Impact of Fermentation Pretreatment on Drying Behaviour and Antioxidant Attributes of Broccoli Waste Powdered Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Preconditioning
2.2. Fermentation with Lactiplantibacillus Plantarum
2.3. Dehydration Conditions and Powders Obtaining
2.4. Drying and Drying Rate Curves—Modelling of the Thin-Layer Drying Curves
2.5. Analytical Determinations
2.5.1. Physicochemical Properties
2.5.2. Antioxidant Properties
2.5.3. Microbial Counts
2.6. Statistical Analysis
3. Results and Discussion
3.1. Impact of Fermentation on Broccoli Wastes Characteristics
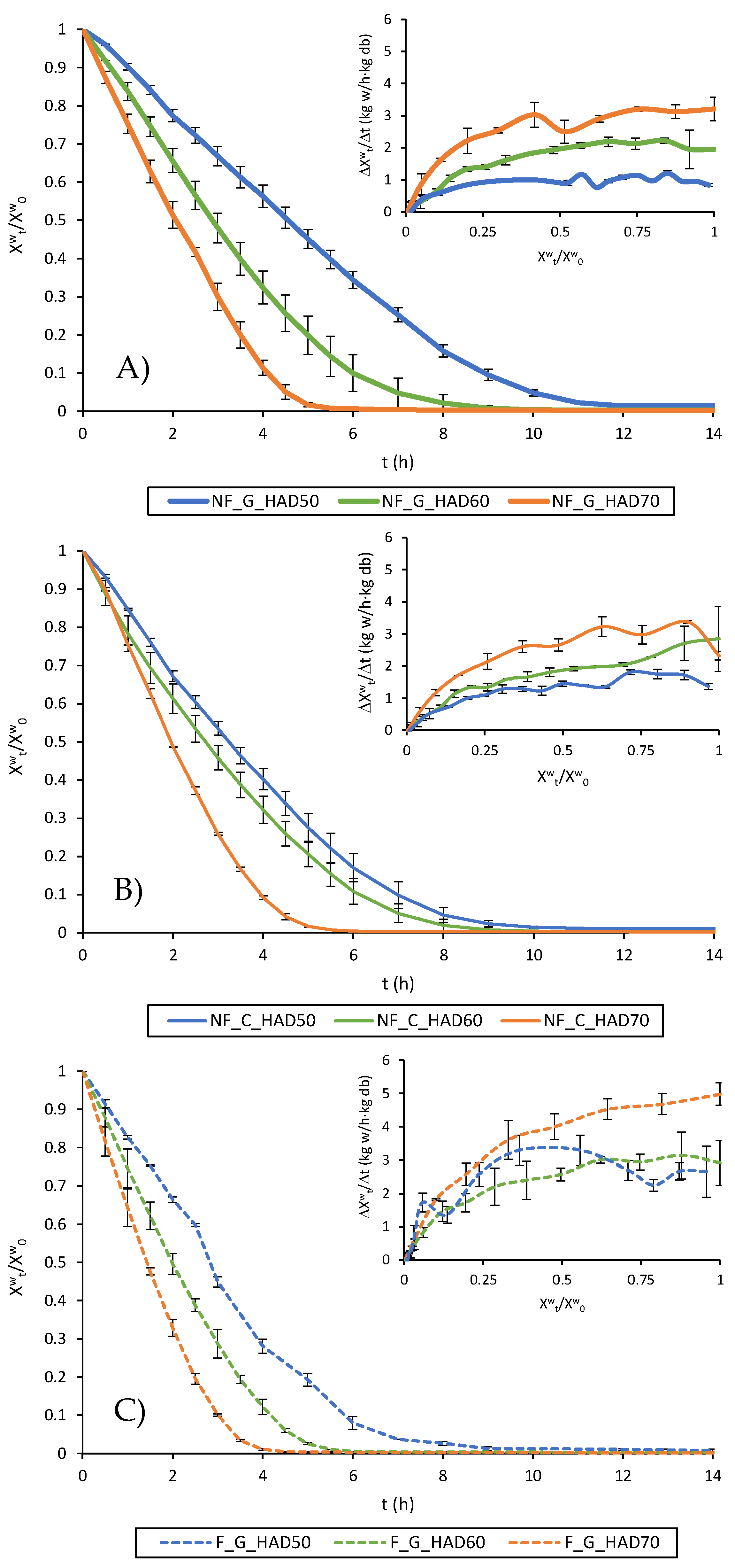
3.2. Drying Curves and Drying Rate Curves
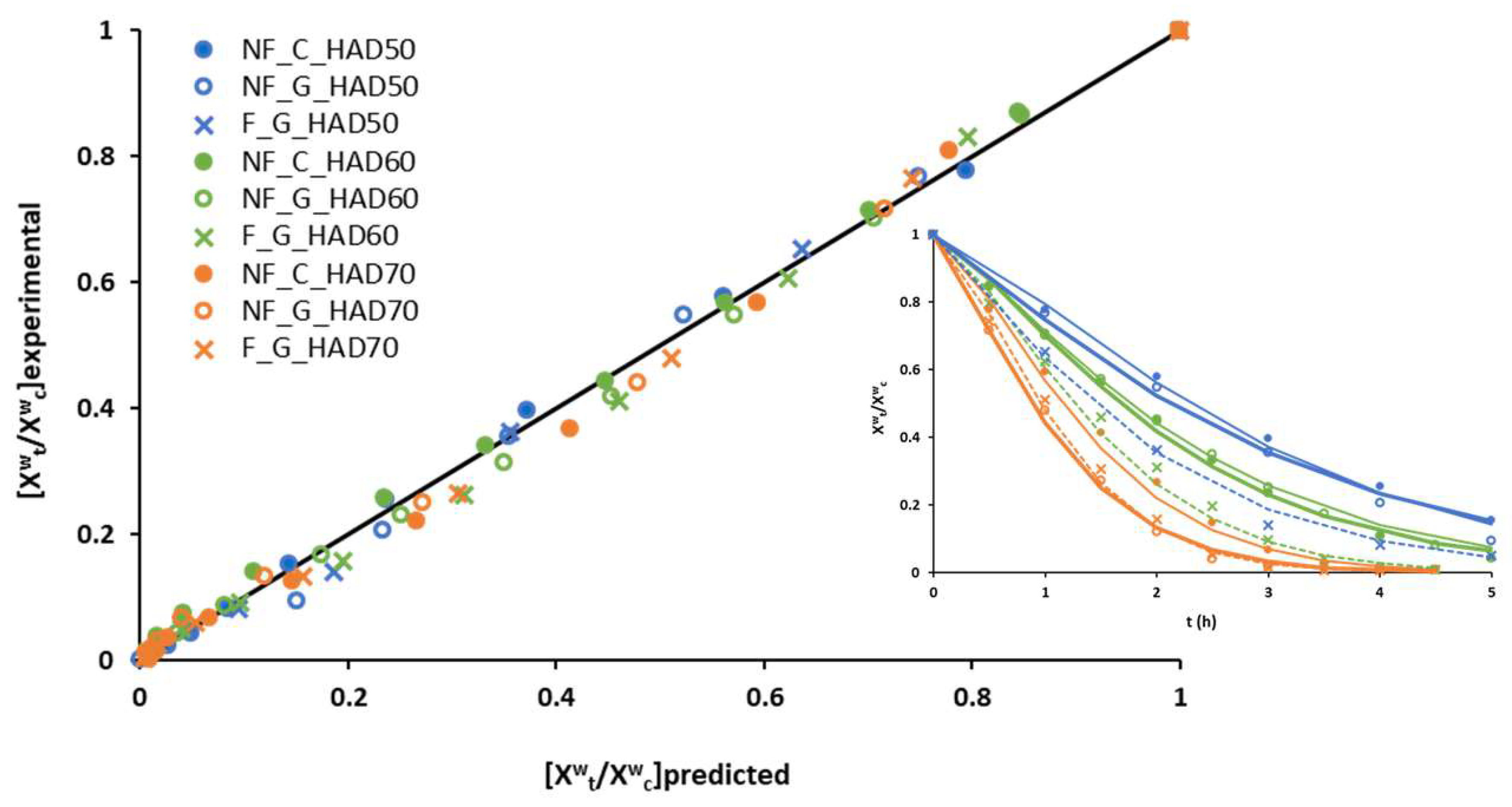
3.3. Modelling of the Drying of Disrupted Broccoli Stems in Thin Layers
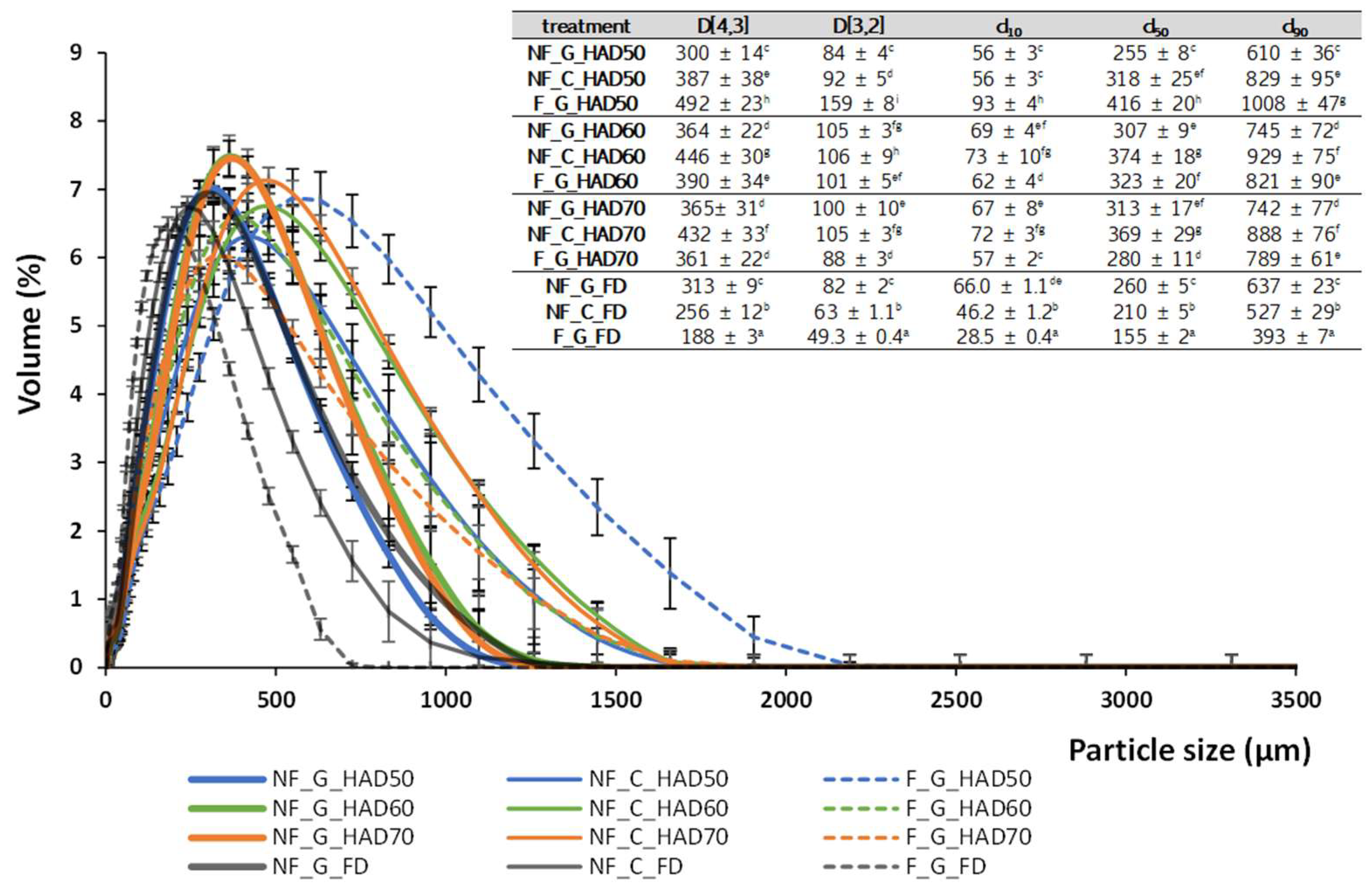
3.4. Characterization of Broccoli Stem Powdered Products
3.4.1. Moisture Content, Water Activity, Antioxidant Properties and Particle Size Characteristics
3.4.2. Potential Probiotic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO and the Sustainable Development Goals. Available online: https://www.fao.org/about/strategy-programme-budget/strategic-framework/fao-sdg/en/ (accessed on 1 December 2022).
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; pp. 1–37. [Google Scholar]
- Sepúlveda, L.; Contreras, E.; Cerro, D.; Quintulén, L. Technical feasibility of natural antioxidant recovery from the mixture of the inedible fractions of vegetables produced in a wholesale market. CYTA J. Food 2021, 19, 418–428. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Santos, M.C.P.; Moro, T.M.A.; Basto, G.J.; Andrade, R.M.S.; Gonçalves, É.C.B.A. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Rusell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Toxqui-Terán, A.; Espinosa-Solis, V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica oleracea var. Italica) Stalk and Floret Juice Powders. Molecules 2021, 26, 1973. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Ediriweera, M.K.; Boo, K.H.; Kim, C.S.; Cho, S.K. Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets. Antioxidants 2021, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Mrkìc, V.; Cocci, E.; Dalla Rosa, M.; Sacchetti, G. Effect of drying conditions on bioactive compounds and antioxidant activity of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2006, 86, 1559–1566. [Google Scholar] [CrossRef]
- Dziki, D.; Habza-Kowalska, E.; Gawlik-Dziki, U.; Miś, A.; Różyło, R.; Krzysiak, Z.; Hassoon, W.H. Drying Kinetics, Grinding Characteristics, and Physicochemical Properties of Broccoli Sprouts. Processes 2020, 8, 97. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QC/visualize (accessed on 1 December 2022).
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Williams, P.A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- Seguí, L.; Bas-Bellver, C.; Barrera, C.; Betoret, N. Valorization of Persimmon (Diospyros kaki) Wastes to Be Used as Functional Ingredients. In Mediterranean Fruits Bio-Wastes; Ramadan, M.F., Farag, M.A., Eds.; Springer Nature: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Md Salim, N.S.; Gariépy, Y.; Raghavan, V. Hot Air Drying and Microwave-Assisted Hot Air Drying of Broccoli Stalk Slices (Brassica oleracea L. Var. Italica). J. Food Process Preserv. 2016, 41, e12905. [Google Scholar] [CrossRef]
- Zura-Bravo, L.; Rodriguez, A.; Stucken, K.; Vega-Gálvez, A. Drying kinetics of probiotic-impregnated murta (Ugni molinae T.) berries. J. Food Sci. Technol. 2019, 56, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, I. Effect of blanching temperature and dipping time on drying time of broccoli. Food Sci. Technol. Int. 2014, 20, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods 2022, 11, 3663. [Google Scholar] [CrossRef]
- Dovene, A.K.; Wang, L.; Bokhary, S.U.F.; Madebo, M.P.; Zheng, Y.; Jin, P. Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars. Foods 2019, 8, 674. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Effect of Processing and In Vitro Digestion on Bioactive Constituents of Powdered IV Range Carrot (Daucus carota, L.) Wastes. Foods 2023, 12, 731. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Gbashi, S.; Phoku, J.Z.; Kayitesi, E. Fermented Pulse-Based Food Products in Developing Nations as Functional Foods and Ingredients. In Functional Food—Improve Health through Adequate Food; Chavarri Hueda, M., Ed.; IntechOpen: London, UK, 2017; pp. 77–109. [Google Scholar] [CrossRef]
- Hutkins, R.W. Microbiology and Technology of Fermented Foods; John Wiley & Sons: London, UK, 2018. [Google Scholar]
- Onimawo, I.A.; Nmerole, E.C.; Idoko, P.I.; Akubor, P.I. Effects of fermentation on nutrient content and some functional properties of pumpkin seed (Telfaria occidentalis). Plant Foods Hum. Nutr. 2003, 58, 1–9. [Google Scholar] [CrossRef]
- Onweluzo, J.C.; Nwabugwu, C.C. Fermentation of millet (Pennisetum americanum) and pigeon pea (Cajanus cajan) seeds for flour production: Effects on composition and selected functional properties. Pak. J. Nutr. 2009, 8, 737–744. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Ifesan, B.O.T. Changes in nutrient and antinutritional contents of sesame seeds during fermentation. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 2407–2410. [Google Scholar]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A.I. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Guan, N.; He, X.; Wang, S.; Liu, F.; Huang, Q.; Fu, X.; Chen, T.; Zhang, B. Cell Wall Integrity of Pulse Modulates the In Vitro Fecal Fermentation Rate and Microbiota Composition. J. Agric. Food Chem. 2020, 68, 1091–1100. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; McCartney, A.L.; Gibson, G.R. An in vitro study of the probiotic potential of a bile-salt-hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl. Environ. Microbiol. 2003, 69, 4743–4752. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Lee, N.K.; Paik, H.D. Bioconversion Using Lactic Acid Bacteria: Ginsenosides, GABA, and Phenolic Compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef]
- Cai, Y.X.; Wang, J.H.; McAuley, C.; Augustin, M.A.; Terefe, N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Pobiega, K.; Nikodem, A.; Gramza-Michałowska, A. Sustainable Production and Characteristics of Dried Fermented Vegetables. Fermentation 2022, 8, 659. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable-fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Bas-Bellver, C.; Andrés, C.; Seguí, L.; Barrera, C.; Jiménez-Hernández, N.; Artacho, A.; Betoret, N.; Gosalbes, M.J. Valorization of Persimmon and Blueberry Byproducts to Obtain Functional Powders: In Vitro Digestion and Fermentation by Gut Microbiota. J. Agric. Food Chem. 2020, 68, 8080–8090. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sarkar, B.C.; Sharma, H.K. Mathematical modelling of thin layer hot air drying of carrot pomace. J. Food Sci. Technol. 2012, 49, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Buzrul, S. Reassessment of Thin-Layer Drying Models for Foods: A Critical Short Communication. Processes 2022, 10, 118. [Google Scholar] [CrossRef]
- Ertekin, C.; Firat, M.Z. A comprehensive review of thin-layer drying models used in agricultural products. Crit. Rev. Food Sci. Nutr. 2017, 57, 701–717. [Google Scholar] [CrossRef]
- Clifford, P.A. Report on Moisture in Dried Fruit. J. AOAC 1934, 17, 215–228. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, W.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant Activities of Phenolic, Proanthocyanidin, and Flavonoid Components in Extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.D.; Lingming, K.; Mason, S.L.; Zhou, J.H.; Sedcole, J.R. Upgrading the utilization of brassica wastes: Physicochemical properties and sensory evaluation of fermented brassica stalks. Int. Food Res. J. 2013, 20, 1961–1969. [Google Scholar]
- Tkacz, K.; Chmielewska, T.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020, 332, 127382. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Gudiño, I.; Martín, A.; Casquete, R.; Prieto, M.H.; Ayuso, M.C.; Córdoba, M.G. Evaluation of broccoli (Brassica oleracea var. italica) crop by-products as sources of bioactive compounds. Sci. Hortic. 2022, 304, 111284. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- Maskan, M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 177–182. [Google Scholar] [CrossRef]
- Tello-Ireland, C.; Lemus-Mondaca, R.; Vega-Gálvez, A.; López, J.; Di Scala, K. Influence of hot-air temperature on drying kinetics, functional properties, colour, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT Food Sci. Technol. 2011, 44, 2112–2118. [Google Scholar] [CrossRef]
- Rodrigues, S.; Silva, L.C.A.; Mulet, A.; Cárcel, J.A.; Fernandes, F.A.N. Development of dried probiotic apple cubes incorporated with Lactobacillus casei NRRL B-442. J. Funct. Foods 2018, 41, 48–54. [Google Scholar] [CrossRef]
- Planinić, M.; Velić, D.; Tomas, S.; Bilić, M.; Bucić, A. Modelling of drying and rehydration of carrots using Peleg’s model. Eur. Food Res. Technol. 2005, 221, 446–451. [Google Scholar] [CrossRef]
- Simpson, R.; Ramírez, C.; Nuñez, H.; Jaques, A.; Almonacid, S. Understanding the success of Page’s model and related empirical equations in fitting experimental data of diffusion phenomena in food matrices. Trends Food Sci. Technol. 2017, 62, 194–201. [Google Scholar] [CrossRef]
- Doymaz, I. Drying characteristics and kinetics of okra. J. Food Eng. 2005, 69, 275–279. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Mahn, A.; Antoine, P.; Reyes, A. Optimization of drying kinetics and quality parameters of broccoli florets. Int. J. Food Eng. 2011, 7. [Google Scholar] [CrossRef]
- Reyes, A.; Mahn, A.; Guzmán, C.; Antoniz, D. Analysis of the Drying of Broccoli Florets in a Fluidized Pulsed Bed. Dry. Technol. 2012, 30, 1368–1376. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Bernaert, N.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). J. Sci. Food Agric. 2013, 93, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Miletic, N.; Mitrovic, O.; Popovic, B.; Nedovic, V.; Zlatkovic, B.; Kandic, M. Polyphenolic content and antioxidant capacity in fruits of plum (Prunus Domestica L.) cultivars ‘Valjevka’ and ‘Mildora’ as influenced by air drying. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Lille, M.; Messagie, I.; Von Loey, A.; Autio, K.; Hendrickx, M. Impact of pretreatment and freezing conditions on the microstructure of frozen carrots: Quantification and relation to texture loss. Eur. Food Res. Technol. 2006, 222, 543–553. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Arilla, A.; Bennár, M.; Barrera, C.; Codoñer, P.; FIto, P. No invasive methodology to produce a probiotic low humid apple snack with potential effect against Helicobacter pylori. J. Food Eng. 2012, 110, 289–293. [Google Scholar] [CrossRef]
- Vesterlund, S.; Salminen, K.; Salminen, S. Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed. Int. J. Food Microbiol. 2012, 157, 319–321. [Google Scholar] [CrossRef]
- Cui, L.; Niu, L.; Li, D.; Liu, Y.; Liu, C.; Song, J. Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks. J. Integr. Agric. 2018, 17, 247–255. [Google Scholar] [CrossRef]

| Model | Equation |
|---|---|
| Lewis | MR = exp(−k · t) |
| Henderson and Pabis | MR = a · exp(−k · t) |
| Linear | MR = −k · t + a |
| Page | MR = exp(−k · tn) |
| Diffusional |
| Property | Non-Fermented | Fermented |
|---|---|---|
| xw (%) | 90.5 ± 1.0 a | 92 ± 2 a |
| aw | 0.989 ± 0.005 a | 0.991 ± 0.003 a |
| xss (g/g) | 0.696 ± 0.062 a | 0.615 ± 0.035 a |
| Total Phenol Content (mg GAE/gdm) | 3.62 ± 0.17 a | 3.7 ± 0.4 a |
| Total Flavonoid Content (mg QE/gdm) | 0.75 ± 0.15 a | 1.2 ± 0.3 b |
| DPPH (mg TE/gdm) | 0.46 ± 0.16 a | 1.1 ± 0.3 b |
| ABTS (mg TE/gdm) | 3.66 ± 0.06 a | 5.46 ± 0.11 b |
| tc (h) | (kg w/kg db) | RateCDRP (kg w/h·kg db) | |
|---|---|---|---|
| NF_G_HAD50 | 7 | 4.1 ± 0.2 a | 0.97 ± 0.02 a |
| NF_C_HAD50 | 7 | 4.4 ± 0.3 a | 1.10 ± 0.07 a |
| F_G_HAD50 | 3 | 5.5 ± 0.6 b | 2.6 ± 0.4 cd |
| NF_G_HAD60 | 2.5 | 6.3 ± 0.4 c | 2.13 ± 0.12 b |
| NF_C_HAD60 | 3 | 5.36 ± 0.08 b | 2.2 ± 0.3 bc |
| F_G_HAD60 | 1.5 | 6.7 ± 0.2 c | 3.0 ± 0.3 d |
| NF_G_HAD70 | 2.5 | 4.6 ± 0.4 a | 2.99 ± 0.12 d |
| NF_C_HAD70 | 1.5 | 6.44 ± 0.15 c | 2.98 ± 0.03 d |
| F_G_HAD70 | 1 | 7.6 ± 0.3 d | 4.73 ± 0.11 e |
| Model | Parameters | NF_G_HAD50 | NF_C_HAD50 | F_G_HAD50 | NF_G_HAD60 | NF_C_HAD60 | F_G_HAD60 | NF_G_HAD70 | NF_C_HAD70 | F_G_HAD70 |
|---|---|---|---|---|---|---|---|---|---|---|
| Page | k (h−1) | 0.29 ± 0.05 a | 0.23 ± 0.02 a | 0.45 ± 0.04 bc | 0.35 ± 0.05 ab | 0.36 ± 0.04 ab | 0.503 ± 0.005 bc | 0.8 ± 0.2 d | 0.57 ± 0.02 c | 0.73 ± 0.08 d |
| n | 1.17 ± 0.08 a | 1.32 ± 0.08 ab | 1.19 ± 0.13 a | 1.30 ± 0.08 ab | 1.31 ± 0.06 ab | 1.420 ± 0.005 b | 1.31 ± 0.10 ab | 1.41 ± 0.02 b | 1.46 ± 0.05 b | |
| SSE | 0.0007 | 0.0059 | 0.0075 | 0.0057 | 0.0059 | 0.0089 | 0.0060 | 0.0080 | 0.0069 | |
| R2 | 0.983 | 0.996 | 0.975 | 0.992 | 0.992 | 0.984 | 0.979 | 0.988 | 0.990 | |
| Diffusional | Def × 10−9 (m2/s) | 1.34 ± 0.03 a | 1.16 ± 0.06 a | 1.5 ± 0.13 a | 1.5 ± 0.4 a | 1.6 ± 0.6 a | 2.35 ± 0.02 b | 3.0 ± 0.2 cd | 2.58 ± 0.07 bc | 3.34 ± 0.14 d |
| SSE | 0.0246 | 0.0388 | 0.0398 | 0.0445 | 0.0492 | 0.0516 | 0.0277 | 0.0497 | 0.0531 | |
| R2 | 0.849 | 0.938 | 0.968 | 0.902 | 0.901 | 0.879 | 0.935 | 0.903 | 0.919 | |
| Lewis | k (h−1) | 0.40 ± 0.04 a | 0.44 ± 0.02 ab | 0.59 ± 0.05 b | 0.6 ± 0.2 b | 0.61 ± 0.12 b | 0.80 ± 0.13 c | 1.14 ± 0.08 de | 0.985 ± 0.010 cd | 1.26 ± 0.06 e |
| SSE | 0.0254 | 0.0246 | 0.0171 | 0.0294 | 0.0318 | 0.0379 | 0.0266 | 0.0362 | 0.0393 | |
| R2 | 0.986 | 0.963 | 0.981 | 0.939 | 0.940 | 0.911 | 0.947 | 0.931 | 0.941 | |
| Henderson and Pabis | k (h−1) | 0.40 ± 0.05 a | 0.50 ± 0.03 ab | 0.61 ± 0.05 bc | 0.7 ± 0.2 c | 0.71 ± 0.15 c | 1.07 ± 0.02 d | 1.20 ± 0.04 d | 1.155 ± 0.003 d | 1.45 ± 0.05 e |
| a | 1.1 ± 0.2 a | 1.44 ± 0.05 bc | 1.05 ± 0.02 a | 1.5 ± 0.2 c | 1.5 ± 0.2 bc | 1.74 ± 0.06 c | 1.2 ± 0.2 ab | 1.77 ± 0.07 c | 1.72 ± 0.03 c | |
| SSE | 0.0023 | 0.0469 | 0.0158 | 0.0497 | 0.0519 | 0.0791 | 0.0265 | 0.0755 | 0.0822 | |
| R2 | 0.989 | 0.980 | 0.982 | 0.965 | 0.968 | 0.946 | 0.951 | 0.960 | 0.957 | |
| Linear | k (h−1) | 0.183 ± 0.004 c | 0.154 ± 0.006 a | 0.157 ± 0.004 ab | 0.154 ± 0.003 a | 0.167 ± 0.002 b | 0.224 ± 0.007 d | 0.282 ± 0.009 e | 0.282 ± 0.002 e | 0.334 ± 0.003 f |
| a | 0.953 ± 0.014 e | 0.93 ± 0.02 de | 0.801 ± 0.004 a | 0.83 ± 0.03 ab | 1.36 ± 0.02 f | 0.860 ± 0.005 bc | 0.83 ± 0.05 ab | 1.329 ± 0.005 f | 0.90 ± 0.02 cd | |
| SSE | 0.0016 | 0.0030 | 0.0517 | 0.0315 | 0.1612 | 0.0289 | 0.0625 | 0.1228 | 0.0624 | |
| R2 | 0.984 | 0.969 | 0.836 | 0.894 | 0.912 | 0.925 | 0.880 | 0.960 | 0.949 |
| Treatment | xw (%) | aw | Total Phenol Content (mg GAE/gdm) | Total Flavonoid Content (mg QE/gdm) | DPPH (mg TE/gdm) | ABTS (mg TE/gdm) |
|---|---|---|---|---|---|---|
| NF_G_HAD50 | 3.7 ± 0.2 f | 0.28 ± 0.04 cde | 3.0 ± 0.4 a | 2.48 ± 0.13 ab | 1.65 ± 0.03 a | 12.2 ± 0.4 a |
| NF_C_HAD50 | 3.3 ± 0.2 ef | 0.239 ± 0.008 ab | 5.04 ± 0.10 cd | 3.24 ± 0.12 cd | 2.77 ± 0.14 bcd | 13.1 ± 0.3 ab |
| F_G_HAD50 | 3.92 ± 0.12 g | 0.292 ± 0.005 e | 6.8 ± 0.6 f | 3.5 ± 0.4 d | 3.4 ± 0.4 de | 19.2 ± 0.7 f |
| NF_G_HAD60 | 3.3 ± 0.4 ef | 0.264 ± 0.014 bcd | 3.9 ± 0.5 b | 2.9 ± 0.3 c | 2.6 ± 0.6 bc | 12.1 ± 1.1 a |
| NF_C_HAD60 | 3.2 ± 0.5 def | 0.27 ± 0.03 cd | 4.9 ± 0.5 c | 4.6 ± 0.2 e | 2.6 ± 0.3 bc | 26.7 ± 1.5 g |
| F_G_HAD60 | 2.57 ± 0.04 abcd | 0.253 ± 0.004 abc | 6.4 ± 0.3 ef | 4.5 ± 0.5 e | 3.0 ± 0.5 bcd | 19.2 ± 0.8 f |
| NF_G_HAD70 | 2.9 ± 0.6 bcde | 0.267 ± 0.012 cd | 6.1 ± 0.9 e | 4.6 ± 0.4 e | 3.1 ± 0.3 cd | 14.5 ± 0.9 bc |
| NF_C_HAD70 | 2.5 ± 0.4 abc | 0.26 ± 0.02 cd | 6.3 ± 0.9 ef | 4.4 ± 0.4 e | 3.1 ± 0.4 d | 15.3 ± 0.8 cd |
| F_G_HAD70 | 3.197 ± 0.011 cdef | 0.237 ± 0.007 a | 8.7 ± 0.3 g | 5.7 ± 0.4 f | 2.48 ± 0.11 b | 13.0 ± 1.1 a |
| NF_G_FD | 2.40 ± 0.03 ab | 0.262 ± 0.005 abcd | 4.2 ± 0.2 c | 3.27 ± 0.05 d | 3.3 ± 0.4 d | 17 ± 3 de |
| NF_C_FD | 2.76 ± 0.04 abcde | 0.289 ± 0.005 de | 3.5 ± 0.2 ab | 2.17 ± 0.13 a | 3.9 ± 0.4 e | 12.8 ± 0.3 a |
| F_G_FD | 2.096 ± 0.006 a | 0.2490 ± 0.0007 abc | 5.88 ± 0.11 de | 2.5 ± 0.2 b | 2.6 ± 0.2 bc | 17.2 ± 0.8 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Fermentation Pretreatment on Drying Behaviour and Antioxidant Attributes of Broccoli Waste Powdered Ingredients. Foods 2023, 12, 3526. https://doi.org/10.3390/foods12193526
Bas-Bellver C, Barrera C, Betoret N, Seguí L. Impact of Fermentation Pretreatment on Drying Behaviour and Antioxidant Attributes of Broccoli Waste Powdered Ingredients. Foods. 2023; 12(19):3526. https://doi.org/10.3390/foods12193526
Chicago/Turabian StyleBas-Bellver, Claudia, Cristina Barrera, Noelia Betoret, and Lucía Seguí. 2023. "Impact of Fermentation Pretreatment on Drying Behaviour and Antioxidant Attributes of Broccoli Waste Powdered Ingredients" Foods 12, no. 19: 3526. https://doi.org/10.3390/foods12193526









