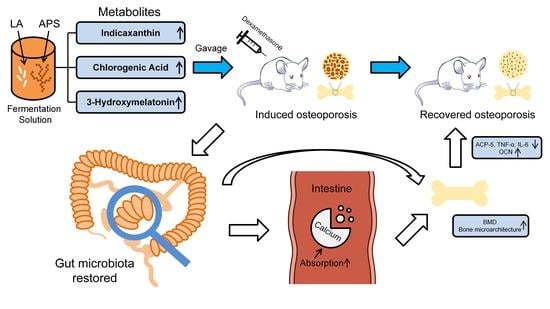
Lactobacillus acidophilus (LA) Fermenting Astragalus Polysaccharides (APS) Improves Calcium Absorption and Osteoporosis by Altering Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Crude APS
2.2. Preparation of LA
2.3. Preparation of Fermentation Solution (FS)
2.4. Analysis of Metabolomics in FS
2.5. Preparation of Mixed Solution (MS)
2.6. Animal Experiments
2.7. Detection of Apparent Calcium Absorption
2.8. Detection of Bone Mineral Density (BMD)
2.9. Analysis of Tibia Paraffin Section, H&E Staining, and Bone Microarchitecture
2.10. Detection of ACP-5, OCN, TNF-α, and IL-6
2.11. Analysis of 16S rDNA Sequencing and Bioinformatics
2.12. Statistics
3. Results
3.1. FS More Significantly Improved Apparent Calcium Absorption in Osteoporotic Rats
3.2. FS More Significantly Improved Rat Osteoporosis Induced by Dexamethasone
3.2.1. FS More Significantly Improved BMD
3.2.2. FS More Significantly Improved the Restoration of Bone Microarchitecture
3.3. FS More Significantly Decreased the Osteoclast Differentiation Biomarker ACP5 and Increased the Osteoblast Differentiation Marker OCN
3.4. FS More Significantly Decreased the Levels of Pro-Inflammatory Cytokines TNF-α and IL-6
3.5. Changes in the Gut Microbiota Profile of Rats with Osteoporosis
3.5.1. Composition Changes of Gut Microbiota in Osteoporotic Rats
3.5.2. Specific Bacteria Served as Bacterial Markers of FS to Improve Calcium Absorption and Osteoporosis
3.6. Key Bacteria in FS-Improved Calcium Absorption and Osteoporosis
3.7. Functional Changes of Gut Microbiota Related to Calcium Absorption in Rats with Osteoporosis Treated by FS
3.8. Key Differential Metabolites of FS to Improve Calcium Absorption and Osteoporosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Carey, J.J.; Wu, P.C.-H.; Bergin, D. Risk Assessment Tools for Osteoporosis and Fractures in 2022. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101775. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.K.; Mahalle, N. Calcium Supplementation: Why, Which, and How? Indian J. Endocrinol. Metab. 2019, 23, 387. [Google Scholar] [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The Abundance and Variety of Carbohydrate-Active Enzymes in the Human Gut Microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, J.; Liu, L.; Zhang, G.; Zhou, A.; Peng, X. The Gut Microbiota Alteration and the Key Bacteria in Astragalus Polysaccharides (APS)-Improved Osteoporosis. Food Res. Int. 2020, 138, 109811. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Cheng, H.; Wang, H.; Tan, Y.; Feng, W.; Peng, C. Interactions between Polysaccharides and Gut Microbiota: A Metabolomic and Microbial Review. Food Res. Int. 2022, 160, 111653. [Google Scholar] [CrossRef]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017, 5, 20. [Google Scholar] [CrossRef]
- Yang, L.-C.; Lin, S.-W.; Li, I.-C.; Chen, Y.-P.; Tzu, S.-Y.; Chou, W.; Chen, C.-C.; Lin, W.-C.; Chen, Y.-L.; Lin, W.-H. Lactobacillus Plantarum GKM3 and Lactobacillus Paracasei GKS6 Supplementation Ameliorates Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation and Inhibiting Osteoclast Formation. Nutrients 2020, 12, 1914. [Google Scholar] [CrossRef]
- Sapra, L.; Dar, H.Y.; Bhardwaj, A.; Pandey, A.; Kumari, S.; Azam, Z.; Upmanyu, V.; Anwar, A.; Shukla, P.; Mishra, P.K. Lactobacillus Rhamnosus Attenuates Bone Loss and Maintains Bone Health by Skewing Treg-Th17 Cell Balance in Ovx Mice. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus Acidophilus Inhibits Bone Loss and Increases Bone Heterogeneity in Osteoporotic Mice via Modulating Treg-Th17 Cell Balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2010; pp. 47–53. [Google Scholar]
- Feng, R.; Feng, L.; Yuan, Z.; Wang, D.; Wang, F.; Tan, B.; Han, S.; Li, T.; Li, D.; Han, Y. Icariin Protects against Glucocorticoid-Induced Osteoporosis in Vitro and Prevents Glucocorticoid-Induced Osteocyte Apoptosis in Vivo. Cell Biochem. Biophys. 2013, 67, 189–197. [Google Scholar] [CrossRef]
- Chonan, O.; Takahashi, R.; Watanuki, M. Role of Activity of Gastrointestinal Microflora in Absorption of Calcium and Magnesium in Rats Fed Β1-4 Linked Galactooligosaccharides. Biosci. Biotechnol. Biochem. 2001, 65, 1872–1875. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone Histomorphometry: Standardization of Nomenclature, Symbols, and Units: Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Chen, Q.; Zhang, Z.; Zhao, X.; Hou, H. Functional Calcium Binding Peptides from Pacific Cod (Gadus Macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model. Nutrients 2018, 10, 1325. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Nagpal, M.; Singh, M. Osteoblast-n-Osteoclast: Making Headway to Osteoporosis Treatment. Curr. Drug Targets 2020, 21, 1640–1651. [Google Scholar] [CrossRef]
- He, H.-P.; Gu, S. The PPAR-γ/SFRP5/Wnt/β-Catenin Signal Axis Regulates the Dexamethasone-Induced Osteoporosis. Cytokine 2021, 143, 155488. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Zhang, H.; Jie, Q.; Li, X.; Shi, Q.; Huang, Q.; Gao, B.; Han, Y.; Guo, K. Glucocorticoids: Dose-Related Effects on Osteoclast Formation and Function via Reactive Oxygen Species and Autophagy. Bone 2015, 79, 222–232. [Google Scholar] [CrossRef]
- Xavier, A.; Toumi, H.; Lespessailles, E. Animal Model for Glucocorticoid Induced Osteoporosis: A Systematic Review from 2011 to 2021. Int. J. Mol. Sci. 2021, 23, 377. [Google Scholar] [CrossRef]
- Hayman, A.R.; Bune, A.J.; Bradley, J.R.; Rashbass, J.; Cox, T.M. Osteoclastic Tartrate-Resistant Acid Phosphatase (Acp 5): Its Localization to Dendritic Cells and Diverse Murine Tissues. J. Histochem. Cytochem. 2000, 48, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, C.; Yue, J.; Liu, W. Broadening the Role of Osteocalcin in the Hypothalamic-Pituitary-Gonadal Axis. J. Endocrinol. 2021, 249, R43–R51. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.; Gravallese, E.M. Impact of Inflammation on the Osteoblast in Rheumatic Diseases. Curr. Osteoporos. Rep. 2014, 12, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, D.; Zhou, Z.; Zhu, L.; Hu, X.; Lu, J.; Shi, C.; Chen, F.; Chen, A. TNF-α Suppresses Osteogenic Differentiation of MSCs by Accelerating P2Y2 Receptor in Estrogen-Deficiency Induced Osteoporosis. Bone 2018, 117, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A. Influences of the IL-6 Cytokine Family on Bone Structure and Function. Cytokine 2021, 146, 155655. [Google Scholar] [CrossRef] [PubMed]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The Role of Gut Microbiota in Bone Homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Raveschot, C.; Coutte, F.; Frémont, M.; Vaeremans, M.; Dugersuren, J.; Demberel, S.; Drider, D.; Dhulster, P.; Flahaut, C.; Cudennec, B. Probiotic Lactobacillus Strains from Mongolia Improve Calcium Transport and Uptake by Intestinal Cells in Vitro. Food Res. Int. 2020, 133, 109201. [Google Scholar] [CrossRef]
- Ghanem, K.Z.; Badawy, I.H.; Abdel-Salam, A.M. Influence of Yoghurt and Probiotic Yoghurt on the Absorption of Calcium, Magnesium, Iron and Bone Mineralization in Rats. Milchwissenschaft 2004, 59, 472–475. [Google Scholar]
- Wan, J.; Hu, S.; Ni, K.; Chang, G.; Sun, X.; Yu, L. Characterisation of Fecal Soap Fatty Acids, Calcium Contents, Bacterial Community and Short-Chain Fatty Acids in Sprague Dawley Rats Fed with Different Sn-2 Palmitic Triacylglycerols Diets. PLoS ONE 2016, 11, e0164894. [Google Scholar] [CrossRef]
- Li, Y.; Lv, L.; Ye, J.; Fang, D.; Shi, D.; Wu, W.; Wang, Q.; Wu, J.; Yang, L.; Bian, X. Bifidobacterium Adolescentis CGMCC 15058 Alleviates Liver Injury, Enhances the Intestinal Barrier and Modifies the Gut Microbiota in D-Galactosamine-Treated Rats. Appl. Microbiol. Biotechnol. 2019, 103, 375–393. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chou, P.-L.; Yang, J.-C.; Chien, C.-T. Silicon-Containing Water Intake Confers Antioxidant Effect, Gastrointestinal Protection, and Gut Microbiota Modulation in the Rodents. PLoS ONE 2021, 16, e0248508. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, F.; Yin, X.; Cao, H.; Wang, X.; Liu, D.; Chen, J.; Chen, X. Bone Homeostasis and Gut Microbial-Dependent Signaling Pathways. J. Microbiol. Biotechnol. 2021, 31, 765–774. [Google Scholar] [CrossRef]
- Wei, X.; Ouyang, K.; Long, T.; Liu, Z.; Li, Y.; Qiu, Q. Dynamic Variations in Rumen Fermentation Characteristics and Bacterial Community Composition during in Vitro Fermentation. Fermentation 2022, 8, 276. [Google Scholar] [CrossRef]
- Kishi, M.; Fukaya, M.; Tsukamoto, Y.; Nagasawa, T.; Takehana, K.; Nishizawa, N. Enhancing Effect of Dietary Vinegar on the Intestinal Absorption of Calcium in Ovariectomized Rats. Biosci. Biotechnol. Biochem. 1999, 63, 905–910. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L. Spermidine Improves Gut Barrier Integrity and Gut Microbiota Function in Diet-Induced Obese Mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity Analysis of Gut Microbiota in Osteoporosis and Osteopenia Patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Qin, J.; Hao, Y.; Shi, Y.; Fu, L. Structural and Functional Changes of Gut Microbiota in Ovariectomized Rats and Their Correlations with Altered Bone Mass. Aging 2020, 12, 10736. [Google Scholar] [CrossRef]
- Ibrahim, M.; Anishetty, S. A Meta-Metabolome Network of Carbohydrate Metabolism: Interactions between Gut Microbiota and Host. Biochem. Biophys. Res. Commun. 2012, 428, 278–284. [Google Scholar] [CrossRef]
- Wawrzyniak, N.; Suliburska, J. Nutritional and Health Factors Affecting the Bioavailability of Calcium: A Narrative Review. Nutr. Rev. 2021, 79, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host–Microbe Interplay. Trends Endocrinol. Metab. 2020, 31, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New Aspects of Vitamin D Metabolism and Action—Addressing the Skin as Source and Target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Polysaccharide from Fermented Momordica charantia L. with Lactobacillus plantarum NCU116 Ameliorates Type 2 Diabetes in Rats. Carbohydr. Polym. 2018, 201, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kang, N.; Kim, E.-A.; Yang, H.-W.; Oh, J.-Y.; Fernando, I.P.S.; Kim, K.-N.; Ahn, G.; Jeon, Y.-J. Radioprotective Effects of a Polysaccharide Purified from Lactobacillus Plantarum-Fermented Ishige Okamurae against Oxidative Stress Caused by Gamma Ray-Irradiation in Zebrafish in Vivo Model. J. Funct. Foods 2017, 28, 83–89. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin Inhibits NADPH Oxidase (NOX)-1 Activation and NF-ΚB-Dependent Release of Inflammatory Mediators and Prevents the Increase of Epithelial Permeability in IL-1β-Exposed Caco-2 Cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; Kim, I.H. Difructose Dianhydride Improves Intestinal Calcium Absorption, Wound Healing, and Barrier Function. Sci. Rep. 2018, 8, 7813. [Google Scholar] [CrossRef]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic Acid Prevents Osteoporosis by Shp2/PI3K/Akt Pathway in Ovariectomized Rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef] [Green Version]
- Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mondelo, N.; Mastaglia, S.; Somoza, J.; Cardinali, D.P. Melatonin Effect on Bone Metabolism in Rats Treated with Methylprednisolone. J. Pineal Res. 2006, 40, 297–304. [Google Scholar] [CrossRef]










| Con | Mod | Fer | Mix | |
|---|---|---|---|---|
| Tb.Ar (mm2) | 8.373 ± 2.482 a | 6.422 ± 1.841 a | 8.239 ± 1.698 a | 7.705 ± 1.577 a |
| Tb.Pm (mm) | 283.528 ± 62.196 b | 169.300 ± 24.429 a | 233.897 ± 77.859 ab | 171.904 ± 21.450 a |
| BV/TV (%) | 65.613 ± 22.370 b | 32.837 ± 11.319 a | 53.187 ± 21.070 ab | 39.872 ± 8.866 ab |
| Tb.N (mm−1) | 13.296 ± 3.709 b | 5.288 ± 1.442 a | 8.646 ± 2.573 a | 5.369 ± 1.005 a |
| Tb.Sp (μm) | 28.644 ± 19.799 b | 136.691 ± 38.530 a | 59.987 ± 32.019 b | 119.035 ± 42.584 a |
| Genus | Relative Abundance (%) | |||
|---|---|---|---|---|
| Con | Mod | Fer | Mix | |
| Lactobacillus | 18.2040 | 9.7956 | 12.6682 | 11.1472 |
| Allobaculum | 4.0409 | 3.7342 | 14.6471 | 5.5833 |
| unclassified_f__Lachnospiraceae | 7.5366 | 9.3171 | 3.4125 | 5.4050 |
| Lachnospiraceae_NK4A136_group | 5.5491 | 7.0952 | 0.7910 | 2.5797 |
| UCG-005 | 1.4815 | 0.8556 | 3.7246 | 2.8082 |
| norank_f__Lachnospiraceae | 1.9242 | 2.9739 | 0.6871 | 1.3591 |
| Lachnoclostridium | 2.0744 | 2.3404 | 0.9932 | 1.0434 |
| Blautia | 1.9770 | 0.2813 | 3.3311 | 0.7532 |
| Christensenellaceae_R-7_group | 1.2659 | 0.9645 | 1.7921 | 1.3322 |
| Ruminococcus | 0.9681 | 1.6450 | 0.4389 | 1.8254 |
| unclassified_f__Oscillospiraceae | 0.6484 | 2.5963 | 0.3425 | 1.2051 |
| norank_f__Muribaculaceae | 0.7546 | 1.0063 | 0.2851 | 1.7442 |
| norank_f__Oscillospiraceae | 0.5453 | 1.5403 | 0.1912 | 0.9099 |
| Roseburia | 1.1361 | 1.3167 | 0.1218 | 0.5946 |
| norank_f__Erysipelotrichaceae | 1.0560 | 0.4637 | 1.1465 | 0.4231 |
| Pathways | Relative Abundance (%) | |||
|---|---|---|---|---|
| Con | Mod | Fer | Mix | |
| KEGG: Carbohydrate metabolism | 10.4620 | 10.1761 | 10.5526 | 10.3166 |
| KEGG: Amino acid metabolism | 6.5946 | 6.8001 | 6.7853 | 6.8195 |
| KEGG: Metabolism of cofactors and vitamins | 3.7919 | 3.8326 | 3.7920 | 3.8840 |
| KEGG: Cell motility | 1.0894 | 1.5041 | 0.8104 | 1.0483 |
| KEGG: Cell growth and death | 0.7518 | 0.7693 | 0.7483 | 0.7595 |
| KEGG: Endocrine system | 0.6319 | 0.6315 | 0.6638 | 0.6498 |
| KEGG: Aging | 0.2654 | 0.2752 | 0.2680 | 0.2878 |
| KEGG: Immune system | 0.2660 | 0.2925 | 0.2607 | 0.2754 |
| Differential Metabolite | FC(Fer/Mix) | p Value | Regulated |
|---|---|---|---|
| Indicaxanthin | 1.2746 | 0.000000453 | up |
| Chlorogenic Acid | 1.2231 | 0.01733 | up |
| 3-Hydroxymelatonin | 1.1687 | 0.0002299 | up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Cheng, J.; Liu, L.; Luo, J.; Peng, X. Lactobacillus acidophilus (LA) Fermenting Astragalus Polysaccharides (APS) Improves Calcium Absorption and Osteoporosis by Altering Gut Microbiota. Foods 2023, 12, 275. https://doi.org/10.3390/foods12020275
Zhou J, Cheng J, Liu L, Luo J, Peng X. Lactobacillus acidophilus (LA) Fermenting Astragalus Polysaccharides (APS) Improves Calcium Absorption and Osteoporosis by Altering Gut Microbiota. Foods. 2023; 12(2):275. https://doi.org/10.3390/foods12020275
Chicago/Turabian StyleZhou, Junhua, Jing Cheng, Liu Liu, Jianming Luo, and Xichun Peng. 2023. "Lactobacillus acidophilus (LA) Fermenting Astragalus Polysaccharides (APS) Improves Calcium Absorption and Osteoporosis by Altering Gut Microbiota" Foods 12, no. 2: 275. https://doi.org/10.3390/foods12020275