Development and Application of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Film Containing Salicylic Acid for Banana Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MCM-41 Loaded with SA Powder
2.3. Preparation of Film
2.4. Characterization of Film
2.4.1. Morphological Properties
2.4.2. Mechanical Properties
2.4.3. Barrier Properties
2.4.4. Optical Properties
2.5. Application to Banana Preservation
2.5.1. Samples Preparation
2.5.2. Determination of CO2 and O2 Contents
2.5.3. Rate of Weight Loss
2.5.4. Hardness
2.5.5. Soluble Solids Content
2.5.6. Malonic Dialdehyde
2.5.7. Polyphenol Oxidase
2.5.8. Sensory Evaluation
2.6. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Film
3.1.1. Microstructure Analysis
3.1.2. Mechanical Properties
3.1.3. Barrier Properties
3.1.4. Optical Properties
3.2. Qualities of Bananas
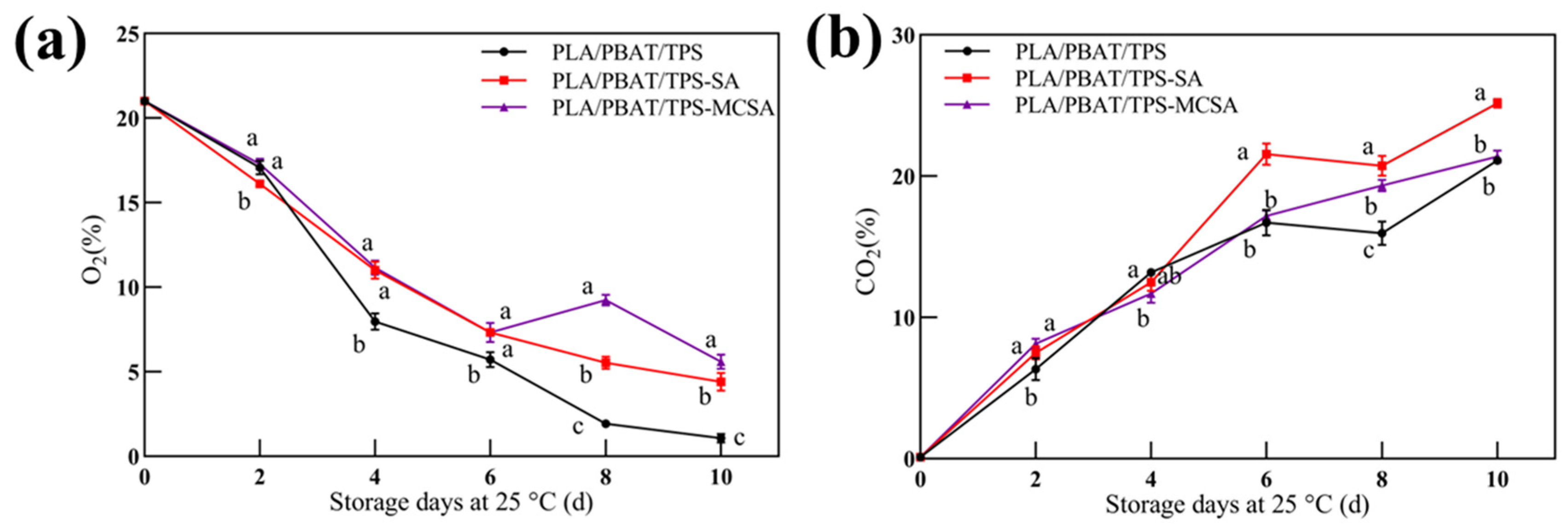
3.2.1. Volume Fractions of O2 and CO2 Contents
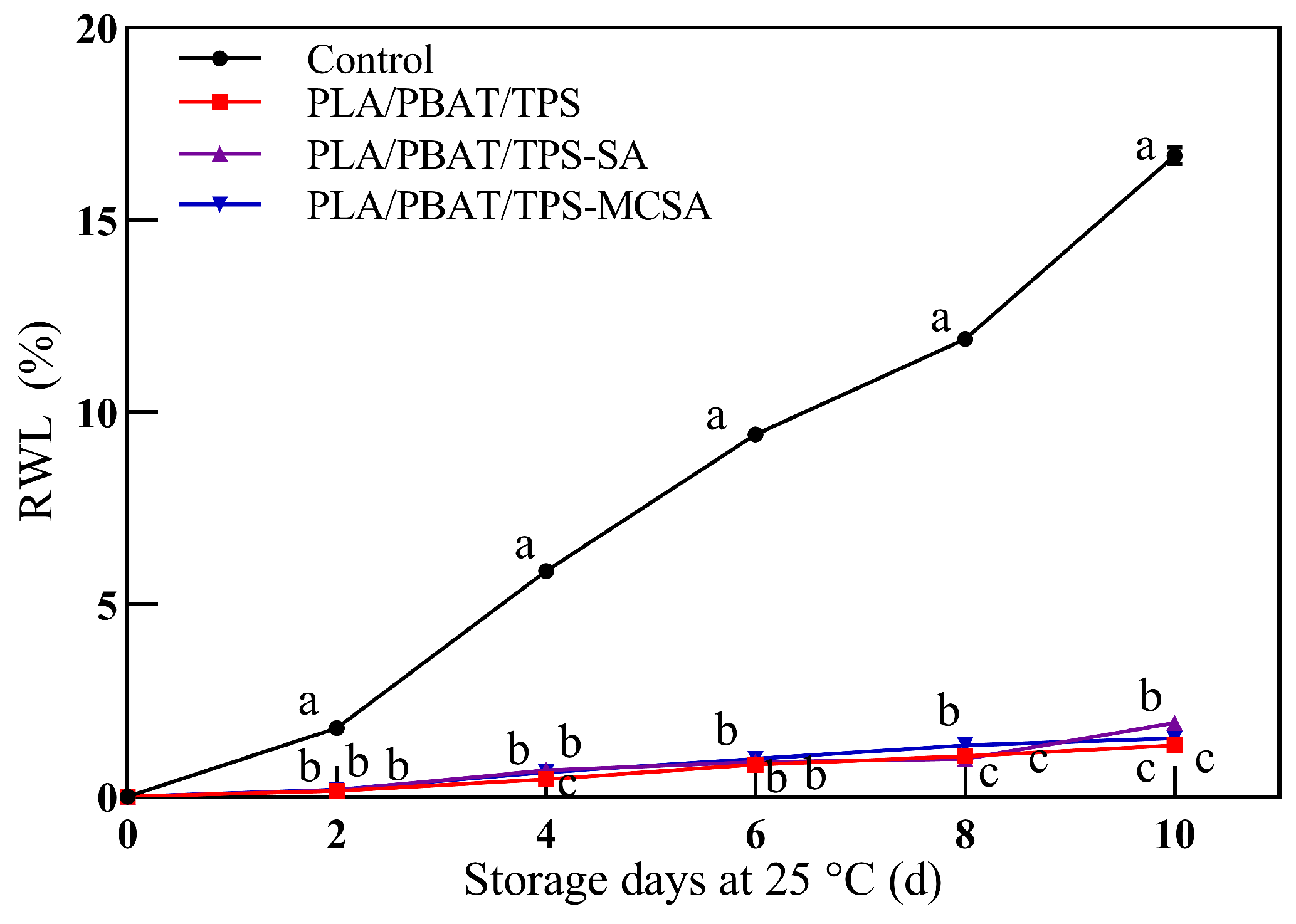
3.2.2. Rate of Weight Loss
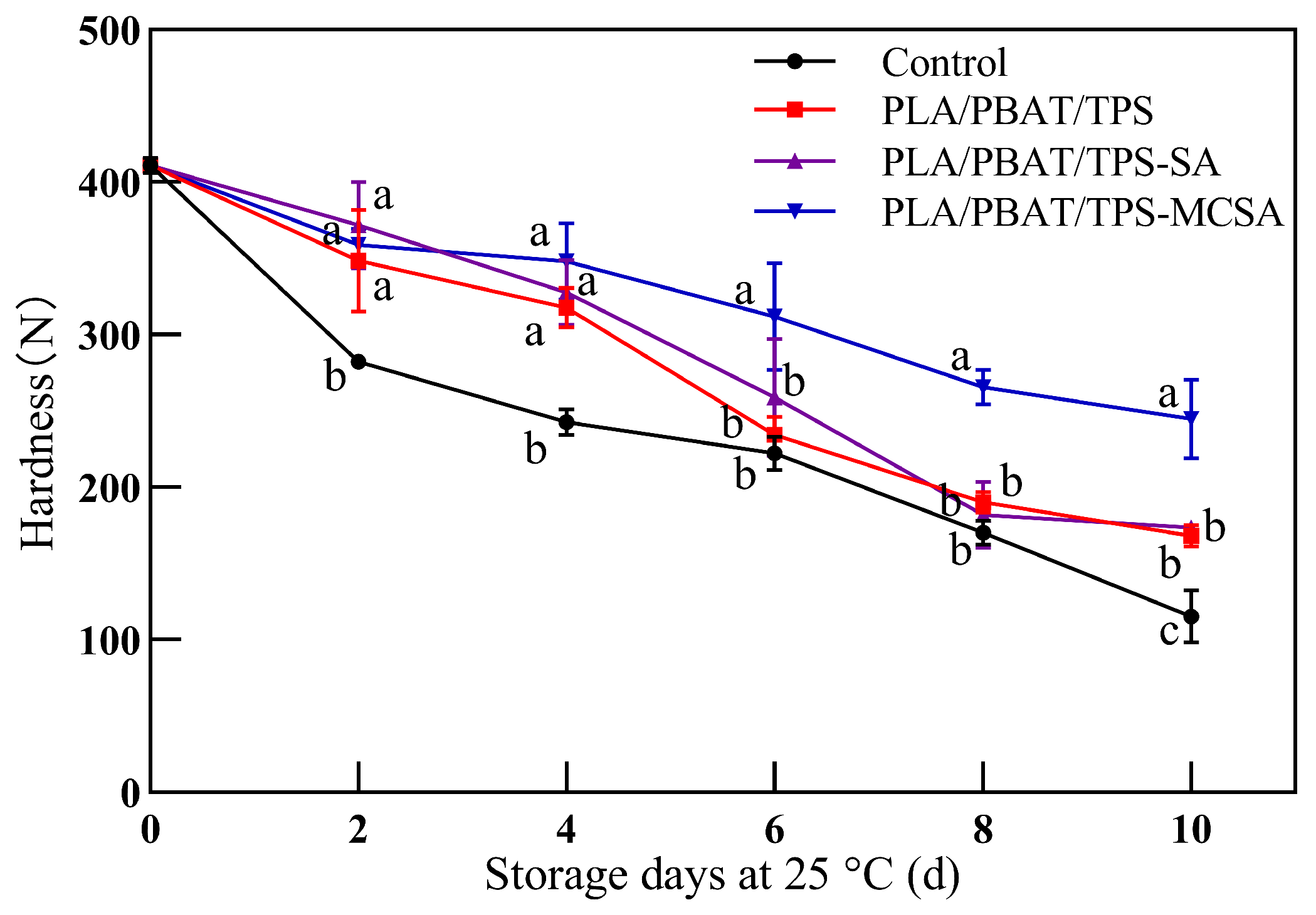
3.2.3. Hardness
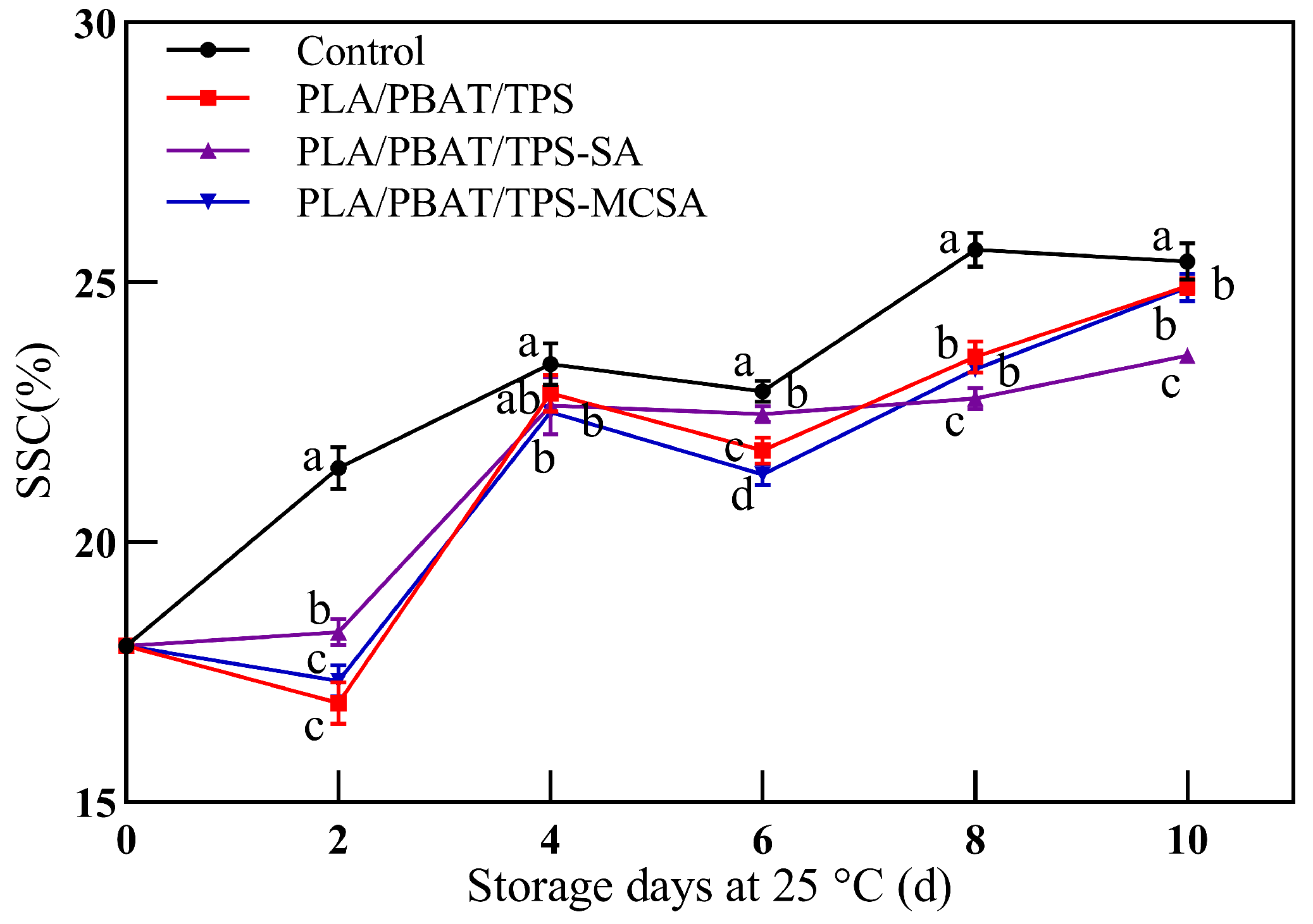
3.2.4. Soluble Solids Content
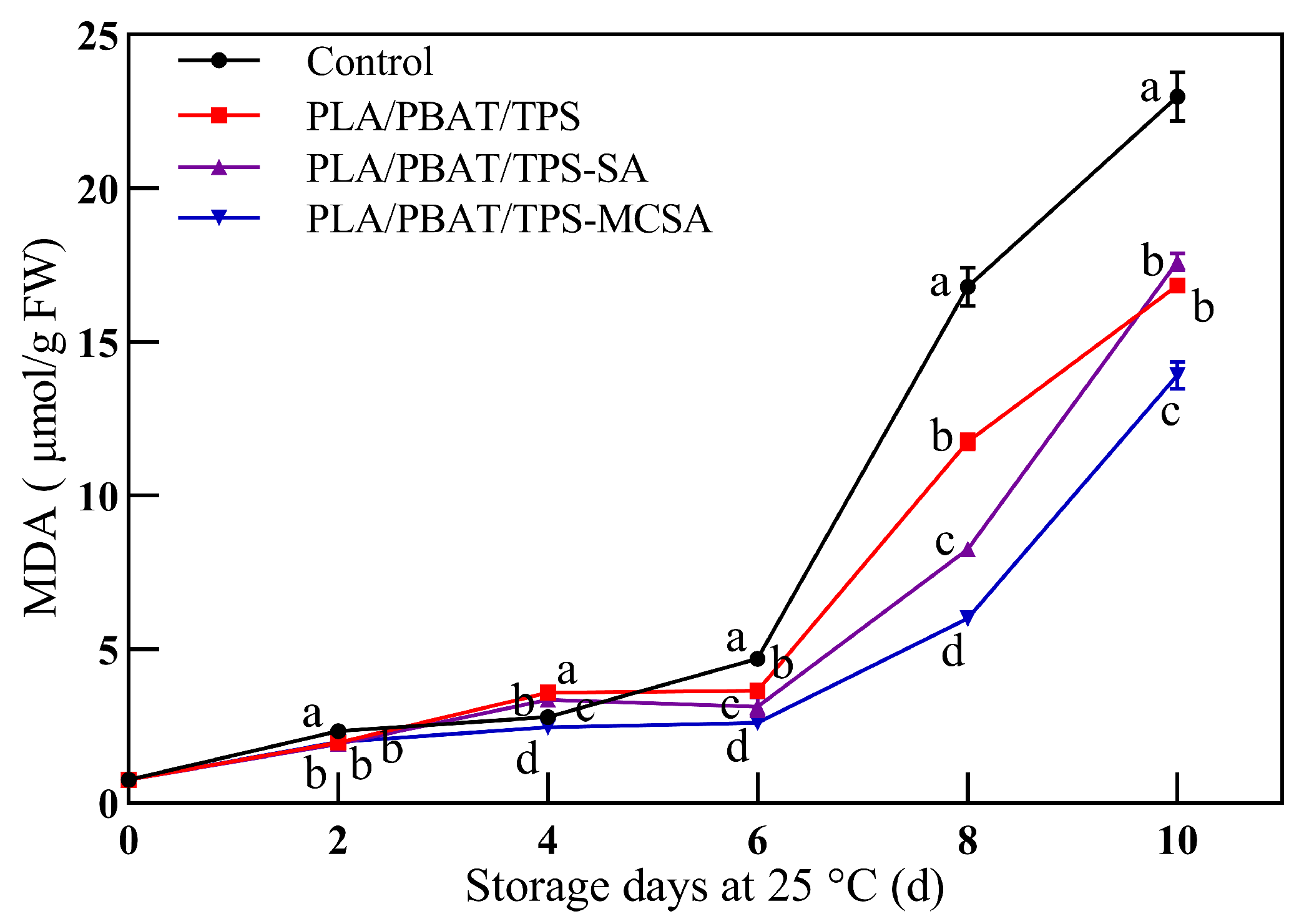
3.2.5. Malonic Dialdehyde
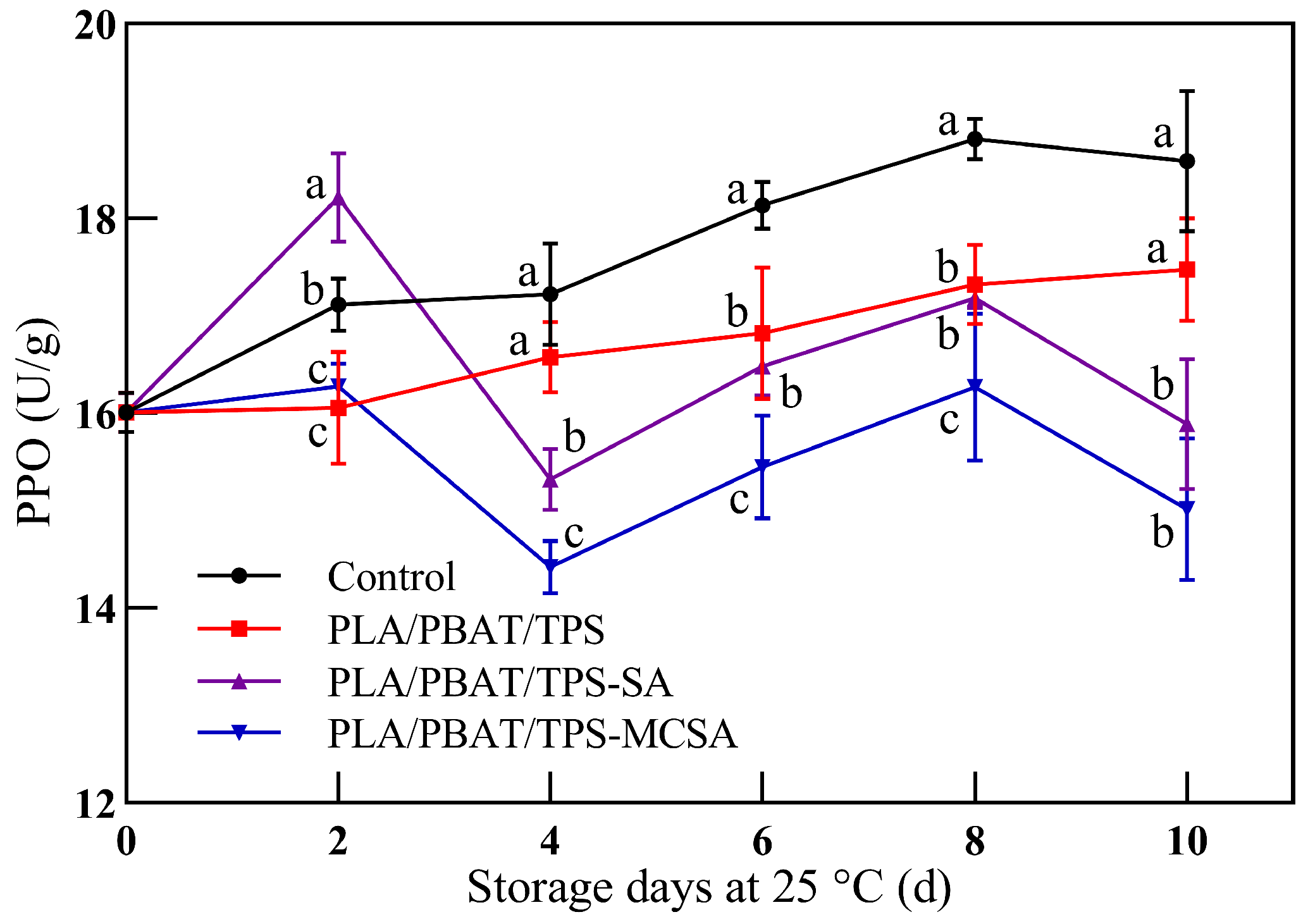
3.2.6. Polyphenol Oxidase
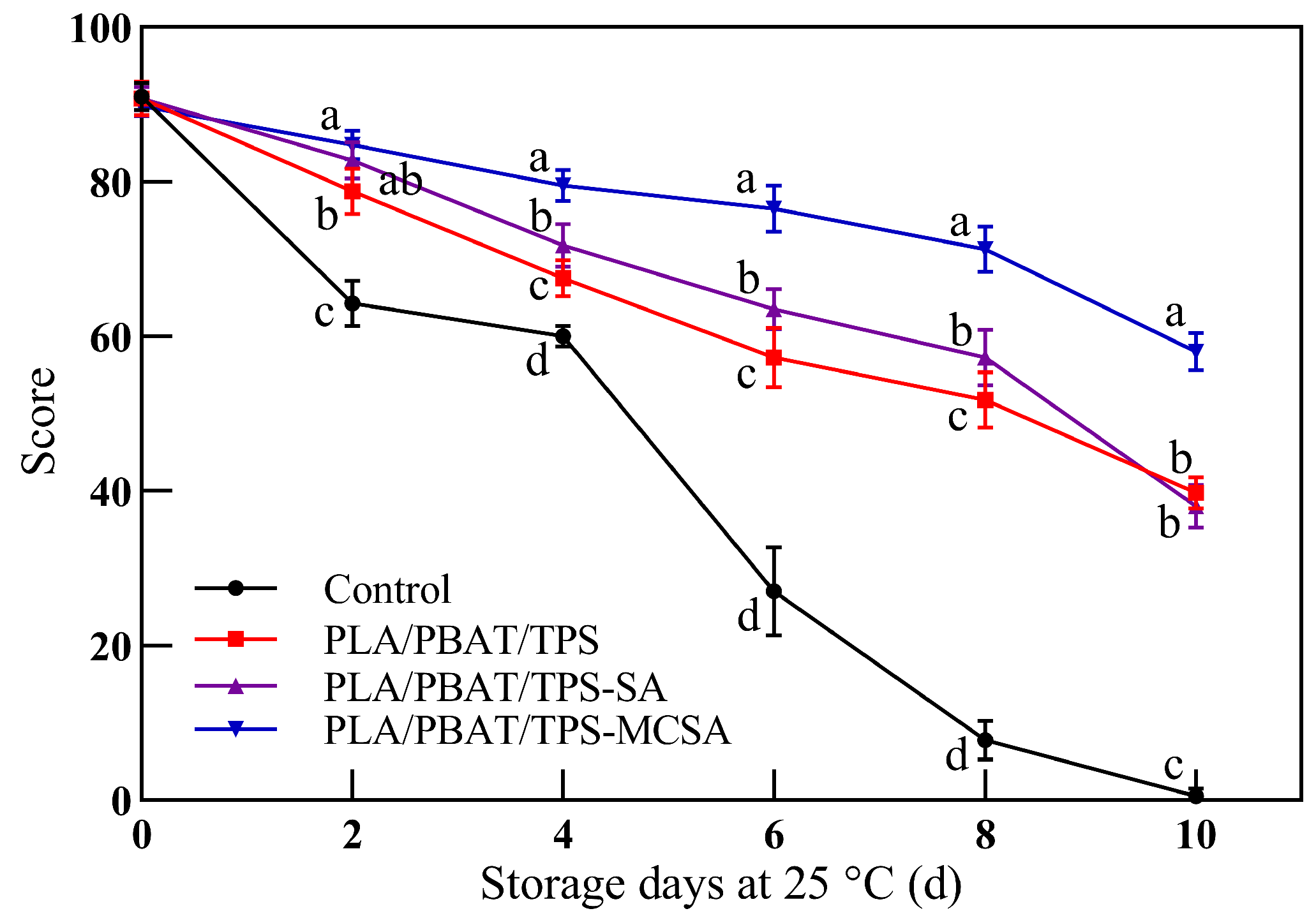
3.2.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.C.; Li, Y.N. Mechanical and Antibacterial Properties of Modified Nano-ZnO/High-Density Polyethylene Composite Films with a Low Doped Content of Nano-ZnO. J. Appl. Polym. Sci. 2010, 116, 2965–2969. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. Post-Harvest Shelf-Life of Banana and Guava: Mechanisms of Common Degradation Problems and Emerging Counteracting Strategies. Innov. Food Sci. Emerg. Technol. 2018, 49, 20–30. [Google Scholar] [CrossRef]
- Brat, P.; Bugaud, C.; Guillermet, C.; Salmon, F. Review of Banana Green Life throughout the Food Chain: From Auto-Catalytic Induction to the Optimisation of Shipping and Storage Conditions. Sci. Hortic. 2020, 262, 109054. [Google Scholar] [CrossRef]
- Huang, H.; Wang, L.; Qiu, D.; Zhang, N.; Bi, F. Changes of Morphology, Chemical Compositions, and the Biosynthesis Regulations of Cuticle in Response to Chilling Injury of Banana Fruit During Storage. Front. Plant Sci. 2021, 12, 792384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Chen, J.; Song, T.; Jiang, Y.; Zhang, Y.; Wang, L.; Li, F. Preharvest Spraying Calcium Ameliorated Aroma Weakening and Kept Higher Aroma-Related Genes Expression Level in Postharvest ‘Nanguo’ Pears after Long-Term Refrigerated Storage. Sci. Hortic. 2019, 247, 287–295. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of Exogenous Salicylic Acid under Changing Environment: A Review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of Salicylic Acid on Post-Harvest Physiology of Horticultural Crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Xue, C.; Yu, Z.; Yang, R.; Cai, Z.; Si, L. Biocontrol of Gray Mold in Grapes with the Yeast Hanseniaspora uvarum Alone and in Combination with Salicylic Acid or Sodium Bicarbonate. Postharvest Biol. Technol. 2015, 100, 160–167. [Google Scholar] [CrossRef]
- Gačnik, S.; Veberič, R.; Hudina, M.; Koron, D.; Mikulič-Petkovšek, M. Salicylate Treatment Affects Fruit Quality and Also Alters the Composition of Metabolites in Strawberries. Horticulturae 2021, 7, 400. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic Acid Treatment Mitigates Chilling Injury in Peach Fruit by Regulation of Sucrose Metabolism and Soluble Sugar Content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Inhibition of Ethylene Biosynthesis by Salicylic Acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M.; et al. Salicylic Acid and Melatonin Alleviate the Effects of Heat Stress on Essential Oil Composition and Antioxidant Enzyme Activity in Mentha × piperita and Mentha arvensis L. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Kaur, P.; Grewal, S.K. Salicylic Acid Enriched Beeswax Coatings Suppress Fruit Softening in Pears by Modulation of Cell Wall Degrading Enzymes under Different Storage Conditions. Food Packag. Shelf Life 2022, 32, 100821. [Google Scholar] [CrossRef]
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite Supported by CaO/MgO Nanocomposite as Heterogeneous Catalyst for Biodiesel Production from Waste Cooking Oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Dardir, F.M.; Ahmed, E.A.; Soliman, M.F.; Othman, S.I.; Allam, A.A.; Alwail, M.A.; Abukhadra, M.R. Synthesis of Chitosan/Al-MCM-41 Nanocomposite from Natural Microcline as a Carrier for Levofloxacin Drug of Controlled Loading and Release Properties; Equilibrium, Release Kinetic, and Cytotoxicity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126805. [Google Scholar] [CrossRef]
- Vilaça, N.; Morais-Santos, F.; Machado, A.F.; Sirkecioğlu, A.; Pereira, M.F.R.; Sardo, M.; Rocha, J.; Parpot, P.; Fonseca, A.M.; Baltazar, F.; et al. Micro- and Mesoporous Structures as Drug Delivery Carriers for Salicylic Acid. J. Phys. Chem. C 2015, 119, 3589–3595. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Z.; Cai, H.; Wang, L.; Hu, C.; Li, D.; Chen, Y.; Kang, Y.; Li, L. Controlled Moisture Permeability of Thermoplastic Starch/Polylactic Acid/Poly Butylene Adipate-Co-Terephthalate Film for the Autolysis of Straw Mushroom Volvariella volvacea. Food Chem. 2022, 373, 131409. [Google Scholar] [CrossRef]
- Amorim, R.; Vilaça, N.; Martinho, O.; Reis, R.M.; Sardo, M.; Rocha, J.; Fonseca, A.M.; Baltazar, F.; Neves, I.C. Zeolite Structures Loading with an Anticancer Compound As Drug Delivery Systems. J. Phys. Chem. C 2012, 116, 25642–25650. [Google Scholar] [CrossRef]
- Gui, H.; Zhao, M.; Zhang, S.; Yin, R.; Hu, C.; Fan, M.; Li, L. Active Antioxidant Packaging from Essential Oils Incorporated Polylactic Acid/Poly(Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch for Preserving Straw Mushroom. Foods 2022, 11, 2252. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, S.; Waterhouse, G.I.N.; Zhang, K.; Xu, J. Enhancing the Properties of PBAT/PLA Composites with Novel Phosphorus-Based Ionic Liquid Compatibilizers. Mater. Today Commun. 2021, 27, 102407. [Google Scholar] [CrossRef]
- ASTM-D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM-D1434-82; Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM-E398-13; Standard Test Method for Water Vapor Transmission Rate of Sheet Materials Using Dynamic Relative Humidity Measurement. ASTM International: West Conshohocken, PA, USA, 2013.
- Hao, Y.; Zhang, M.; Liu, B.; Ma, W.; Dong, Q.; Fan, M.; Wang, Y.; Li, L. Nonmigrating Active Antibacterial Packaging: Antimicrobial Mechanism against Staphylococcus aureus and Its Application in Large Yellow Croaker. ACS Sustain. Chem. Eng. 2023, 11, 6220–6229. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Luca, A.; Edelenbos, M. Development of a Small and Flexible Sensor-Based Respirometer for Real-Time Determination of Respiration Rate, Respiratory Quotient and Low O2 Limit of Fresh Produce. Comput. Electron. Agric. 2016, 121, 347–353. [Google Scholar] [CrossRef]
- Fang, D.; Yu, K.; Deng, Z.; Hu, Q.; Zhao, L. Storage Quality and Flavor Evaluation of Volvariella volvacea Packaged with Nanocomposite-Based Packaging Material during Commercial Storage Condition. Food Packag. Shelf Life 2019, 22, 100412. [Google Scholar] [CrossRef]
- Li, L.; Song, W.; Shen, C.; Dong, Q.; Wang, Y.; Zuo, S. Active Packaging Film Containing Oregano Essential Oil Microcapsules and Their Application for Strawberry Preservation. J. Food Process. Preserv. 2020, 44, e14799. [Google Scholar] [CrossRef]
- Terefe, N.S.; Tepper, P.; Ullman, A.; Knoerzer, K.; Juliano, P. High Pressure Thermal Processing of Pears: Effect on Endogenous Enzyme Activity and Related Quality Attributes. Innov. Food Sci. Emerg. Technol. 2016, 33, 56–66. [Google Scholar] [CrossRef]
- Franke, C.; Höll, L.; Langowski, H.-C.; Petermeier, H.; Vogel, R.F. Sensory Evaluation of Chicken Breast Packed in Two Different Modified Atmospheres. Food Packag. Shelf Life 2017, 13, 66–75. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, M.; Dong, Q.; Fan, M.; Wang, L.; Li, L. Extruded Transglutaminase-Modified Gelatin–Beeswax Composite Packaging Film. Food Hydrocoll. 2022, 132, 107849. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kumar, A. Ionic Liquid Assisted Gelatin Films: Green, UV Shielding, Antioxidant, and Antibacterial Food Packaging Materials. ACS Sustain. Chem. Eng. 2019, 7, 8631–8636. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The Multi-Layer Film System Improved the Release and Retention Properties of Cinnamon Essential Oil and Its Application as Coating in Inhibition to Penicillium Expansion of Apple Fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Lopez-Briones, G.; Varoquaux, P.; Bureau, G.; Pascat, B. Modified Atmosphere Packaging of Common Mushroom. Int. J. Food Sci. Technol. 2007, 28, 57–68. [Google Scholar] [CrossRef]
- Bugatti, V.; Bernardo, P.; Clarizia, G.; Viscusi, G.; Vertuccio, L.; Gorrasi, G. Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11. Polymers 2019, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Deng, M.; Gui, R.; Liu, Y.; Chen, X.; Lin, Y.; Li, M.; Wang, Y.; He, W.; et al. Blue Light Combined with Salicylic Acid Treatment Maintained the Postharvest Quality of Strawberry Fruit during Refrigerated Storage. Food Chem. X 2022, 15, 100384. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-Y.; Hazeena, S.H.; Hsieh, S.-L.; Li, B.-H.; Chen, M.-H.; Wang, P.-Y.; Zheng, B.-Q.; Liang, Y.-S. Effect of D-Limonene Nanoemulsion Edible Film on Banana (Musa sapientum Linn.) Post-Harvest Preservation. Molecules 2022, 27, 6157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dong, Q.; Gao, H.; Han, Y.; Li, L. Enhanced Mechanical and Antioxidant Properties of Biodegradable Poly (Lactic) Acid-poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Film Utilizing A-tocopherol for Peach Storage. Packag. Technol. Sci. 2021, 34, 187–199. [Google Scholar] [CrossRef]
- Li, N.; Chen, F.; Cui, F.; Sun, W.; Zhang, J.; Qian, L.; Yang, Y.; Wu, D.; Dong, Y.; Jiang, J.; et al. Improved Postharvest Quality and Respiratory Activity of Straw Mushroom (Volvariella volvacea) with Ultrasound Treatment and Controlled Relative Humidity. Sci. Hortic. 2017, 225, 56–64. [Google Scholar] [CrossRef]
- Othman, S.H.; Abdullah, N.A.; Nordin, N.; Abdul Karim Shah, N.N.; Mohd Nor, M.Z.; Md Yunos, K.F. Shelf Life Extension of Saba Banana: Effect of Preparation, Vacuum Packaging, and Storage Temperature. Food Packag. Shelf Life 2021, 28, 100667. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.-Y.; Kim, J.; Moon, K.-D. Effect of Edible Coating with Morus Alba Root Extract and Carboxymethyl Cellulose for Enhancing the Quality and Preventing the Browning of Banana (Musa acuminata Cavendish) during Storage. Food Packag. Shelf Life 2022, 31, 100809. [Google Scholar] [CrossRef]
- Hu, W.; Gong, F.; Dong, Q.; Kang, Y.; Zhou, Y.; Liu, B.; Li, L. Antioxidant Mechanism of a Continuous Microenvironment Regulation System for Prolonging the Shelf-life of Red Grapes. J. Food Sci. 2023, 88, 2496–2511. [Google Scholar] [CrossRef]
- Qu, T.; Li, B.; Huang, X.; Li, X.; Ding, Y.; Chen, J.; Tang, X. Effect of Peppermint Oil on the Storage Quality of White Button Mushrooms (Agaricus bisporus). Food Bioprocess Technol. 2020, 13, 404–418. [Google Scholar] [CrossRef]
- Wei, W.; Lv, P.; Xia, Q.; Tan, F.; Sun, F.; Yu, W.; Jia, L.; Cheng, J. Fresh-Keeping Effects of Three Types of Modified Atmosphere Packaging of Pine-Mushrooms. Postharvest Biol. Technol. 2017, 132, 62–70. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin Treatment Reduces Ethylene Production and Maintains Fruit Quality in Apple during Postharvest Storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, W.; Hu, S.; Kang, Y.; Zhang, Y.; Ng, T.B. Effects of Oudemansiella radicata Polysaccharide on Postharvest Quality of Oyster Mushroom (Pleurotus ostreatus) and Its Antifungal Activity against Penicillium digitatum. Postharvest Biol. Technol. 2020, 166, 111207. [Google Scholar] [CrossRef]
- Giboreau, A.; Dacremont, C.; Egoroff, C.; Guerrand, S.; Urdapilleta, I.; Candel, D.; Dubois, D. Defining Sensory Descriptors: Towards Writing Guidelines Based on Terminology. Food Qual. Prefer. 2007, 18, 265–274. [Google Scholar] [CrossRef]

| Sample | PLA (wt%) | PBAT (wt%) | TPS (wt%) | SA (wt%) | MCSA (wt%) |
|---|---|---|---|---|---|
| PLA/PBAT/TPS | 15 | 45 | 40 | 0 | 0 |
| PLA/PBAT/TPS-SA | 14.7 | 44.1 | 39.2 | 2 | 0 |
| PLA/PBAT/TPS-MCSA | 14.7 | 44.1 | 39.2 | 0 | 2 |
| Score | Basis of Scoring |
|---|---|
| 81~100 | Good taste of flesh, bright yellow peel color, no mechanical damage, no angles, no brown. |
| 61~80 | The peel is dark yellow, with tiny needle-like brown spots, the flesh has a good taste and no mechanical damage. |
| 41~60 | Sesame-sized brown spots on the peel, soft flesh texture. |
| 21~40 | Large, localized brown spots on the epidermis, tawny, slight mechanical damage to the flesh. |
| 1~20 | Large browning on the surface, soft flesh, and unpleasant odor. |
| 0 | Large browning of the epidermis, severe flesh decay, irritating odor, inedible. |
| Sample | TS (Vertical) (MPa) | EAB (Vertical) (%) | TS (Lateral) (MPa) | EAB (Lateral) (%) |
|---|---|---|---|---|
| PLA/PBAT/TPS | 30.28 ± 0.958 c | 436.56 ± 23.780 c | 26.11 ± 2.732 c | 356.44 ± 16.670 c |
| PLA/PBAT/TPS-SA | 21.20 ± 0.549 a | 288.79 ± 29.550 a | 12.12 ± 2.025 a | 211.34 ± 13.770 a |
| PLA/PBAT/TPS-MCSA | 26.93 ± 0.828 b | 379.56 ± 35.460 b | 16.86 ± 1.421 b | 256.79 ± 23.670 b |
| Sample | OTR (cm3·m−2·24 h−1·0.1 MPa−1) | WVTP (g·m−1·Pa−1·s−1) |
|---|---|---|
| PLA/PBAT/TPS | 384.36 ± 22.06 b | 860.08 ± 4.11 a |
| PLA/PBAT/TPS-SA | 361.29 ± 3.12 a | 1178.13 ± 37.67 c |
| PLA/PBAT/TPS-MCSA | 543.10 ± 3.47 c | 938.50 ± 12.38 b |
| Sample | T (%) | H (%) |
|---|---|---|
| PLA/PBAT/TPS | 30.61 ± 0.81 b | 43.58 ± 0.74 a |
| PLA/PBAT/TPS-SA | 29.90 ± 0.70 b | 42.15 ± 0.93 a |
| PLA/PBAT/TPS-MCSA | 23.37 ± 1.07 a | 53.20 ± 0.39 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Hao, Y.; Liu, B.; Chen, Y.; Li, L. Development and Application of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Film Containing Salicylic Acid for Banana Preservation. Foods 2023, 12, 3397. https://doi.org/10.3390/foods12183397
Ding J, Hao Y, Liu B, Chen Y, Li L. Development and Application of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Film Containing Salicylic Acid for Banana Preservation. Foods. 2023; 12(18):3397. https://doi.org/10.3390/foods12183397
Chicago/Turabian StyleDing, Jian, Yi Hao, Boqiang Liu, Yunxia Chen, and Li Li. 2023. "Development and Application of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Film Containing Salicylic Acid for Banana Preservation" Foods 12, no. 18: 3397. https://doi.org/10.3390/foods12183397




