Investigation of the Potential Phlorotannins and Mechanism of Six Brown Algae in Treating Type II Diabetes Mellitus Based on Biological Activity, UPLC-QE-MS/MS, and Network Pharmacology
Abstract
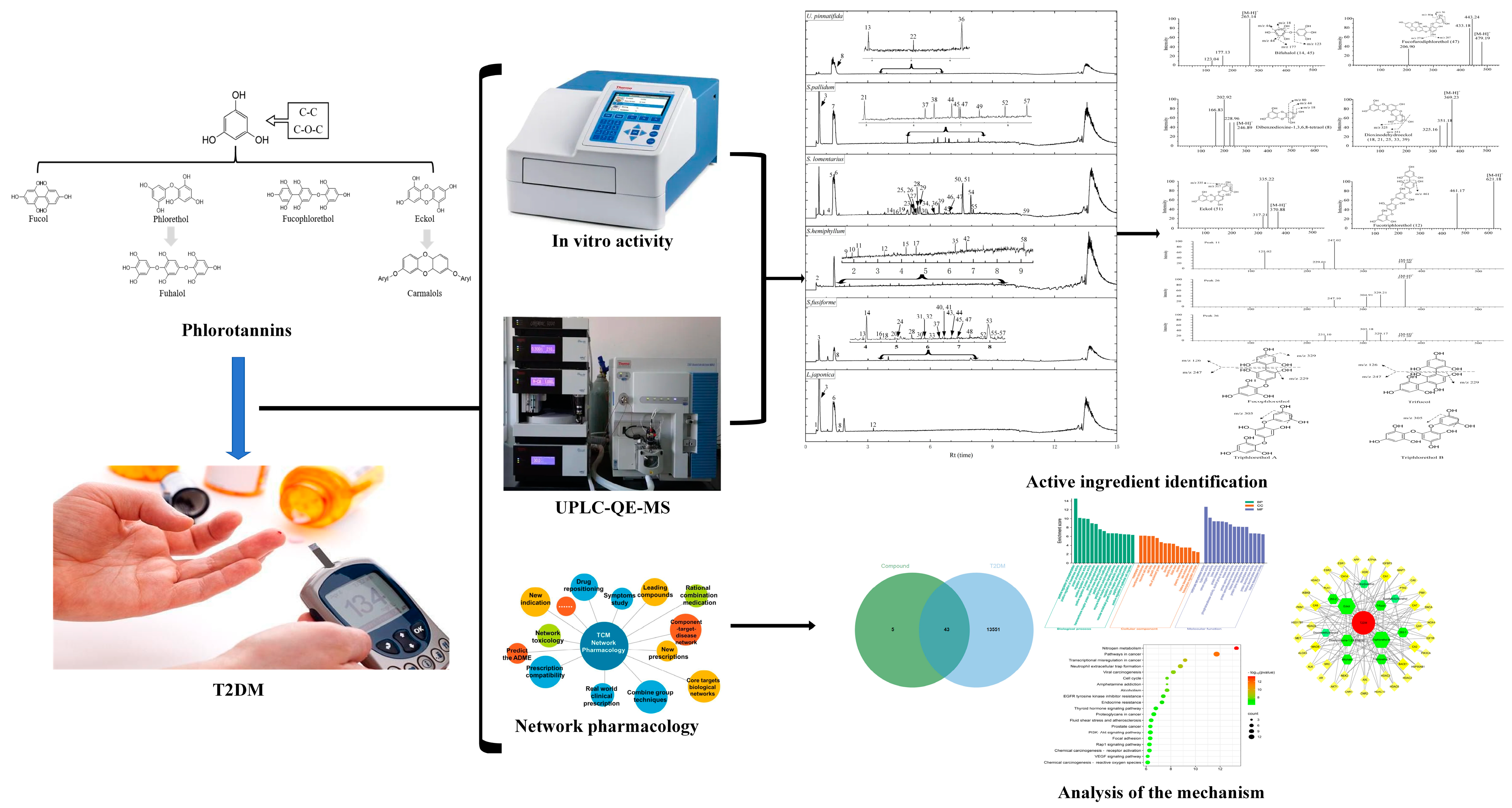
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Databases and Software
2.3. Brown Algae Samples
2.4. Sample Preparation and Extraction
2.5. Determination of Total Phenolic Content
2.6. Antioxidant Activities
2.6.1. DPPH· Scavenging Test
2.6.2. ABTS· Scavenging Test
2.6.3. Iron Ion Reduction Force Experiment
2.7. Enzyme Inhibition Assay
2.7.1. α-Glucosidase Inhibition Assay
2.7.2. α-Amylase Assay
2.8. Characterization of Phlorotannins
2.9. Network Pharmacological Analysis
2.9.1. Potential Target Prediction and Screening
2.9.2. GO Function and KEGG Pathway Enrichment Analysis
2.9.3. Network Construction of Compound-Target-Disease
2.10. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Total Phenolic Content
3.2. Antioxidant Activities
3.3. Enzyme Inhibition Assay
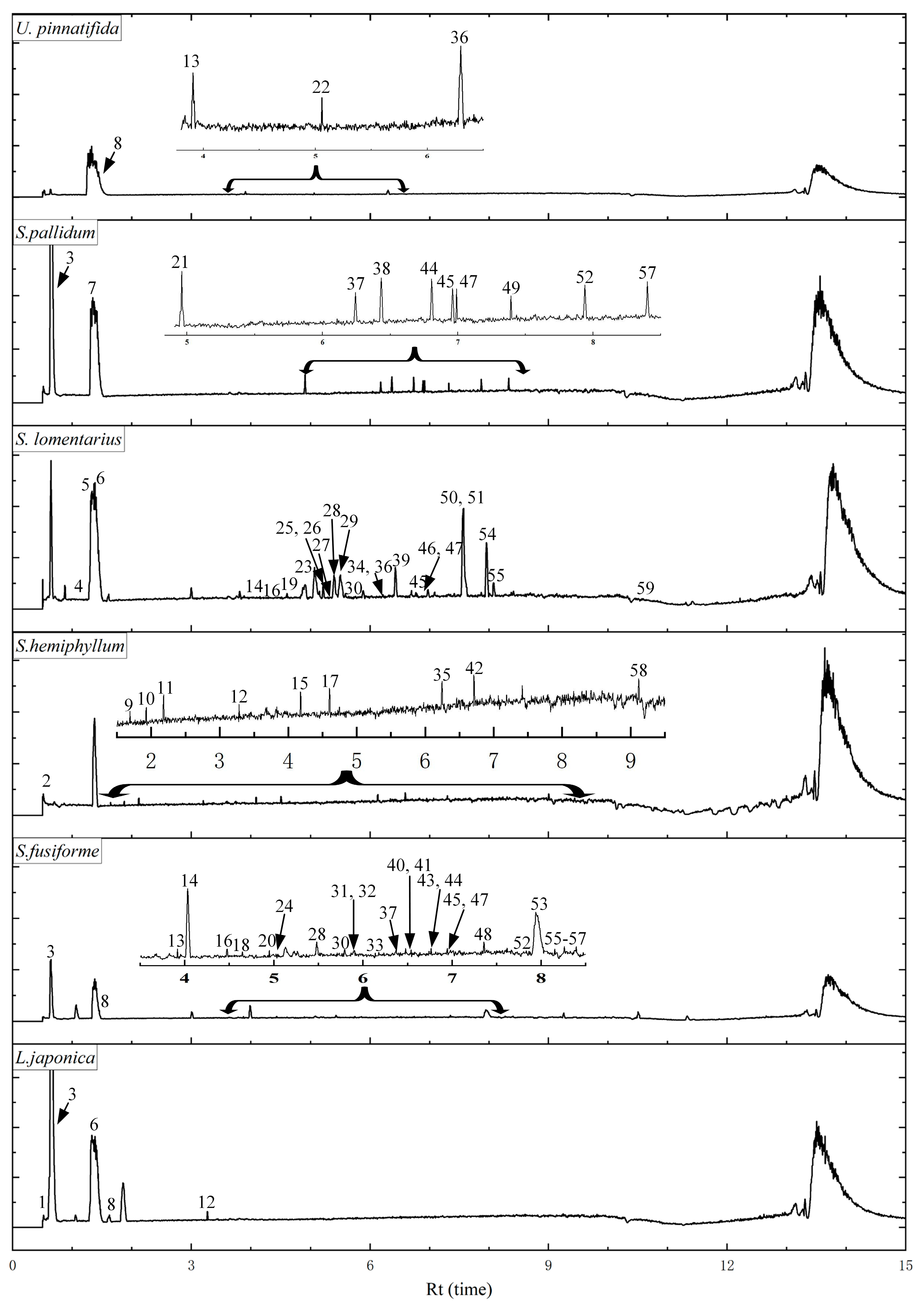
3.4. UPLC Analysis of Different Components
3.5. Network Pharmacological Analysis
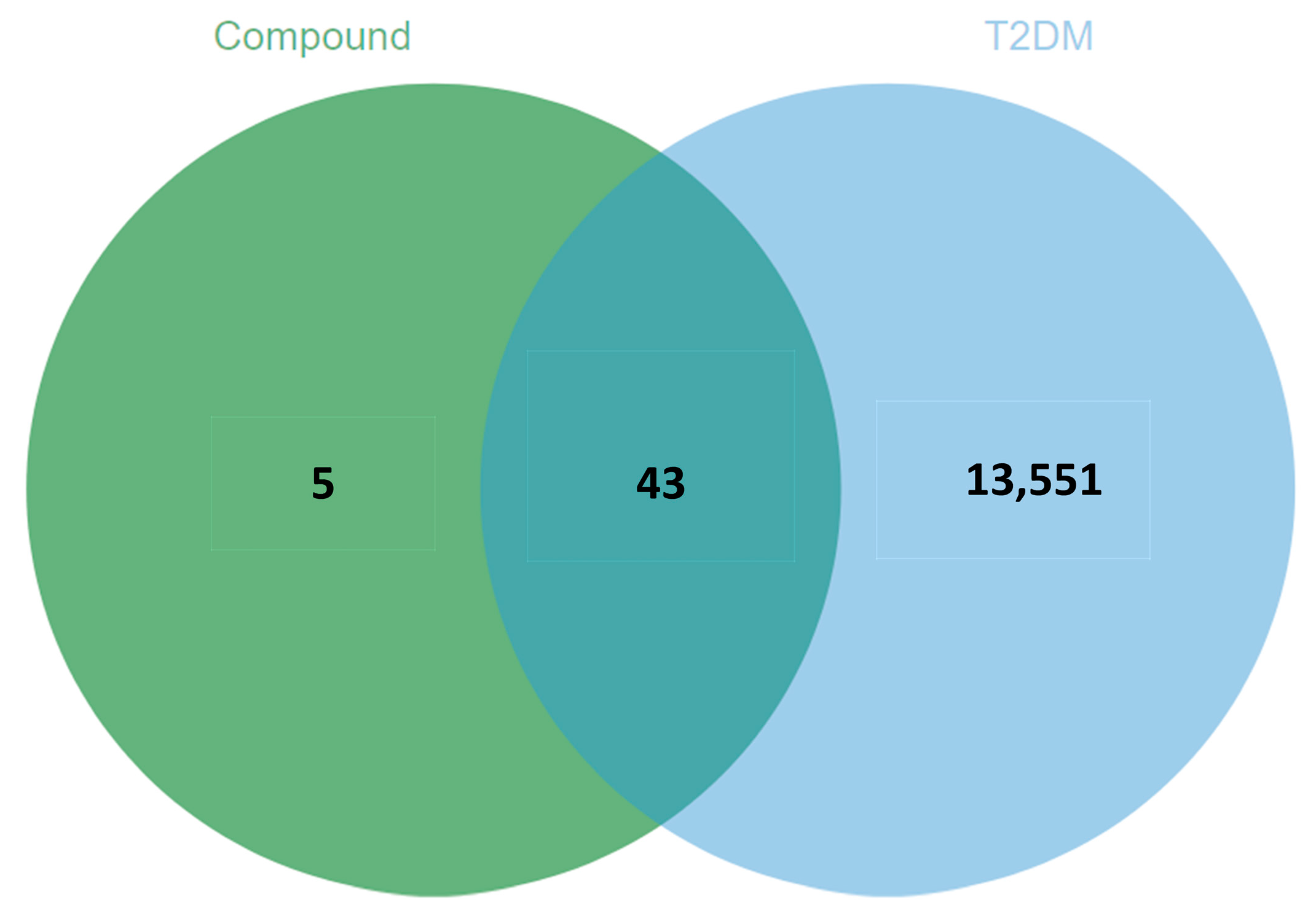
3.5.1. Potential Target Prediction and Screening
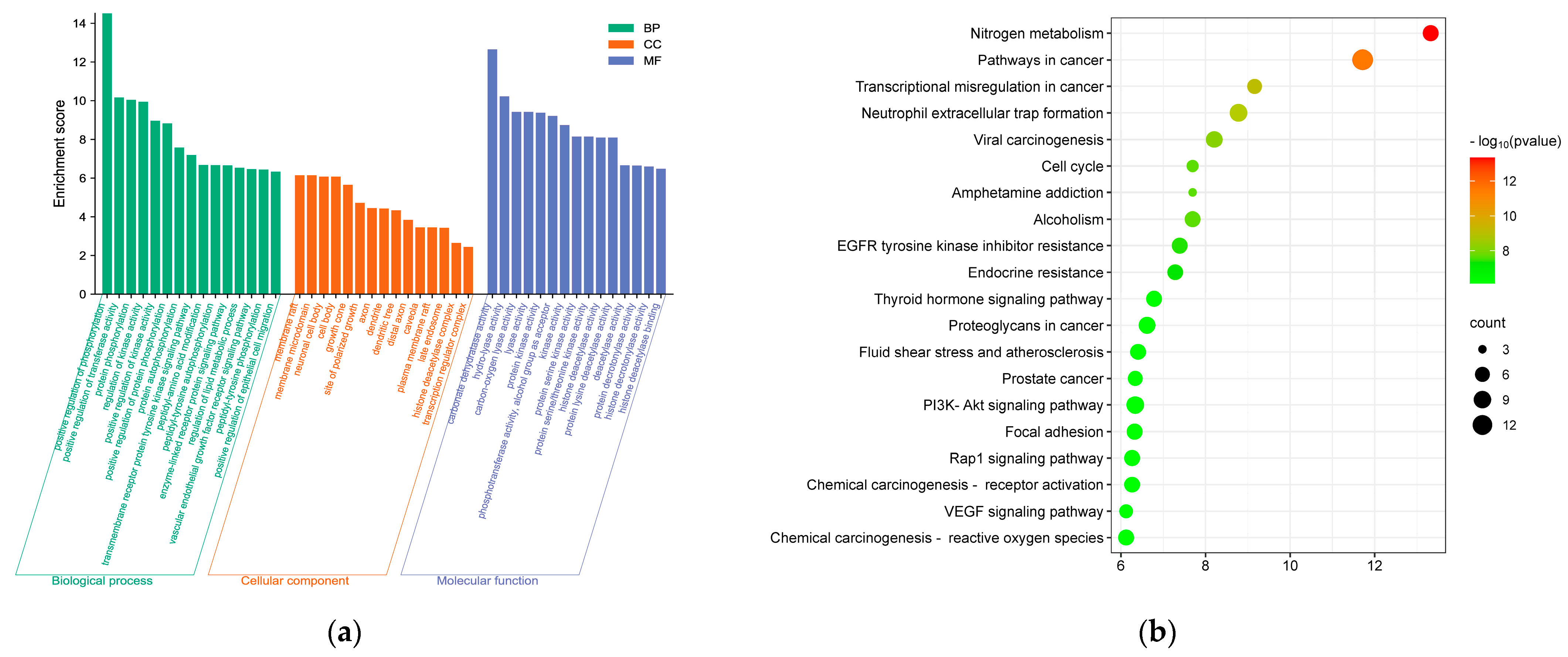
3.5.2. GO Function and KEGG Pathway Enrichment Analysis
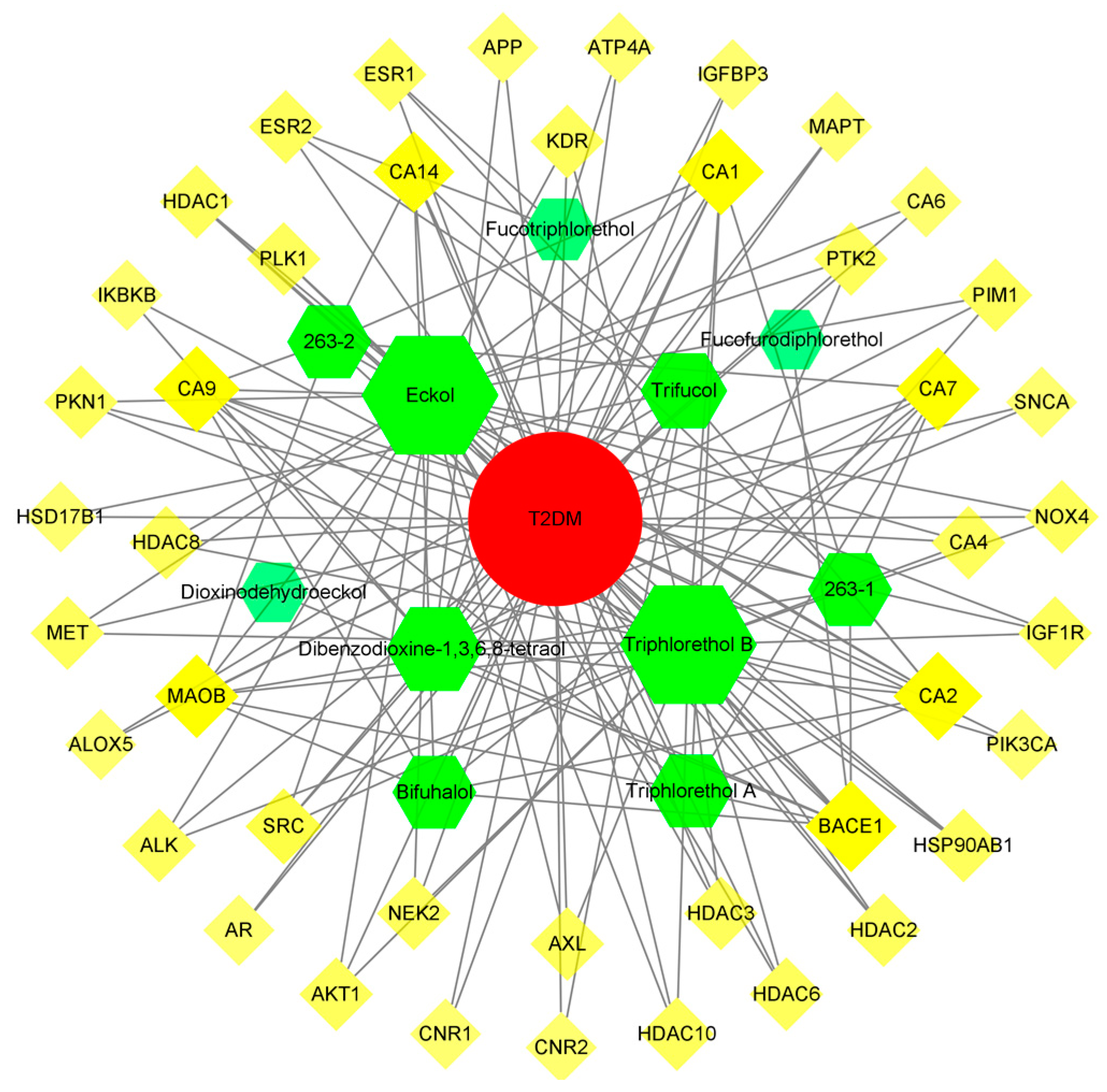
3.5.3. Compound-Target-Disease Network Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Jain, S.; Saraf, S. Type 2 diabetes mellitus-Its global prevalence and therapeutic strategies. Diabetes Metab. Syndr. 2010, 4, 48–56. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40 (Suppl. S1), S11–S24. [Google Scholar] [CrossRef] [Green Version]
- Nasab, S.B.; Homaei, A.; Pletschke, B.I.; Salinas-Salazar, C.; Castillo-Zacarias, C.; Parra-Saldívar, R. Marine resources effective in controlling and treating diabetes and its associated complications. Process Biochem. 2020, 92, 313–342. [Google Scholar] [CrossRef]
- Olaokun, O.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complement. Med. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Feng, J.; Peng, Q.; Liu, X.; Fan, Z.C. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxidative Med. Cell. Longev. 2019, 2019, 9570616. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Kim, K.R.; Lee, J.H.; Kim, S.J.; Rhee, S.D.; Jung, W.H.; Yang, S.D.; Kim, S.S.; Ahn, J.H.; Cheon, H.G. KR-62980: A novel peroxisome proliferator-activated receptor gamma agonist with weak adipogenic effects. Biochem. Pharmacol. 2006, 72, 446–454. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, J.H.; Han, J.S. A phlorotannin constituent of Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Pharm. Biol. 2017, 55, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, M.H.; Heo, S.J.; Kang, S.M.; Ko, S.C.; Han, J.S.; Jeon, Y.J. Dieckol isolated from Ecklonia cava inhibits alpha-glucosidase and alpha-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, J.H.I.; Castañeda, H.G.T. Preparation and chromatographic analysis of phlorotannins. J. Chromatorgr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskl) C. Agardh. Arab. J. Chem. 2017, 10, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Park, S.R.; Kim, J.H.; Jang, H.D.; Yang, S.Y.; Kim, Y.H. Inhibitory activity of minor phlorotannins from Ecklonia cava on α-glucosidase. Food Chem. 2018, 257, 128–134. [Google Scholar] [CrossRef]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins of the Brown Algae Sargassum vulgare from the Mediterranean Sea Coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, S.H.; Yoon, N.Y.; Jung, W.K.; Jeon, Y.J.; Kim, S.K.; Lee, M.S.; Kim, Y.M. α-Glucosidase- and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agr. 2012, 92, 2084–2090. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Nickavar, B.; Esbati, N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to control hyperglycemia and diabetes-related vascular complications. J. Appl. Phycol. 2019, 31, 3143–3152. [Google Scholar] [CrossRef]
- Shen, P.; Gu, Y.; Zhang, C.X.; Sun, C.H.; Qin, L.; Yu, C.X.; Qi, H. Metabolomic Approach for Characterization of Polyphenolic Compounds in Laminaria japonica, Undaria pinnatifida, Sargassum fusiforme and Ascophyllum nodosum. Foods 2021, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Stein, T.I.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef] [Green Version]
- Consortium, U.P. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293–299. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [Green Version]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological. Networks 2010, 696, 291–303. [Google Scholar]
- Barros Santos, M.C.; Ribeiro da Silva Lima, L.; Ramos Nascimento, F.; Pimenta do Nascimento, T.; Cameron, L.C.; Simões Larraz Ferreira, M. Metabolomic approach for characterization of phenolic compounds in different wheat genotypes during grain development. Food Res. Int. 2019, 124, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.F.; Bai, Y.; Xu, Z.; Shi, Y.X.; Sun, Y.H.; Janaswamy, S.; Yu, C.X.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef] [Green Version]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. 2019, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch. Pharm. Res. 2006, 29, 165–171. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Gall, E.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Catarino, M.D.; Marçal, C.; Bonifácio-Lopes, T.; Campos, D.; Mateus, N.; Silva, A.M.S.; Pintado, M.M.; Cardoso, S.M. Impact of Phlorotannin Extracts from Fucus vesiculosus on Human Gut Microbiota. Mar. Drugs 2021, 19, 375. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, J.; Fan, J.J.; Clark, J.; Shen, P.L.; Li, Y.Q.; Zhang, C.S. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Fu, X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT-Food Sci. Technol. 2021, 138, 110654. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, P.; Ye, S.H.; Li, W.; Li, M.; Ding, Y. Systems pharmacology-based drug discovery and active mechanism of phlorotannins for type 2 diabetes mellitus by integrating network pharmacology and experimental evaluation. J. Food Biochem. 2022, 46, e14492. [Google Scholar] [CrossRef]
- Ismail, G.A.; Gheda, S.F.; Abo-Shady, A.M.; Abdel-Karim, O.H. In vitropotential activity of some seaweeds as antioxidants and inhibitors of diabetic enzymes. Food Sci. Tech. 2020, 40, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.J.; Fu, X.T.; Duan, D.L.; Liu, X.Y.; Xu, J.C.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Zhang, R.; Yuen, A.K.L.; Magnusson, M.; Wright, J.T.; Nys, R.D.; Masters, A.F.; Maschmeyer, T. A comparative assessment of the activity and structure of phlorotannins from the brown seaweed Carpophyllum flexuosum. Algal Res. 2018, 29, 130–141. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. Dev. Appl. Phycol. 2007, 20, 255–261. [Google Scholar]
- Li, Z.; Li, Y.; Overstreet, J.M.; Chung, S.J.; Niu, A.L.; Fan, X.F.; Wang, S.W.; Wang, Y.Q.; Zhang, M.Z.; Harris, R.C. Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes 2018, 67, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Song, M.; Wang, P.; Zhao, R.; Chen, H.Y.; Zhang, M.; Shi, Y.Y.; Liu, K.D.; Liu, F.F.; Yang, R.; et al. Targeted therapy of the AKT kinase inhibits esophageal squamous cell carcinoma growth in vitro and in vivo. Int. J. Cancer 2019, 145, 1007–1019. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, K.C.R.; Laurindo, C.P.; Machado, U.F. Estrogen and Glycemic Homeostasis: The Fundamental Role of Nuclear Estrogen Receptors ESR1/ESR2 in Glucose Transporter GLUT4 Regulation. Cells 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, S.M.; Santos, F.F.; Costa, B.P.; Ceravolo, G.S.; Santos-Eichler, R.; Carvalho, M.H.C.; Fortes, Z.B.; Akamine, E.H. Involvement of inducible nitric oxide synthase and estrogen receptor ESR2 (ERβ) in the vascular dysfunction in female type 1 diabetic rats. Life Sci. 2019, 216, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nogal-Ávila, M.D.; Troyano-Suárez, N.; Román-García, P.; Cannata-Andía, J.B.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D.; Kuro-O, M.; Ruiz-Torres, M.P. Amadori products promote cellular senescence activating insulin-like growth factor-1 receptor and down-regulating the antioxidant enzyme catalase. Int. J. Biochem. Cell B 2013, 45, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Obesity and Diabetic Kidney Disease: Role of Oxidant Stress and Redox Balance. Antioxid. Redox Signal. 2016, 25, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Xia, L.; Goldberg, H.; Lee, K.W.; Quaggin, S.E.; Fantus, I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013, 288, 6835–6848. [Google Scholar] [CrossRef] [Green Version]
| No. | Sample | Total Solids (mg) | TPC (g PGE/100 g DW) | Enrichment (Fold) | |
|---|---|---|---|---|---|
| 1 | U. pinnatifida | EE | 4534.8 | 0.50 | / |
| 2 | HEX | 1164.4 | 0.64 | 1.28 | |
| 3 | EA | 810.4 | 1.56 | 3.12 | |
| 4 | AQ | 2560.0 | 0.11 | 0.23 | |
| 5 | S. pallidum | EE | 2956.0 | 0.74 | / |
| 6 | HEX | 350.3 | 3.49 | 4.72 | |
| 7 | EA | 206.7 | 2.34 | 3.16 | |
| 8 | AQ | 2399 | 0.20 | 0.06 | |
| 9 | S. lomentarius | EE | 6948.4 | 0.30 | / |
| 10 | HEX | 754.9 | 0.68 | 2.25 | |
| 11 | EA | 326.5 | 1.60 | 5.32 | |
| 12 | AQ | 5867.0 | 0.24 | 0.78 | |
| 13 | S. hemiphyllum | EE | 1842.4 | 2.28 | / |
| 14 | HEX | 1021.4 | 2.85 | 1.25 | |
| 15 | EA | 132.6 | 6.32 | 2.78 | |
| 16 | AQ | 688.4 | 0.58 | 0.25 | |
| 17 | S. fusiforme | EE | 1359.6 | 1.12 | / |
| 18 | HEX | 82.8 | 1.80 | 1.60 | |
| 19 | EA | 114.0 | 1.90 | 1.70 | |
| 20 | AQ | 1162.8 | 1.02 | 0.91 | |
| 21 | L. japonica | EE | 622.0 | 3.57 | / |
| 22 | HEX | 246.0 | 0.75 | 0.21 | |
| 23 | EA | 64.2 | 0.71 | 0.20 | |
| 24 | AQ | 311.8 | 4.08 | 1.14 | |
| No. | Sample | DPPH (IC50 mg/mL) | ABTS (IC50 mg/mL) | FRAP (Vc mg/g DW) | α-Glucosidase (IC50 mg/mL) | α-Amylase (IC50 mg/mL) | |
|---|---|---|---|---|---|---|---|
| 1 | U. pinnatifida | EE | 4.25 ± 0.53 | 23.08 ± 3.25 | 14.44 ± 1.04 | 11.06 ± 0.39 | 2.44 ± 0.03 |
| 2 | Hex | 1.67 ± 0.15 | 0.40 ± 0.01 | 18.53 ± 2.12 | 0.61 ± 0.08 | 2.16 ± 0.03 | |
| 3 | EA | 2.14 ± 0.03 | 0.51 ± 0.06 | 16.22 ± 0.28 | 0.89 ± 0.10 | 2.17 ± 0.04 | |
| 4 | AQ | 60.37 ± 3.04 | 13.78 ± 1.27 | 14.17 ± 0.28 | 116.90 ± 16.02 | 8.33 ± 1.81 | |
| 5 | S. pallidum | EE | 4.43 ± 0.38 | 2.16 ± 0.08 | 13.50 ± 0.76 | 0.65 ± 0.06 | 3.37 ± 0.26 |
| 6 | Hex | 1.08 ± 0.02 | 0.69 ± 0.04 | 18.80 ± 1.16 | 0.05 ± 0.03 | 2.55 ± 0.02 | |
| 7 | EA | 0.33 ± 0.03 | 0.11 ± 0.01 | 22.94 ± 0.81 | 0.05 ± 0.01 | 0.51 ± 0.01 | |
| 8 | AQ | 8.62 ± 2.88 | 10.97 ± 3.06 | 11.64 ± 0.08 | 5.51 ± 1.15 | 1.64 ± 0.06 | |
| 9 | S. lomentarius | EE | 17.03 ± 1.60 | 7.63 ± 2.17 | 13.24 ± 0.28 | 0.57 ± 0.07 | 148.08 ± 23.41 |
| 10 | Hex | 25.35 ± 2.26 | 5.76 ± 0.98 | 13.46 ± 0.48 | 0.26 ± 0.01 | 21.74 ± 4.40 | |
| 11 | EA | 1.91 ± 0.15 | 2.95 ± 0.41 | 13.68 ± 0.78 | 0.26 ± 0.04 | 2.72 ± 0.04 | |
| 12 | AQ | 27.05 ± 2.05 | 8.78 ± 1.68 | 13.55 ± 0.66 | 65.80 ± 12.24 | 278.08 ± 5.73 | |
| 13 | S. hemiphyllum | EE | 1.08 ± 0.02 | 0.24 ± 0.01 | 17.22 ± 0.28 | 0.15 ± 0.02 | 1.73 ± 0.03 |
| 14 | Hex | 0.96 ± 0.03 | 0.27 ± 0.01 | 21.18 ± 1.82 | 0.06 ± 0.05 | 6.64 ± 0.69 | |
| 15 | EA | 0.35 ± 0.01 | 0.02 ± 0.00 | 53.74 ± 5.52 | 0.02 ± 0.00 | 0.02 ± 0.00 | |
| 16 | AQ | 0.52 ± 0.01 | 0.57 ± 0.00 | 24.01 ± 1.64 | 152.61 ± 17.37 | 8.53 ± 4.34 | |
| 17 | S. fusiforme | EE | 1.08 ± 0.03 | 0.50 ± 0.03 | 18.89 ± 0.20 | 0.38 ± 0.03 | 22.00 ± 1.32 |
| 18 | Hex | 1.49 ± 0.02 | 1.06 ± 0.02 | 19.25 ± 1.17 | 0.61 ± 0.02 | 104.31 ± 23.51 | |
| 19 | EA | 0.94 ± 0.02 | 0.32 ± 0.01 | 19.47 ± 0.63 | 0.19 ± 0.02 | 3.49 ± 0.15 | |
| 20 | AQ | 1.01 ± 0.07 | 0.55 ± 0.05 | 18.80 ± 0.81 | 0.22 ± 0.03 | 5.18 ± 0.18 | |
| 21 | L. japonica | EE | 1.85 ± 0.06 | 0.47 ± 0.02 | 14.35 ± 1.54 | 0.27 ± 0.01 | 1.15 ± 0.03 |
| 22 | Hex | 6.37 ± 0.56 | 1.82 ± 0.03 | 13.99 ± 1.04 | 0.31 ± 0.01 | 0.46 ± 0.01 | |
| 23 | EA | 7.02 ± 1.01 | 21.39 ± 3.67 | 13.77 ± 0.28 | 0.18 ± 0.03 | 3.36 ± 0.15 | |
| 24 | AQ | 2.56 ± 0.08 | 0.43 ± 0.04 | 14.93 ± 0.61 | 1.62 ± 0.26 | 6.69 ± 0.76 | |
| 25 | Vc | 0.01 ± 0.00 | 0.27 ± 0.03 | / | / | / | |
| 26 | Acarbose | / | / | / | 0.63 ± 0.06 | 2.51 ± 0.11 | |
| No. | Rt (min) | Precursor Ion MS 1 | Product Ions MS 2 | Tentative Assignment | Species | Ref. |
|---|---|---|---|---|---|---|
| [M-H]−, m/z | [M-H]−, m/z | |||||
| 1 | 0.61 | 317 | 299.05, 187.03 | Phlorotannin derivative | LJ | [44] |
| 2 | 0.66 | 387 | 261.89 | Phlorethohydroxycarmalol | SH | [10] |
| 3 | 0.66 | 267 | 249.04, 223.04, 221.02 | Phlorotannin derivative | SP, LJ, SF | [19] |
| 4 | 1.01 | 263 | 245.89, 111.02 | Phlorethohydroxycarmalol | SL | [10] |
| 5 | 1.28 | 317 | 272.88 | Phlorotannin derivative | SL | [44] |
| 6 | 1.30 | 385 | 341.01, 312.90, 261.06, 245.04 | Phlorotannin derivative | LJ, SL | [44] |
| 7 | 1.36 | 317 | 298.84 | Phlorotannin derivative | SP | [44] |
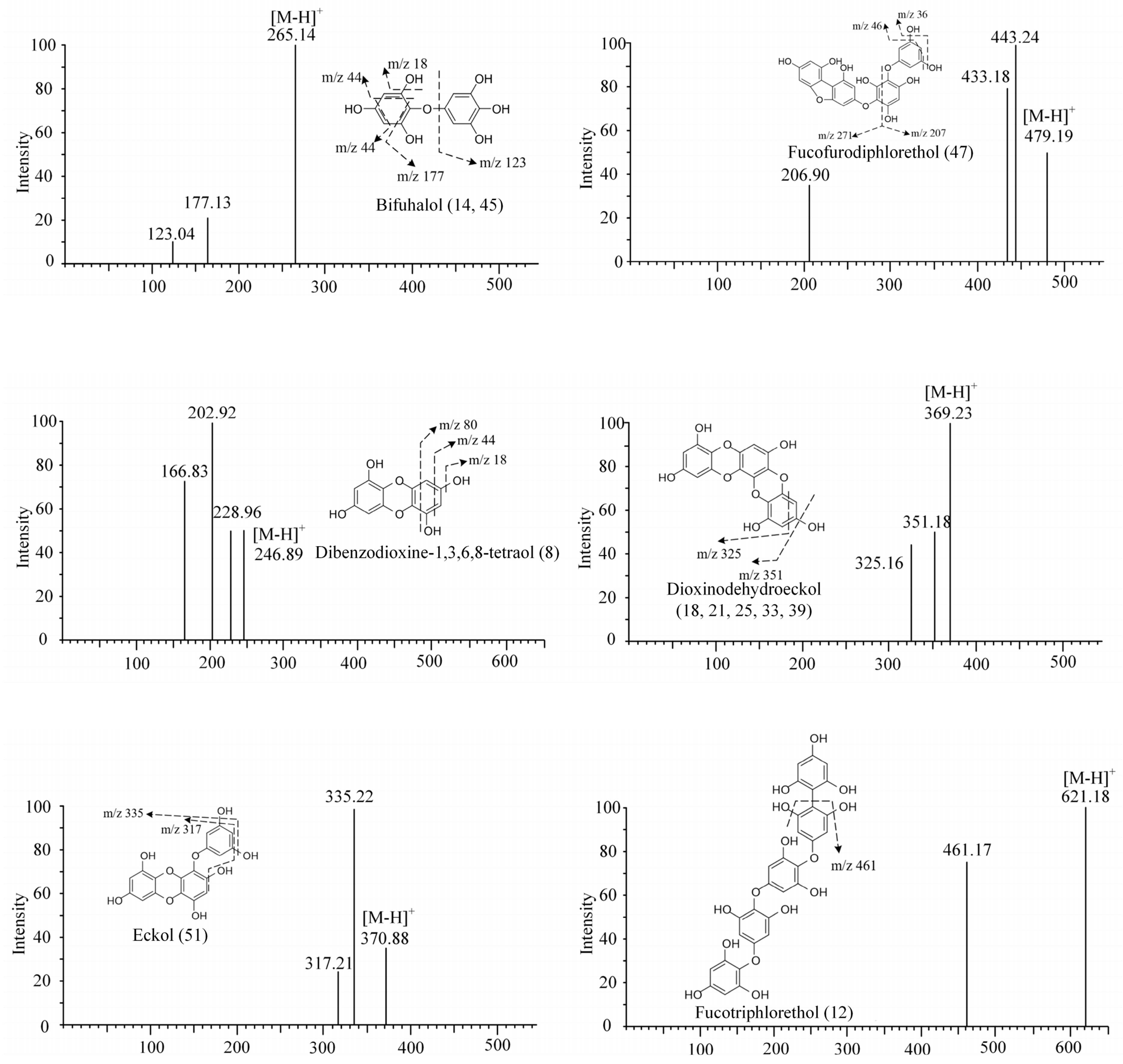
| 8 | 1.47 | 247 | 228.96, 202.92, 166.83 | Dibenzodioxine-1,3,6,8-tetraol | SP, LJ, UP, SF | [19] |
| 9 | 1.69 | 317 | 187.04 | Phlorotannin derivative | SH | [44] |
| 10 | 1.93 | 361 | 317.03, 298.86, 273.04 | Phlorotannin derivative | SH | [44] |
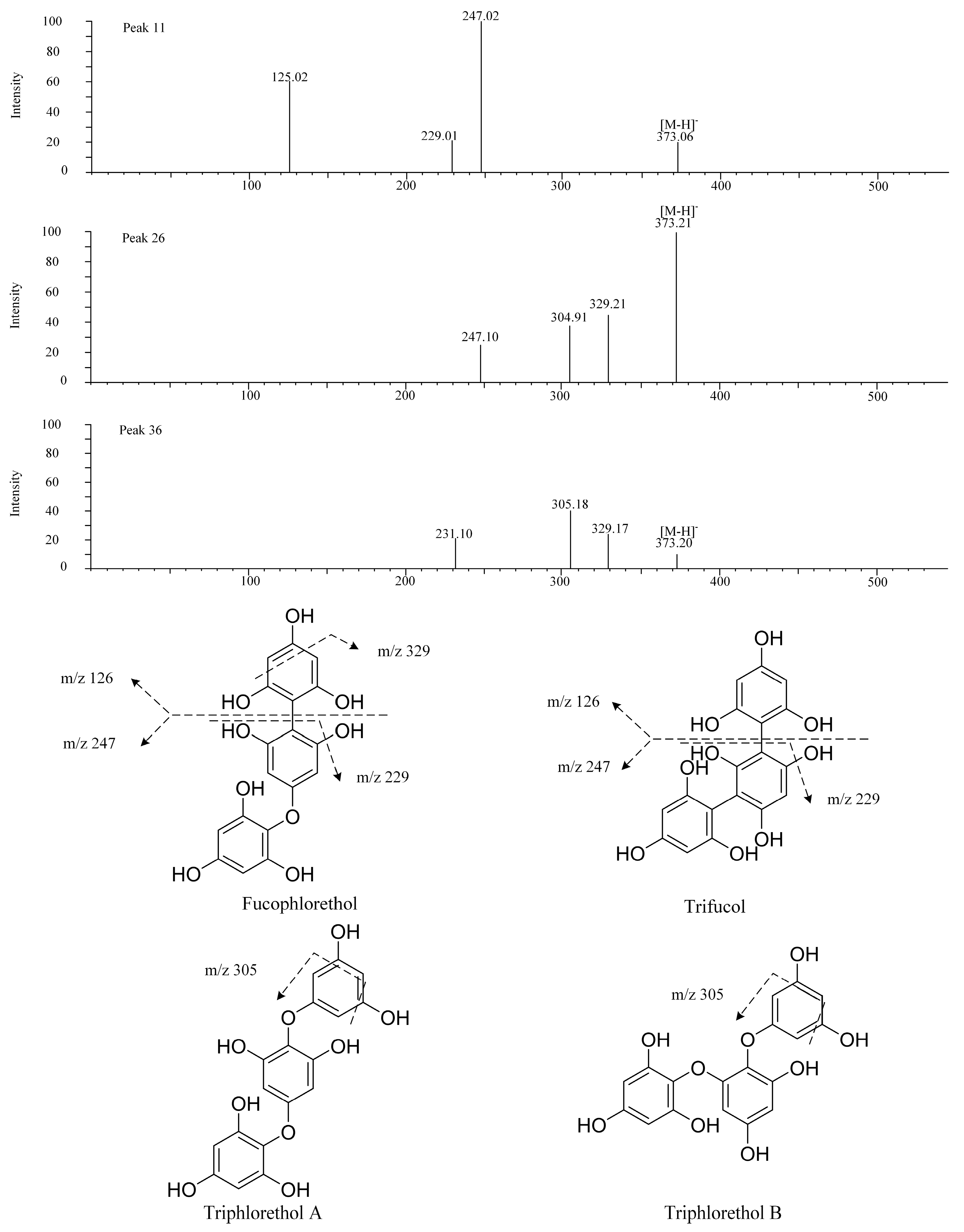
| 11 | 2.18 | 373 | 247.02, 229.01 | Trifucol/fucophlorethol | SH | [10] |
| 12 | 3.28 | 621 | 461.17 | Fucotriphlorethol | SH, LJ | [19] |
| 13 | 3.92 | 317 | 298.92, 228.89 | Phlorotannin derivative | SF, UP | [44] |
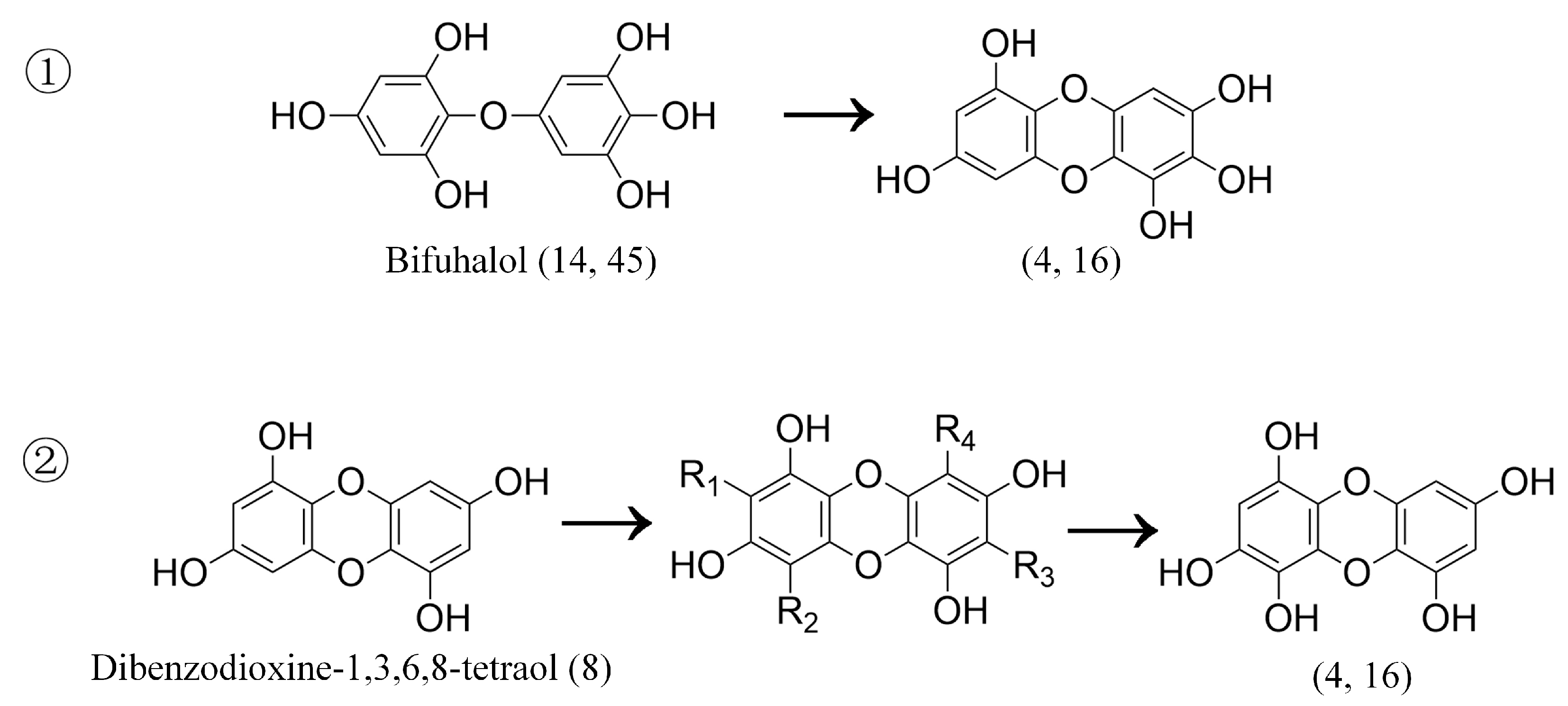
| 14 | 4.06 | 265 | 247.09, 221.12, 193.12 | Bifuhalol | SL, SF | [19] |
| 15 | 4.18 | 267 | 222.96 | Phlorotannin derivative | SH | [19] |
| 16 | 4.37 | 263 | 245.12, 219.14 | Phlorethohydroxycarmalol | SL, SF | [45] |
| 17 | 4.60 | 317 | 298.92, 228.89 | Phlorotannin derivative | SH | [44] |
| 18 | 4.65 | 369 | 325.16 | Dioxinodehydroeckol | SF | [10] |
| 19 | 4.67 | 395 | 351.18 | Phlorotannin derivative | SL | [19] |
| 20 | 4.95 | 361 | 317.03, 298.85, 273.04 | Phlorotannin derivative | SF | [44] |
| 21 | 4.96 | 369 | 351.22 | Dioxinodehydroeckol | SP | [10] |
| 22 | 5.06 | 267 | 222.96, 220.85 | Phlorotannin derivative | UP | [19] |
| 23 | 5.13 | 385 | 261.22 | Phlorotannin derivative | SL | [44] |
| 24 | 5.13 | 395 | 351.18, 249.11 | Phlorotannin derivative | SF | [19] |
| 25 | 5.24 | 369 | 351.18 | Dioxinodehydroeckol | SL | [10] |
| 26 | 5.24 | 373 | 329.21 | Fucophlorethol | SL | [10] |
| 27 | 5.29 | 421 | 403.18, 377.17, 213.16 | Phlorotannin derivative | SL | [19] |
| 28 | 5.46 | 387 | 329.23 | Phlorethohydroxycarmalol | SL, SF | [10] |
| 29 | 5.56 | 267 | 223.13, 195.10, 177.09 | Phlorotannin derivative | SL | [19] |
| 30 | 5.79 | 317 | 299.16 | Phlorotannin derivative | SL, SF | [44] |
| 31 | 5.90 | 385 | 367.21, 260.93 | Phlorotannin derivative | SF | [44] |
| 32 | 5.90 | 421 | 377.19 | Phlorotannin derivative | SF | [19] |
| 33 | 6.14 | 369 | 351.22 | Dioxinodehydroeckol | SF | [10] |
| 34 | 6.24 | 287 | 269.15, 243.18 | Phlorotannin derivative | SL | [19] |
| 35 | 6.24 | 317 | 272.92, 228.93, 187.36 | Phlorotannin derivative | SP, SH | [44] |
| 36 | 6.27 | 373 | 329.21, 305.18, 126.90 | Triphlorethol/trifucol/fucophlorethol | UP, SL | [10] |
| 37 | 6.37 | 363 | 318.91, 274.92 | Phlorotannin derivative | SF | [19] |
| 38 | 6.43 | 363 | 318.91, 274.92 | Phlorotannin derivative | SP | [44] |
| 39 | 6.45 | 369 | 351.22 | Dioxinodehydroeckol | SL | [10] |
| 40 | 6.48 | 267 | 249.19, 222.96 | Phlorotannin derivative | SF | [19] |
| 41 | 6.54 | 469 | 425.25, 264.94 | Phlorotannin derivative | SF | [19] |
| 42 | 6.71 | 361 | 298.86 | Phlorotannin derivative | SH | [44] |
| 43 | 6.76 | 403 | 259.09 | Phlorotannin derivative | SF | [44] |
| 44 | 6.79 | 287 | 269.21, 243.17, 214.93, 172.92 | Phlorotannin derivative | SF, SP | [19] |
| 45 | 6.94 | 265 | 247.17, 221.01, 177.02, 168.78 | Bifuhalol | SF, SP, SL | [19] |
| 46 | 6.95 | 317 | 299.20, 273.22, 255.21, 245.19 | Phlorotannin derivative | SL | [44] |
| 47 | 6.96 | 479 | 443.24, 433.19, 206.90 | Fucofurodiphlorethol | SP, SL, SF | [44] |
| 48 | 7.36 | 711 | 675.36, 371.33 | Phlorotannin derivative | SF | [44] |
| 49 | 7.39 | 267 | 249.19, 222.96, 195.14 | Phlorotannin derivative | SP | [19] |
| 50 | 7.75 | 317 | 299.16, 255.21 | Phlorotannin derivative | SL | [44] |
| 51 | 7.79 | 371 | 335.22, 317.21 | Eckol | SL | [10] |
| 52 | 7.94 | 317 | 299.20, 273.22, 255.21 | Phlorotannin derivative | SP, SF | [44] |
| 53 | 8.00 | 509 | 372.89, 304.91 | Phlorotannin derivative | SF | [10] |
| 54 | 8.05 | 385 | 219.14 | Phlorotannin derivative | SL | [44] |
| 55 | 8.15 | 267 | 249.19, 223.21 | Phlorotannin derivative | SF, SL | [19] |
| 56 | 8.24 | 689 | 653.37, 552.50 | Phlorotannin derivative | SF | [44] |
| 57 | 8.40 | 363 | 317.21 | Phlorotannin derivative | SP, SF | [19] |
| 58 | 9.11 | 711 | 693.47, 267.20, 249.19 | Phlorotannin derivative | SH | [44] |
| 59 | 10.81 | 555 | 164.99 | Phlorotannin derivative | SL | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, Z.; Li, P.; Ye, S.; Li, W.; Li, M.; Zhu, L.; Ding, Y. Investigation of the Potential Phlorotannins and Mechanism of Six Brown Algae in Treating Type II Diabetes Mellitus Based on Biological Activity, UPLC-QE-MS/MS, and Network Pharmacology. Foods 2023, 12, 3000. https://doi.org/10.3390/foods12163000
Chen J, Zhou Z, Li P, Ye S, Li W, Li M, Zhu L, Ding Y. Investigation of the Potential Phlorotannins and Mechanism of Six Brown Algae in Treating Type II Diabetes Mellitus Based on Biological Activity, UPLC-QE-MS/MS, and Network Pharmacology. Foods. 2023; 12(16):3000. https://doi.org/10.3390/foods12163000
Chicago/Turabian StyleChen, Jialiang, Zheng Zhou, Ping Li, Shuhong Ye, Wei Li, Ming Li, Lin Zhu, and Yan Ding. 2023. "Investigation of the Potential Phlorotannins and Mechanism of Six Brown Algae in Treating Type II Diabetes Mellitus Based on Biological Activity, UPLC-QE-MS/MS, and Network Pharmacology" Foods 12, no. 16: 3000. https://doi.org/10.3390/foods12163000





