Effect of Physical Modifications on Physicochemical and Functional Properties of Walnut Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Different Physically Modified Walnut Protein
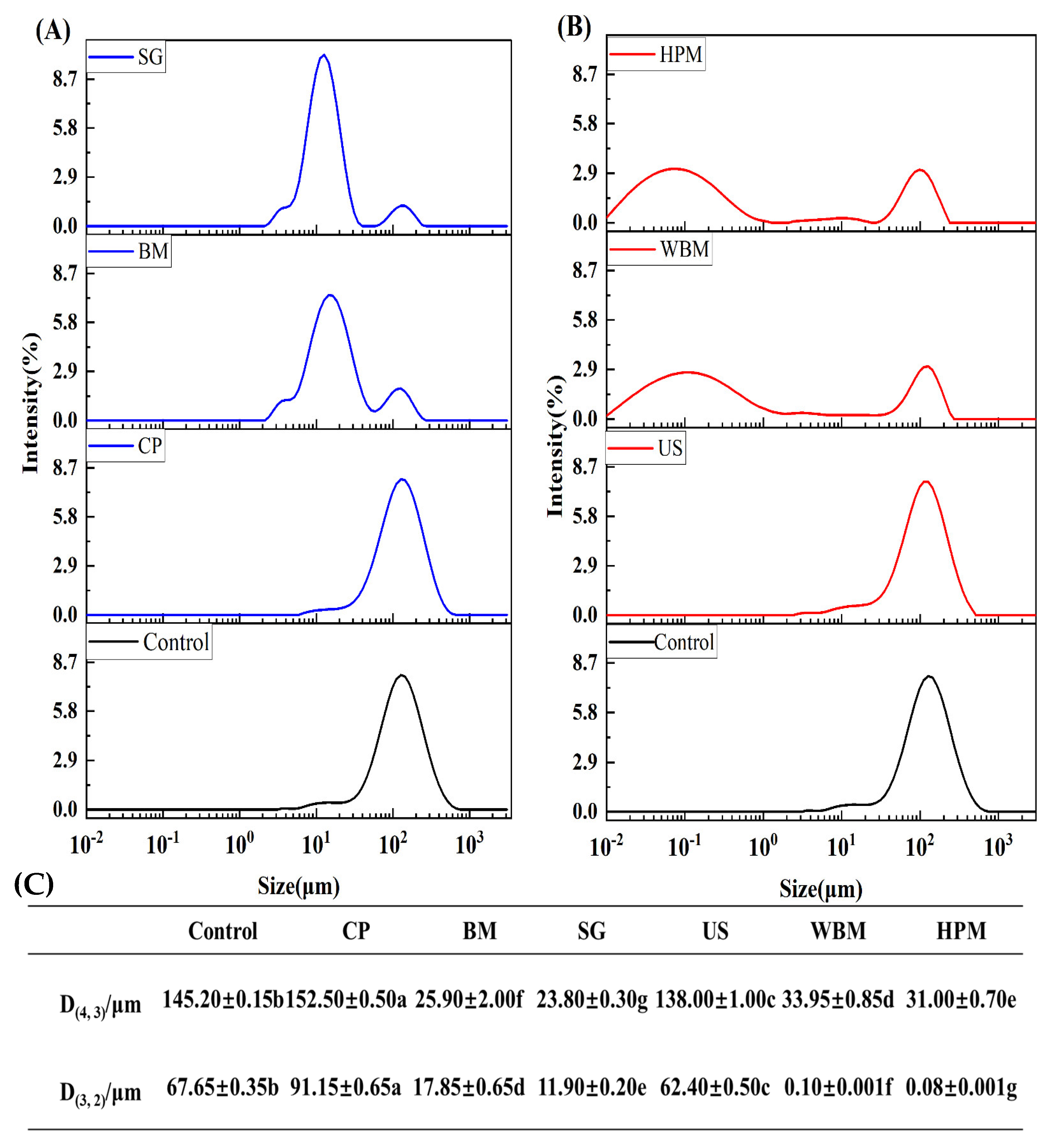
2.3. Measurement of Size Distribution
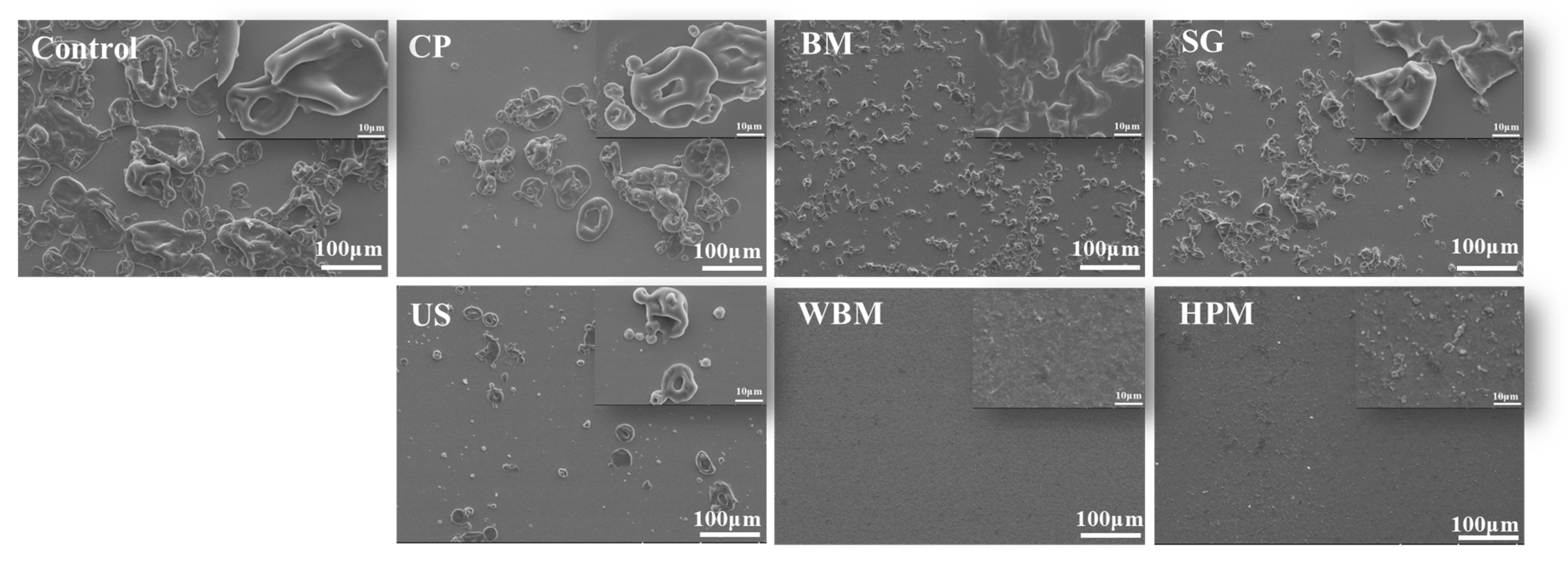
2.4. Scanning Electron Microscopy
2.5. Surface Hydrophobicity
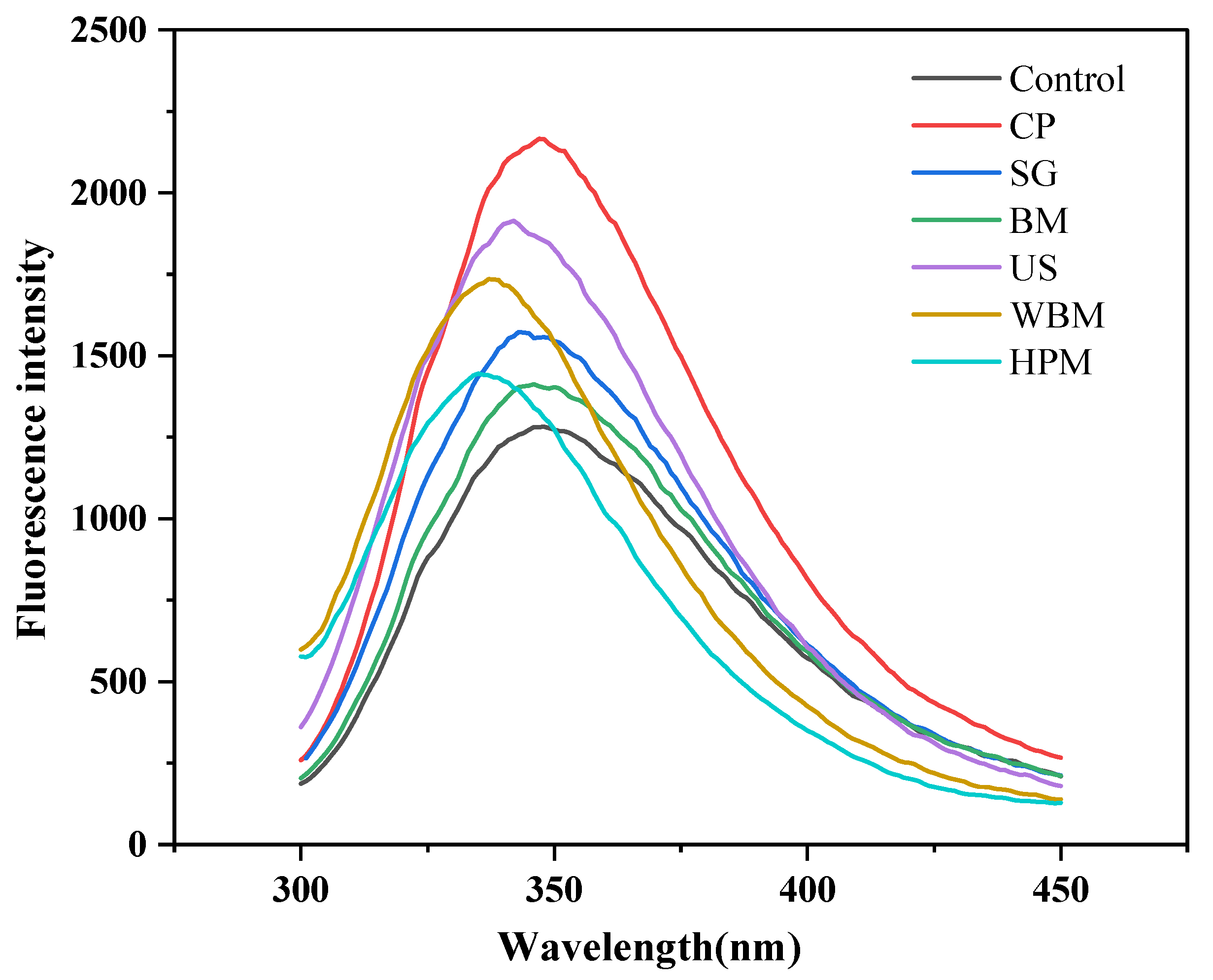
2.6. Intrinsic Fluorescence
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
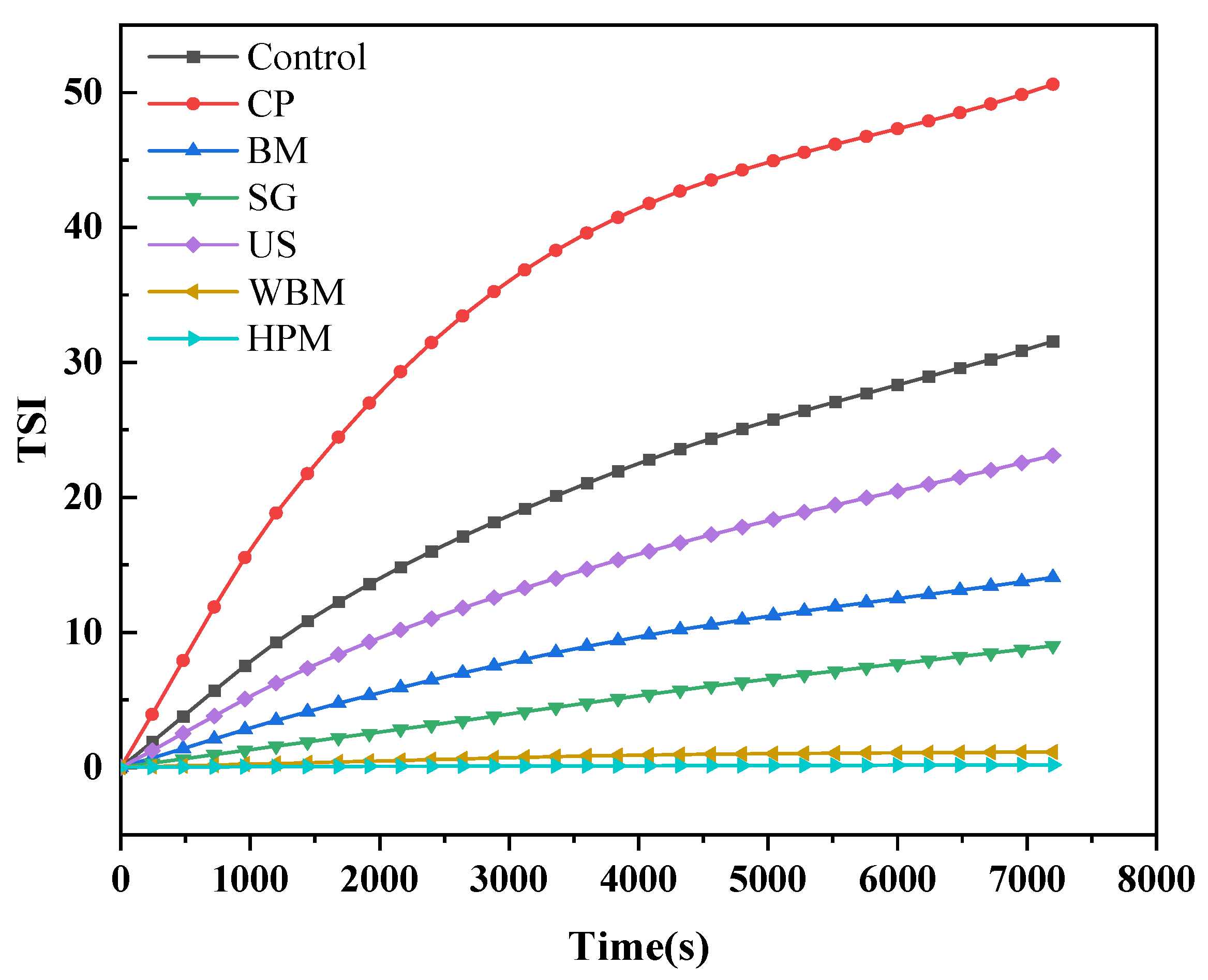
2.8. Determination of the Stability of the Walnut Protein Solution
2.9. Solubility
2.10. Foaming Capacity and Foaming Stability
2.11. Emulsification Activity Index and Emulsion Stability Index
2.12. Observation of the Morphology of Emulsions Stabilized by Walnut Protein
2.13. Statistical Analysis
3. Results and Discussion
3.1. Impact of Physical Modifications on the Physicochemical Properties of Walnut Protein
3.1.1. Size Distribution
3.1.2. Microstructure
3.1.3. Surface Hydrophobicity
3.1.4. Intrinsic Fluorescence
3.1.5. Secondary Structure
3.1.6. Physical Stability in Aqueous Solution
3.2. Impact of Physical Modifications on the Functional Properties of Walnut Protein
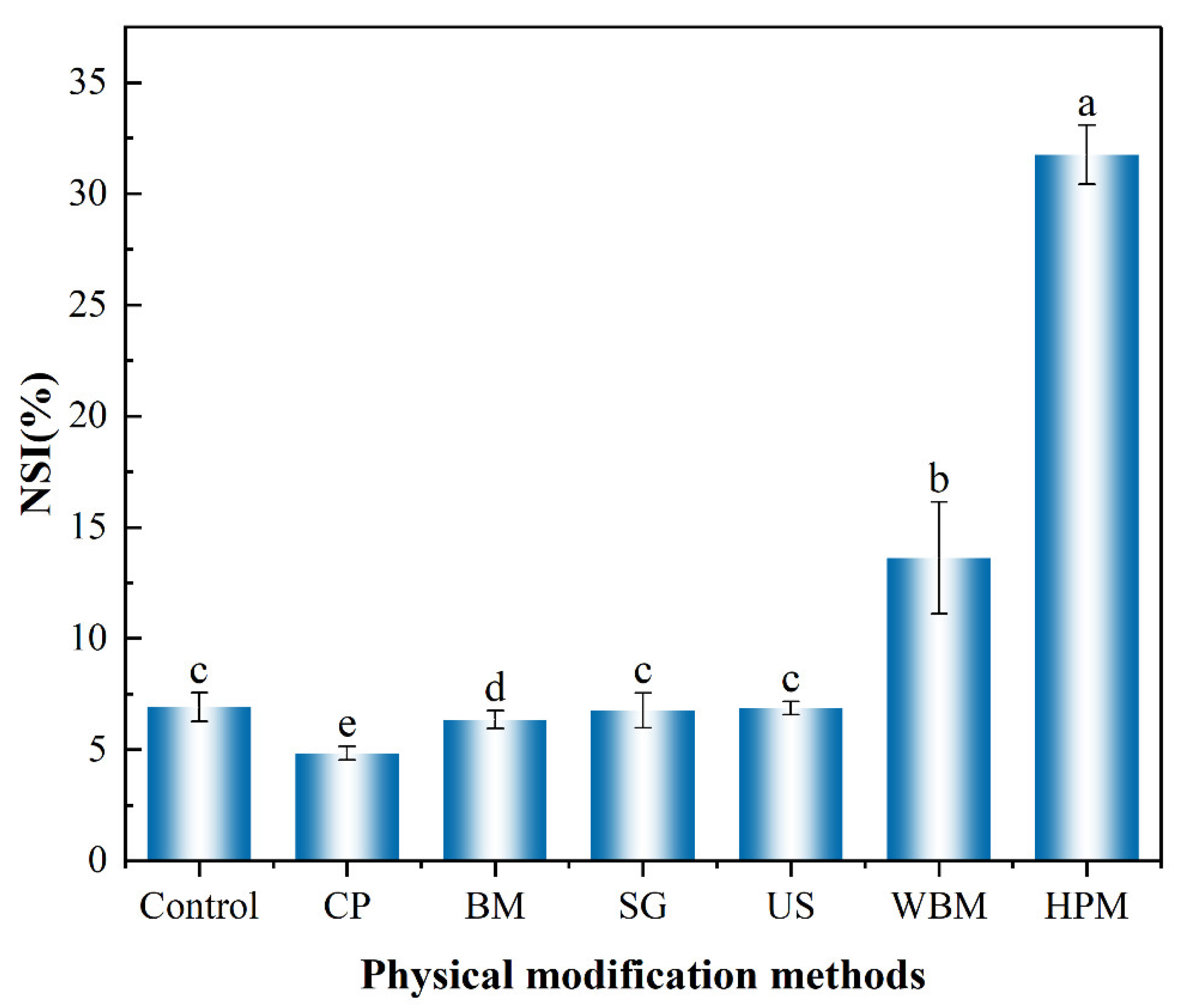
3.2.1. Solubility
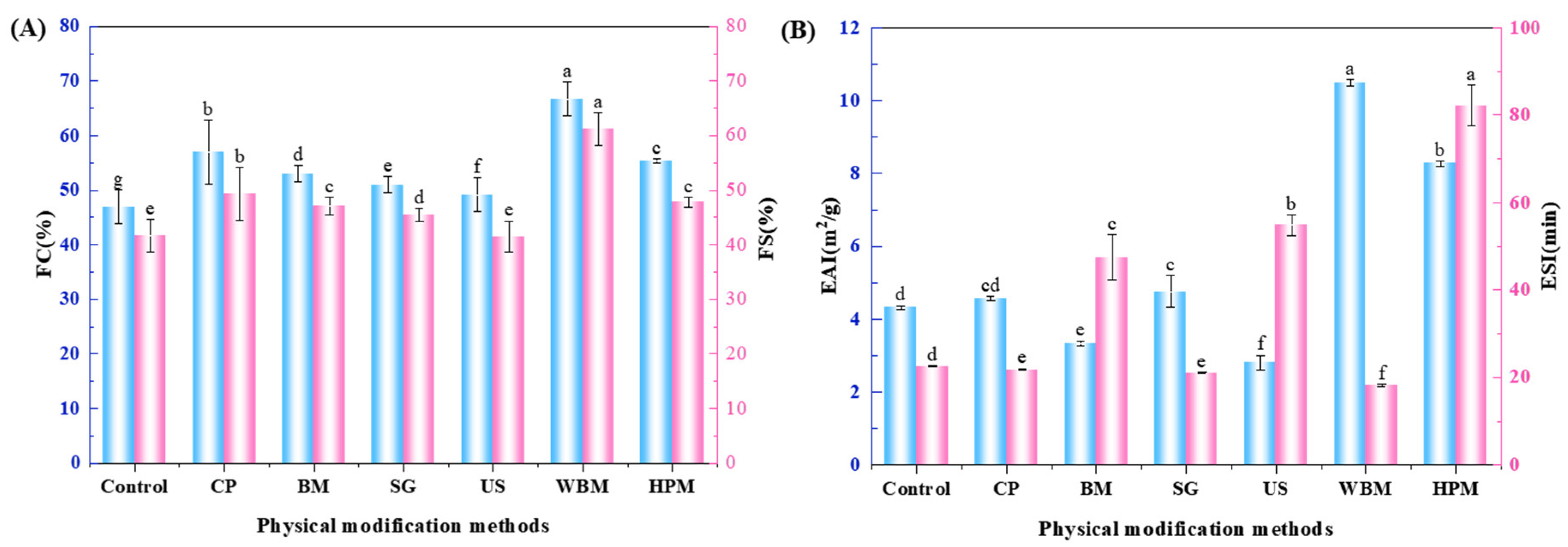
3.2.2. Foaming Properties
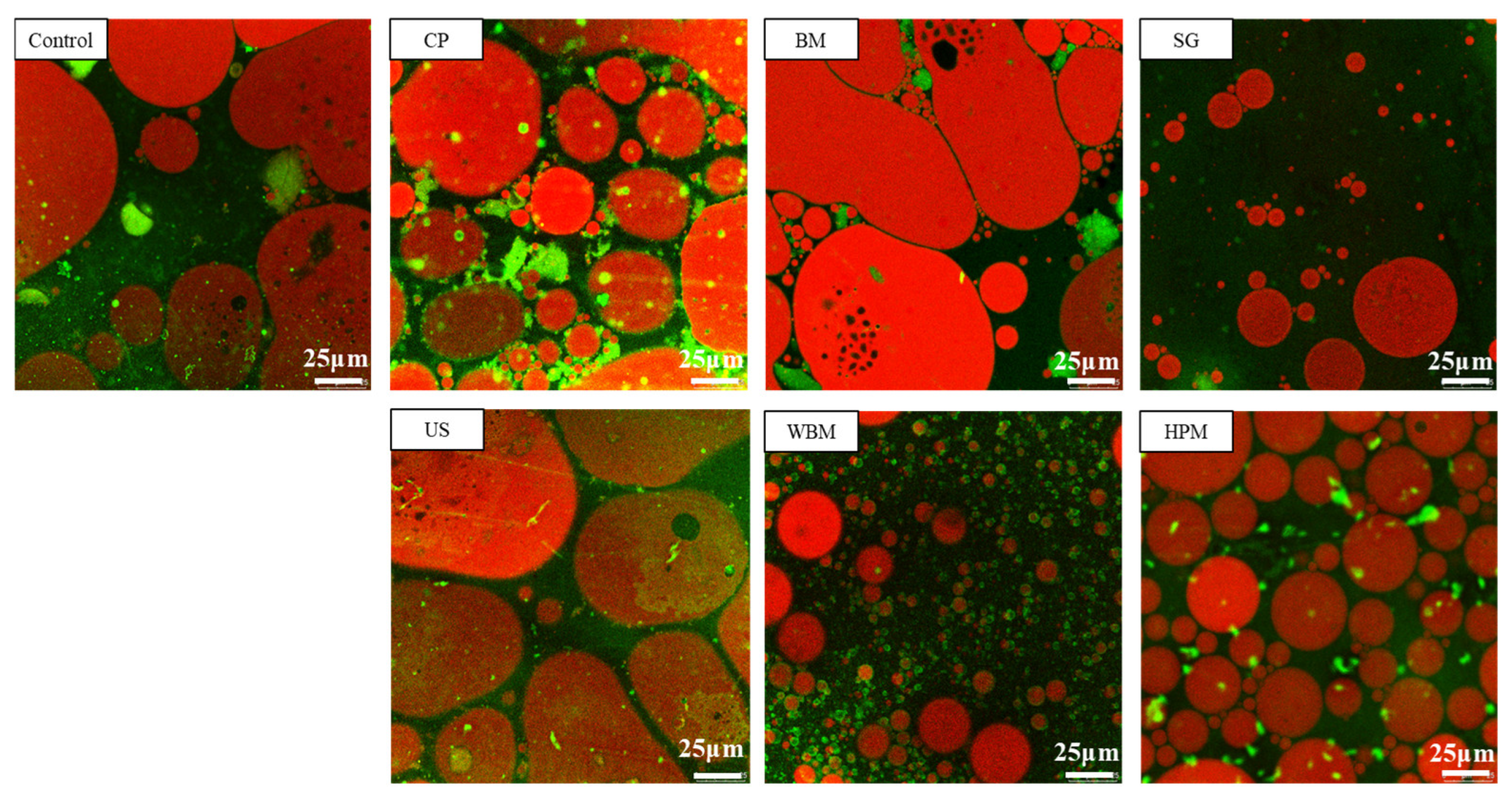
3.2.3. Emulsifying Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rabadán, A.; Pardo, J.E.; Gómez, R.; Álvarez-Ortí, M. Evaluation of physical parameters of walnut and walnut products obtained by cold pressing. LWT Food Sci. Technol. 2018, 91, 308–314. [Google Scholar] [CrossRef]
- Shi, A.; Jiao, B.; Liu, H.; Zhu, S.; Shen, M.; Feng, X.; Hu, H.; Liu, L.; Faisal, S.; Wang, Q.; et al. Effects of proteolysis and transglutaminase crosslinking on physicochemical characteristics of walnut protein isolate. LWT Food Sci. Technol. 2018, 97, 662–667. [Google Scholar] [CrossRef]
- Sze-Tao, K.W.C.; Sathe, S.K. Walnuts (Juglans regia L.): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agric. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.; Mcclements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Liu, Z.; McClements, D.J.; Shi, A.; Zhi, L.; Tian, Y.; Jiao, B.; Liu, H.; Wang, Q. Janus particles: A review of their applications in food and medicine. Crit. Rev. Food Sci. Nutr. 2022, 1–12. [Google Scholar] [CrossRef]
- Hugo, D.V.; Michel, M.; Jean-Michel, S.; Joël, A.; Laurent, C.; Sofiane, G.; Denis, L.; Sofiane, B.; Eric, L.; Gilles, T. Small-scale food process engineering—Challenges and perspectives. Innov. Food Sci. Emerg. Technol. 2018, 46, 122–130. [Google Scholar]
- Dong, S.; Gao, A.; Xu, H.; Chen, Y. Effects of Dielectric Barrier Discharges (DBD) Cold Plasma Treatment on Physicochemical and Structural Properties of Zein Powders. Food Bioprocess Technol. 2017, 10, 434–444. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Wu, T.; Liang, B.; Shi, C.; Zhang, M. Effect of superfine grinding on the structural and physicochemical properties of whey protein and applications for microparticulated proteins. Food Sci. Biotechnol. 2015, 24, 1637–1643. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Lu, X.; Zhang, C.; Hua, Y.; Chen, Y. Effect of high-speed shearing treatment on dehulled walnut proteins. LWT Food Sci. Technol. 2019, 116, 108500. [Google Scholar] [CrossRef]
- Qin, Z.; Guo, X.; Lin, Y.; Chen, J.; Liao, X.; Hu, X.; Wu, J. Effects of high hydrostatic pressure on physicochemical and functional properties of walnut (Juglans regia L.) protein isolate. J. Sci. Food Agric. 2013, 93, 1105–1111. [Google Scholar] [CrossRef]
- Shi, L.; Yang, X.; Gong, T.; Hu, C.; Shen, Y.; Meng, Y. Ultrasonic treatment improves physical and oxidative stabilities of walnut protein isolate-based emulsion by changing protein structure. LWT Food Sci. Technol. 2023, 173, 114269. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, A.; Wu, C.; Hei, X.; Li, S.; Liu, H.; Jiao, B.; Adhikari, B.; Wang, Q. Natural amphiphilic shellac nanoparticle-stabilized novel pickering emulsions with droplets and bi-continuous structures. ACS Appl. Mater. Interfaces 2022, 14, 57350–57361. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Goli, M.; Toghyani, M. The Effects of Phosphorylation and Microwave Treatment on the Functional Characteristics of Freeze-Dried Egg White Powder. Foods 2022, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, T.; Chen, Q.; Liu, H.; Kaplan, D.L.; Wang, Q. Application of transglutaminase modifications for improving protein fibrous structures from different sources by high-moisture extruding. Food Res. Int. 2023, 166, 112623. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Liu, H.; Li, T.; Wang, Q. Mechanism of high-moisture extruded protein fibrous structure formation based on the interactions among pea protein, amylopectin, and stearic acid. Food Hydrocoll. 2023, 136, 108254. [Google Scholar] [CrossRef]
- Li, J.; Shi, A.; Liu, H.; Hu, H.; Wang, Q.; Adhikari, B.; Jiao, B.; Pignitter, M. Effect of Hydrothermal Cooking Combined with High-Pressure Homogenization and Enzymatic Hydrolysis on the Solubility and Stability of Peanut Protein at Low pH. Foods 2022, 11, 1289. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y. Composition, Structure and Functional Properties of Protein Concentrates and Isolates Produced from Walnut (Juglans regia L.). Int. J. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Q.; Mao, X. Effects of Oxidation Modification by Malondialdehyde on the Structure and Functional Properties of Walnut Protein. Foods 2022, 11, 2432. [Google Scholar] [CrossRef]
- Li, S.; Jiao, B.; Faisal, S.; Zhang, Y.; Wu, C.; Li, W.; Shi, A.; Liu, H.; Wang, Q. 50/50 oil/water emulsion stabilized by pea protein isolate microgel particles/xanthan gum complexes and co-emulsifiers. Food Hydrocoll. 2022, 134, 108078. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Zhu, G.; Luo, S.; Zhang, D.; Liu, F.; Shen, Y. Modification of the structural and functional properties of wheat gluten protein using a planetary ball mill. Food Chem. 2021, 363, 130251. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, Z.; He, P.; Wang, M. Microfluidization treatment improve the functional and physicochemical properties of transglutaminase cross-linked groundnut arachin and conarachin. Food Hydrocoll. 2022, 130, 107723. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Zhang, J.; Liu, Y.; Zhou, T.; Chi, S.; Bi, C. High-pressure homogenization modified chickpea protein: Rheological properties, thermal properties and microstructure. J. Food Eng. 2022, 335, 111196. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Z.; Deng, Y.; Wei, Z.; Zhang, Y.; Tang, X.; Liu, G.; Zhou, P.; Zhao, Z.; Zhang, M.; et al. High-pressure homogenization: A potential technique for transforming insoluble pea protein isolates into soluble aggregates. Food Chem. 2022, 397, 133684. [Google Scholar] [CrossRef]
- Kuzminova, A.; Shelemin, A.; Kylian, O.; Choukourov, A.; Valentova, H.; Krakovsky, I.; Nedbal, J.; Slavínská, D.; Biederman, H. Study of the effect of atmospheric pressure air dielectric barrier discharge on nylon 6,6 foils. Polym. Degrad. Stab. 2014, 110, 378–388. [Google Scholar] [CrossRef]
- Mehr, H.M.; Koocheki, A. Effect of atmospheric cold plasma on structure, interfacial and emulsifying properties of Grass pea (Lathyrus sativus L.) protein isolate. Food Hydrocoll. 2020, 106, 105899. [Google Scholar] [CrossRef]
- Tan, M.; Xu, J.; Gao, H.; Yu, Z.; Liang, J.; Mu, D.; Li, X.; Zhong, X.; Luo, S.; Zhao, Y.; et al. Effects of combined high hydrostatic pressure and pH-shifting pretreatment on the structure and emulsifying properties of soy protein isolates. J. Food Eng. 2021, 306, 110622. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Guerrero, P.; Beatty, E.; Kerry, J.P.; Caba, K.D.L. Extrusion of soy protein with gelatin and sugars at low moisture content. J. Food Eng. 2012, 110, 53–59. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Jarpa-Parra, M.; Bamdad, F.; Tian, Z.; Zeng, H.; Temelli, F.; Chen, L. Impact of pH on molecular structure and surface properties of lentil legumin-like protein and its application as foam stabilizer. Colloids Surfaces B 2015, 132, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of Dielectric Barrier Discharge (DBD) Cold Plasma Treatment on Physicochemical and Functional Properties of Peanut Protein. Food Bioprocess Technol. 2018, 11, 344–354. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Bourke, P.; Cullen, P.J. Zein film: Effects of dielectric barrier discharge atmospheric cold plasma. J. Appl. Polym. Sci. 2014, 131, 9541–9546. [Google Scholar] [CrossRef]
- Sharafodin, H.; Soltanizadeh, N. Potential Application of DBD Plasma Technique for Modifying Structural and Physicochemical Properties of Soy Protein Isolate. Food Hydrocoll. 2021, 122, 107077. [Google Scholar] [CrossRef]
- Perez-Andres, J.M.A.; CarlosCullen, P.J.; Tiwari, B.K. Effect of cold plasma on the techno-functional properties of animal protein food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 58, 102205. [Google Scholar] [CrossRef]
- Bigelow, C.C. On the average hydrophobicity of proteins and the relation between it and protein structure. J. Theor. Biol. 1967, 16, 187–211. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of pH on the inter-relationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Liu, H.; Bai, W.; He, L.; Li, X.; Wang, Q. Degradation mechanism of Saccharomyces cerevisiae β-D-glucan by ionic liquid and dynamic high pressure microfluidization. Carbohydr. Polym. 2020, 241, 116123. [Google Scholar] [CrossRef]
- Narsimhan, G.; Xiang, N. Role of Proteins on Formation, Drainage, and Stability of Liquid Food Foams. Annu. Rev. Food Sci. Technol. 2018, 9, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Bai, R.; Lu, F.; Zhang, T.; Gao, Z.; Zhao, S.; Zhu, M.; Ma, H. Effects of high pressure homogenization on the solubility, foaming, and gel properties of soy 11S globulin. Food Hydrocoll. 2021, 124, 107261. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Euston, S.R.; Finnigan, S.R.; Hirst, R.L. Aggregation kinetics of heated whey protein-stabilized emulsions. Food Hydrocoll. 2000, 14, 155–161. [Google Scholar] [CrossRef]
- Arredondo-Parada, I.; Torres-Arreola, W.; Suárez-Jiménez, G.M.; Ramírez-Suárez, J.C.; Juárez-Onofre, J.E.; Rodríguez-Félix, F.; Marquez-Rios, E. Effect of ultrasound on physicochemical and foaming properties of a protein concentrate from giant squid (Dosidicus gigas) mantle. LWT Food Sci. Technol. 2020, 121, 108954. [Google Scholar] [CrossRef]
- Lee, S.H.; Lefevre, T.; Subirade, M.; Paquin, P. Effects of ultra-high pressure homogenization on the properties and structure of interfacial protein layer in whey protein-stabilized emulsion. Food Chem. 2009, 113, 191–195. [Google Scholar] [CrossRef]
| Samples | H0 | λmax (nm) |
|---|---|---|
| Control | 1226.93 ± 16.74 e | 347.67 ± 2.39 a |
| CP | 514.39 ± 9.61 f | 347.93 ± 0.68 a |
| BM | 1287.37 ± 11.51 d | 344.00 ± 0.36 c |
| SG | 548.85 ± 7.41 f | 346.47 ± 0.12 b |
| US | 1543.27 ± 6.20 c | 343.17 ± 0.85 c |
| WBM | 1867.67 ± 16.11 b | 338.50 ± 1.08 d |
| HPM | 3731.57 ± 57.88 a | 336.67 ± 0.94 e |
| Samples | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| Control | 32.63 ± 1.39 b | 23.61 ± 3.26 c | 20.78 ± 2.58 b | 22.99 ± 3.80 a |
| CP | 19.94 ± 1.44 e | 37.26 ± 1.14 b | 27.91 ± 1.28 a | 14.88 ± 0.76 cd |
| BM | 25.18 ± 4.50 cd | 34.53 ± 3.25 b | 26.64 ± 5.60 a | 13.65 ± 0.49 cd |
| SG | 20.13 ± 1.81 e | 44.26 ± 3.95 a | 18.12 ± 2.72 b | 17.48 ± 2.82 bc |
| US | 40.18 ± 2.57 a | 22.53 ± 2.71 c | 27.17 ± 1.54 a | 10.12 ± 2.34 d |
| WBM | 22.88 ± 2.12 de | 33.65 ± 1.43 b | 21.03 ± 2.06 b | 22.43 ± 2.11 ab |
| HPM | 28.13 ± 2.72 c | 36.85 ± 1.66 b | 19.65 ± 1.22 b | 15.36 ± 4.24 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Z.; Hei, X.; Wu, C.; Ma, X.; Hu, H.; Jiao, B.; Zhu, J.; Adhikari, B.; Wang, Q.; et al. Effect of Physical Modifications on Physicochemical and Functional Properties of Walnut Protein. Foods 2023, 12, 3709. https://doi.org/10.3390/foods12193709
Li S, Liu Z, Hei X, Wu C, Ma X, Hu H, Jiao B, Zhu J, Adhikari B, Wang Q, et al. Effect of Physical Modifications on Physicochemical and Functional Properties of Walnut Protein. Foods. 2023; 12(19):3709. https://doi.org/10.3390/foods12193709
Chicago/Turabian StyleLi, Shanshan, Zhe Liu, Xue Hei, Chao Wu, Xiaojie Ma, Hui Hu, Bo Jiao, Jinjin Zhu, Benu Adhikari, Qiang Wang, and et al. 2023. "Effect of Physical Modifications on Physicochemical and Functional Properties of Walnut Protein" Foods 12, no. 19: 3709. https://doi.org/10.3390/foods12193709






