Log CFU/g |
| Ground Chicken 1:6 | 8 | 0.87 | 0.42 | 0.00–3.00 Log CFU/g |
| Ground Turkey 1:4 | 8 | 0.94 | 0.19 | 0.00–2.00 Log CFU/g |
| Ground Turkey 1:6 | 8 | 0.89 | 0.31 | 0.00–3.00 Log CFU/g |
| Matrix | Inoculation Level | SalQuant™ Estimation * | MPN s Estimation * | p Value γ |
|---|---|---|---|---|
| Boot Swab | 1.00 | 1.27 | 0.56 | >0.05 |
| 2.00 | 2.17 | 2.15 | ||
| 3.00 | 2.73 | 4.04 | ||
| 4.00 | 4.17 | 4.04 | ||
| Processing Rinsate | 1.00 | 1.33 | 1.27 | >0.05 |
| 2.00 | 2.97 | 2.27 | ||
| 3.00 | 4.25 | 3.34 | ||
| Ground Chicken 1:4 | 0.00 | −0.83 | −1.52 | >0.05 |
| 1.00 | 0.67 | 0.79 | ||
| 2.00 | 1.65 | 1.32 | ||
| 3.00 | 3.50 | 2.66 | ||
| Ground Chicken 1:6 | 0.00 | −0.16 | −0.57 | >0.05 |
| 1.00 | 1.10 | 1.04 | ||
| 2.00 | 2.23 | 1.63 | ||
| 3.00 | 2.77 | 1.79 | ||
| Ground Turkey 1:4 | 0.00 | 0.11 | 0.36 | >0.05 |
| 1.00 | 0.83 | 0.96 | ||
| 2.00 | 2.01 | 4.04 | ||
| Ground Turkey 1:6 | 0.50 | 0.55 | −0.04 | >0.05 |
| 1.50 | 1.68 | 1.57 | ||
| 2.50 | 2.33 | 2.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Applegate, S.F.; Englishbey, A.K.; Stephens, T.P.; Sanchez-Plata, M.X. Development and Verification of a Poultry Management Tool to Quantify Salmonella from Live to Final Product Utilizing RT-PCR. Foods 2023, 12, 419. https://doi.org/10.3390/foods12020419
Applegate SF, Englishbey AK, Stephens TP, Sanchez-Plata MX. Development and Verification of a Poultry Management Tool to Quantify Salmonella from Live to Final Product Utilizing RT-PCR. Foods. 2023; 12(2):419. https://doi.org/10.3390/foods12020419
Chicago/Turabian StyleApplegate, Savannah F., April K. Englishbey, Tyler P. Stephens, and Marcos X. Sanchez-Plata. 2023. "Development and Verification of a Poultry Management Tool to Quantify Salmonella from Live to Final Product Utilizing RT-PCR" Foods 12, no. 2: 419. https://doi.org/10.3390/foods12020419






