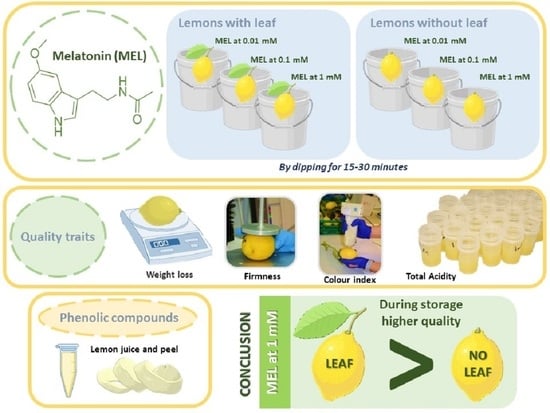
Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Lemon Fruit Quality Characteristics
2.3. Extraction and Quantification of Total Phenolic Content
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Melatonin on Respiration Rate and Weight Loss
3.2. Effect of Melatonin on Lemon Quality Characteristics
3.3. Effect of Melatonin on Total Phenolic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, Z.; Wang, H.; Liu, T.; Jia, Y.; Prasad, K.N.; Qu, H.; Duan, X.; Jiang, Y. Changes in quality attributes of mandarin with and without leaf during refrigerated storage. J. Food Process. Preserv. 2014, 38, 11–20. [Google Scholar] [CrossRef]
- Murai, K.; Chen, N.J.; Paull, R.E. Pineapple crown and slip removal on fruit quality and translucency. Sci. Hortic. 2021, 283, 110087. [Google Scholar] [CrossRef]
- Badiche, F.; Valverde, J.M.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Preharvest Use of γ-Aminobutyric Acid (GABA) as an Innovative Treatment to Enhance Yield and Quality in Lemon Fruit. Horticulturae 2023, 9, 93. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30. [Google Scholar] [CrossRef]
- Sepulveda, M.; Cuevas, I.I.; Smilanick, J.L.; Cerioni, L.; Rapisarda, V.A.; Ramallo, J. Improvement in imazalil treatments in commercial packinglines to control green mold on lemon fruit. Sci. Hortic. 2015, 192, 387–390. [Google Scholar] [CrossRef]
- Lemessa, A.; Popardowski, E.; Hebda, T.; Jakubowski, T. The Effect of UV-C Irradiation on the Mechanical and Physio-Logical Properties of Potato Tuber and Different Products. Appl. Sci. 2022, 12, 5907. [Google Scholar] [CrossRef]
- Klonsky, K. Comparison of production costs and resource use for organic and conventional production systems. Am. J. Agric. Econ. 2012, 94, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in plants and other phototrophs: Advances and gaps concerning the diversity of functions. J. Exper. Bot. 2015, 66, 627–646. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Arnao, M.B. Phytomelatonin: An emerging new hormone in plants. J. Exper. Bot. 2022, 73, 5773–5778. [Google Scholar] [CrossRef] [PubMed]
- Tijero, V.; Muñoz, P.; Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, M.E.; Zapata, P.J.; Valero, D.; Guillén, F. Melatonin Treatment of Pomegranate Trees Increases Crop Yield and Quality Parameters at Harvest and during Storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valverde, J.M.; Valero, D.; Serrano, M. Melatonin Treatment to Pomegranate Trees Enhances Fruit Bioactive Compounds and Quality Traits at Harvest and during Postharvest Storage. Antioxidants 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Antolí, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of Melatonin Treatment on Sweet Cherry Tree Yield and Fruit Quality. Agronomy 2022, 12, 3. [Google Scholar] [CrossRef]
- Mubarok, S.; Suminar, E.; Abidat, A.H.; Setyawati, C.A.; Setiawan, E.; Buswar, A.S. Overview of Melatonin’s Impact on Postharvest Physiology and Quality of Fruits. Horticulturae 2023, 9, 586. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Melatonin: A blooming biomolecule for postharvest management of perishable fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 17, 110236. [Google Scholar] [CrossRef]
- Jiménez-Cuesta, M.J.; Cuquerella, J.; Martínez-Jávega, J.M. Determination of a Color Index for Citrus Fruit Degreening. In Proceedings of the International Society of Citriculture, Tokyo, Japan, 9–12 November 1981; Volume 2, pp. 750–753. [Google Scholar]
- Aboryia, M.S.; Lo’ay, A.A.; Omar, A.S.M. Reduction of chilling injury of ‘Washington’ navel orange fruits by melatonin treatments during cold storage. Folia Hortic. 2021, 33, 343–353. [Google Scholar] [CrossRef]
- McCollum, T.G.; McDonald, R.E. Electrolyte leakage, respiration, and ethylene production as indices of chilling injury in grapefruit. HortScience 1991, 26, 1191–1192. [Google Scholar] [CrossRef] [Green Version]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Giménez, M.J.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Preharvest application of methyl jasmonate increases crop yield, fruit quality and bioactive compounds in pomegranate ‘Mollar de Elche’ at harvest and during postharvest storage. J. Sci. Food Agric. 2020, 100, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC-Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Hussain, S.; Khalid, M.F.; Ali, M.A.; Ahmed, N.; Hasanuzzaman, M.; Ahmad, S. Citrus Production: Technological Advancements and Adaptation to Changing Climate, 1st ed.; CRC-Taylor & Francis: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Murata, T.; Yamawaki, K. Respiratory changes of several varieties of citrus fruits during and after conditioning with two different humidities. J. Jpn. Soc. Hortic. Sci. 1989, 58, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, M.F.; Iglesias, D.J.; Primo-Millo, E.; Hernández, F.; Amorós, A. Efecto de la temperatura sobre la respiración de los órganos reproductivos y vegetativos del limón. Actas Hortic. 2015, 71, 283–285. [Google Scholar]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Miranda, S.; Vilches, P.; Suazo, M.; Pavez, L.; Garcia, K.; Mendez, M.A.; Gonzalez, M.; Meisel, L.A.; Defilippi, B.G.; Del Pozo, T. Melatonin triggers metabolic and gene expression changes leading to improved quality traits of two sweet cherry cultivars during cold storage. Food Chem. 2020, 319, 126360. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Wilson, K.B.; Hanson, P.J. Environmental control of whole-plant transpiration, canopy conductance and estimates of the decoupling coefficient for large red maple trees. Agric. For. Meteorol. 2000, 104, 157–168. [Google Scholar] [CrossRef]
- Bal, E. Effect of melatonin treatments on biochemical quality and postharvest life of nectarines. J. Food Meas. Charact. 2021, 15, 288–295. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Paniagua, C.; Posé, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit softening and pectin disassembly: An overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Yang, H.; Tie, W.W.; Yan, Y.; Ding, Z.; Liu, Y. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Núñez-Gómez, D.; Forner-Giner, M.Á.; Hernández, F.; Pastor-Pérez, J.J.; Legua, P. Quality Parameters of Spanish Lemons with Commercial Interest. Foods 2021, 10, 62. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, W.; Zhao, M.; Wang, J.; Wang, Q. Effect of melatonin treatment on visual quality and health-promoting properties of broccoli florets under room temperature. Food Chem. 2020, 319, 126498. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, L.; Zhang, L.; Lv, H.; He, Q.; Guo, L.; Zhang, X.; He, H.; Ren, S.; Zhang, N.; et al. Melatonin promotes carotenoid biosynthesis in an ethylene-dependent manner in tomato fruits. Plant Sci. 2020, 298, 110580. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Zhao, Y.T.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G.; Laskshmanan, P.; Chen, J.Y. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Kuniga, T.; Nakajima, N.; Yoshida, T. Quantification of carotenoids in citrus fruit by LC-MS and comparison of patterns of seasonal changes for carotenoids among citrus varieties. J. Agric. Food Chem. 2007, 55, 2356–2368. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Terol, J.; Rodrigo, M.J.; Licciardello, C.; Sadka, A. Fruit growth and development. In The Genus Citrus; Talón, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Oxford, UK, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatment with glycine betaine enhances chilling tolerance of blood orange fruit by increasing antioxidant defence systems and osmoregulation during cold storage. Sci. Hortic. 2022, 305, 111352. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Jankowski, P.; Radzanowska, J. Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruit (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol. Technol. 2014, 88, 21–33. [Google Scholar] [CrossRef]
- Parkin, K.L.; Marangoni, A.; Jackman, R.L.; Yada, R.Y.; Stanley, D.W. Chilling injury. A review of possible mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- Liu, J.; He, C.; Shen, F.; Zhang, K.; Zhu, S. The crown plays an important role in maintaining quality of harvested pineapple. Postharvest Biol. Technol. 2017, 124, 18–24. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef] [Green Version]
- Ye, X. Phytochemicals in Citrus: Applications in Functional Foods; CRC Press-Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Ho, C.T. Dietary bioactives and essential oils of lemon and lime fruits. Food Sci. Hum. Well 2022, 11, 753–764. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badiche-El Hilali, F.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds. Foods 2023, 12, 2979. https://doi.org/10.3390/foods12152979
Badiche-El Hilali F, Valverde JM, García-Pastor ME, Serrano M, Castillo S, Valero D. Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds. Foods. 2023; 12(15):2979. https://doi.org/10.3390/foods12152979
Chicago/Turabian StyleBadiche-El Hilali, Fátima, Juan Miguel Valverde, María E. García-Pastor, María Serrano, Salvador Castillo, and Daniel Valero. 2023. "Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds" Foods 12, no. 15: 2979. https://doi.org/10.3390/foods12152979