Auricularia auricular Adsorbs Aflatoxin B1 and Ameliorates Aflatoxin B1-Induced Liver Damage in Sprague Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Chemicals, and Reagents
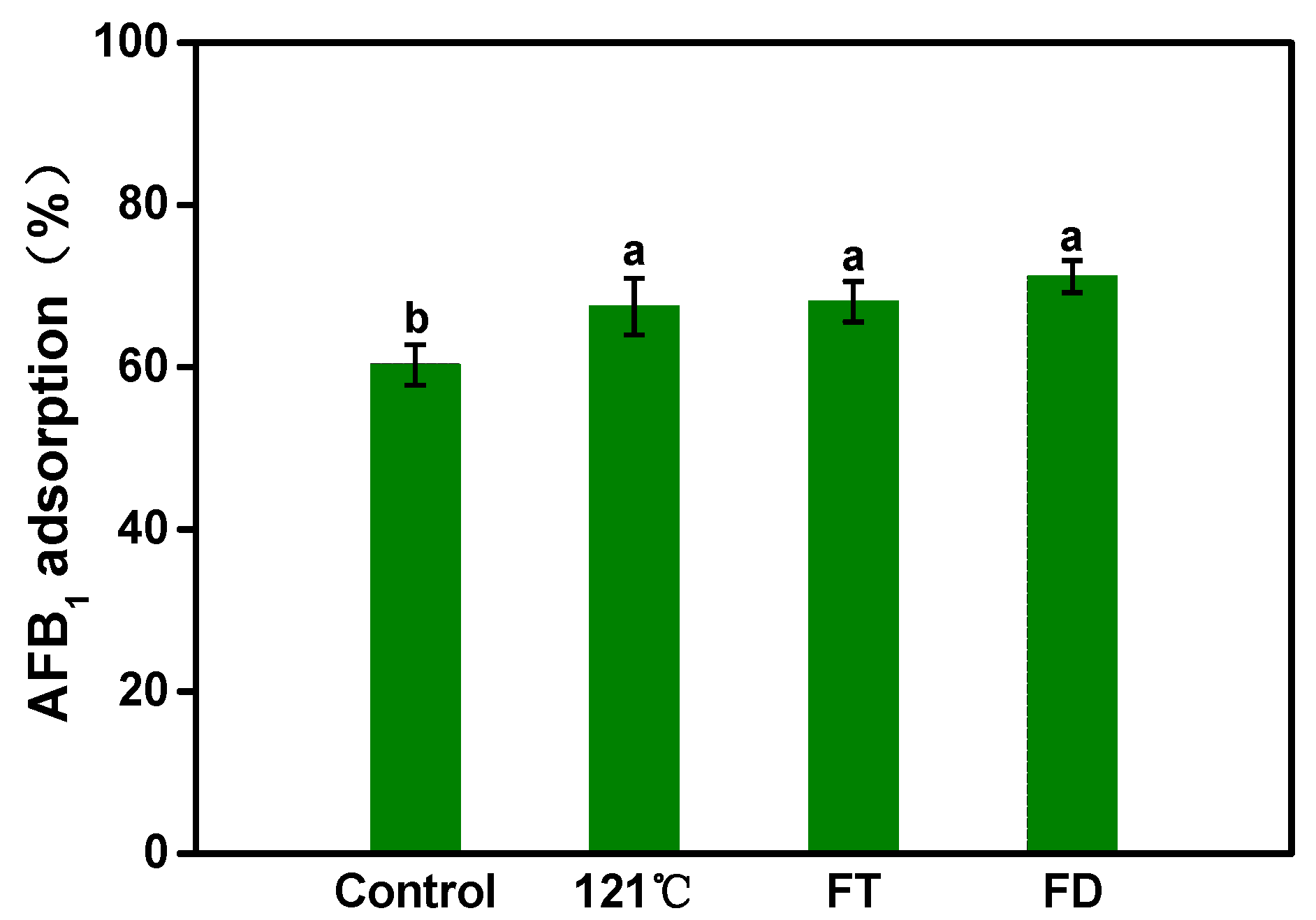
2.2. Assessment of Adsorption Capacity under Different Pretreatments
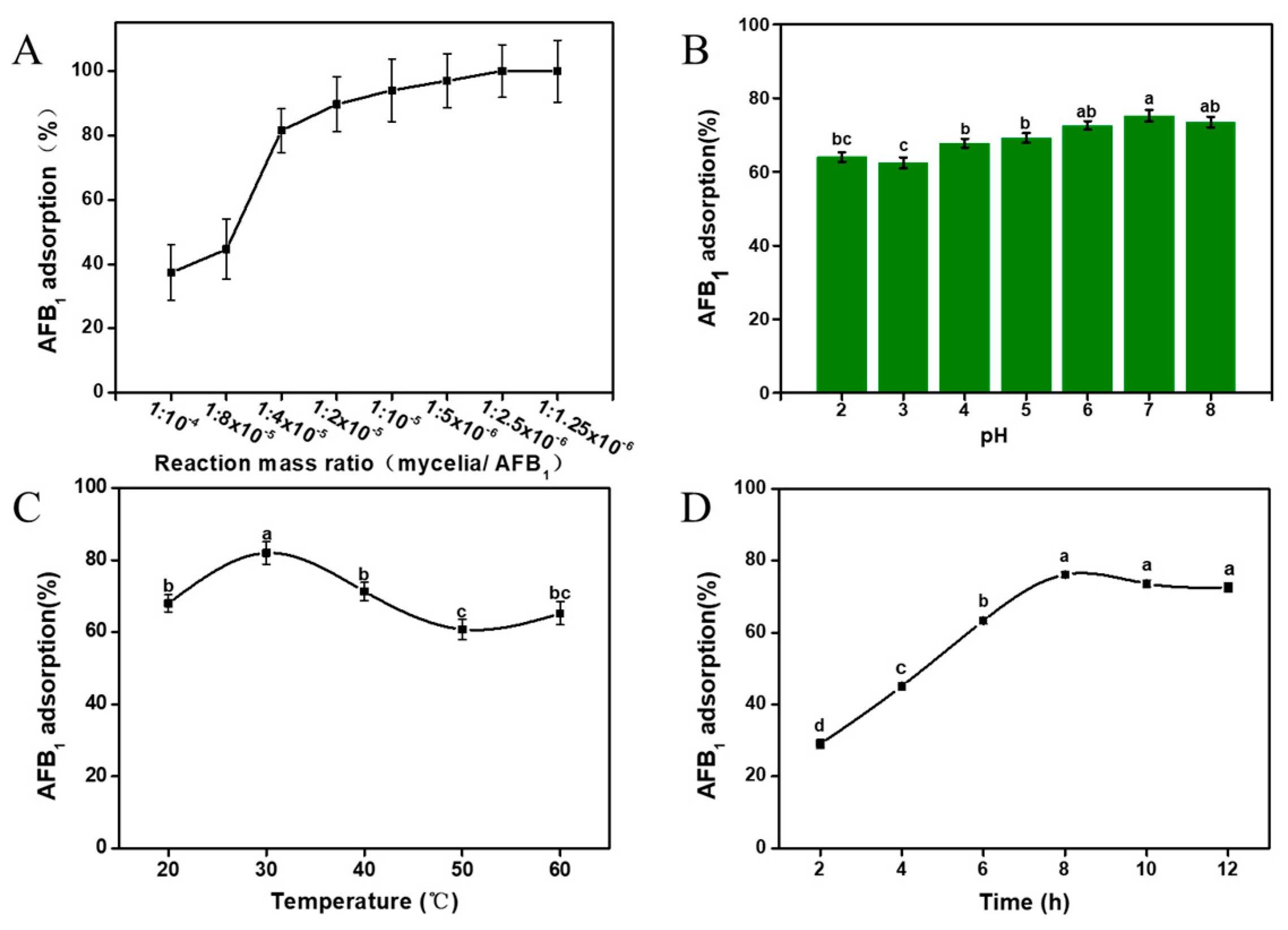
2.3. Effects of Reaction Ratio (Mycelia/AFB1), pH, Temperature and Incubation Time on AFB1 Adsorption Capacity
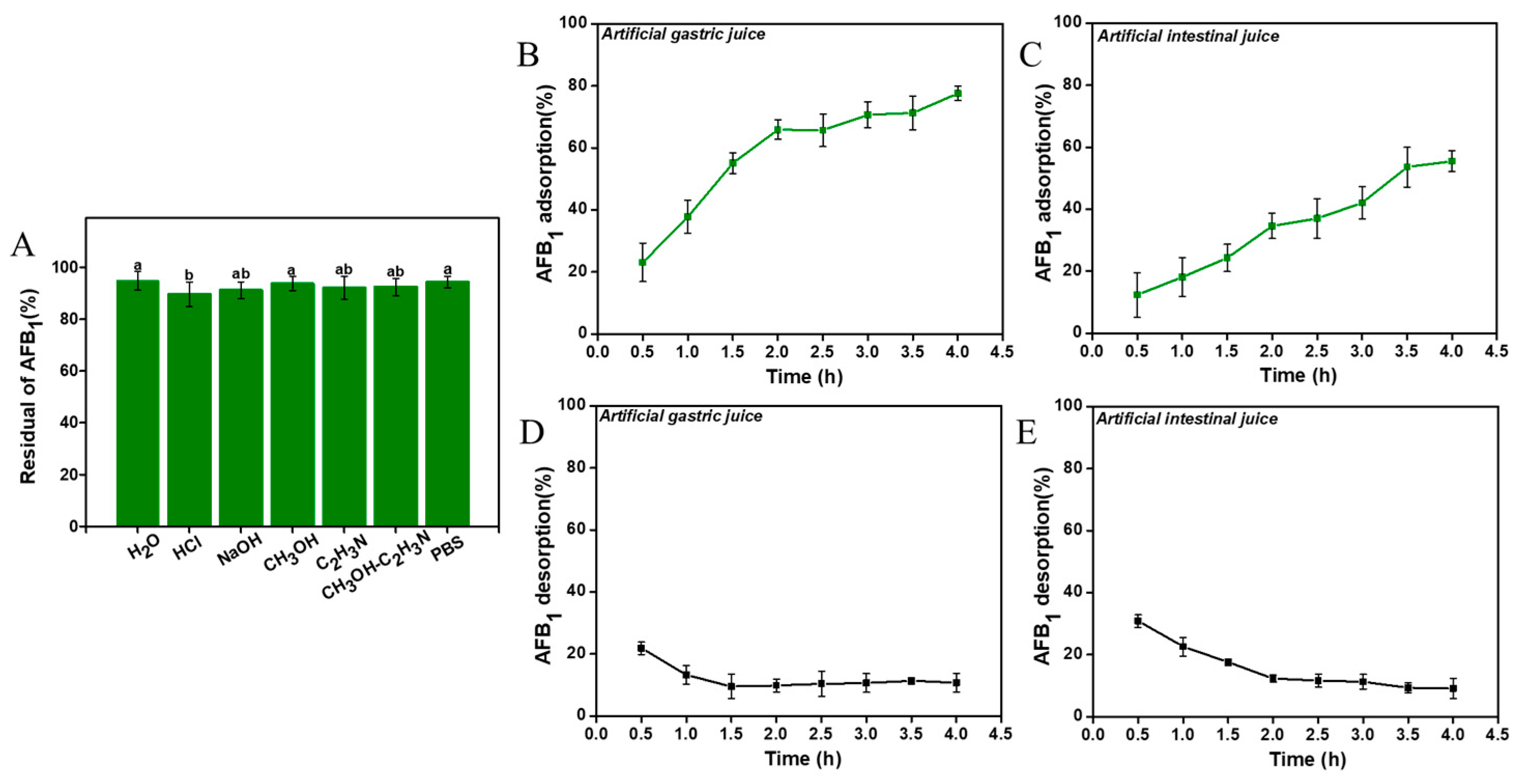
2.4. Stability of the Adsorption
2.5. Adsorption Kinetics
2.6. Adsorption Isotherm
2.7. Adsorption Thermodynamics
2.8. The In Vivo Evaluation of the Intervention Effect of A. auricular on AFB1-Induced Liver Injury
2.9. Statistics Analysis
3. Results
3.1. Characterization of AFB1 Adsorption by A. auricular Mycelia
3.1.1. Assessment of Adsorption Capacity under Different Pretreatments
3.1.2. Effects of Reaction Ratio (Mycelia/AFB1), pH, Temperature and Incubation Time on AFB1 Adsorption Capacity
3.1.3. Stability Evaluation of AFB1 Adsorption
3.2. Analysis of Adsorption Behavior
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherm
3.2.3. Adsorption Thermodynamics
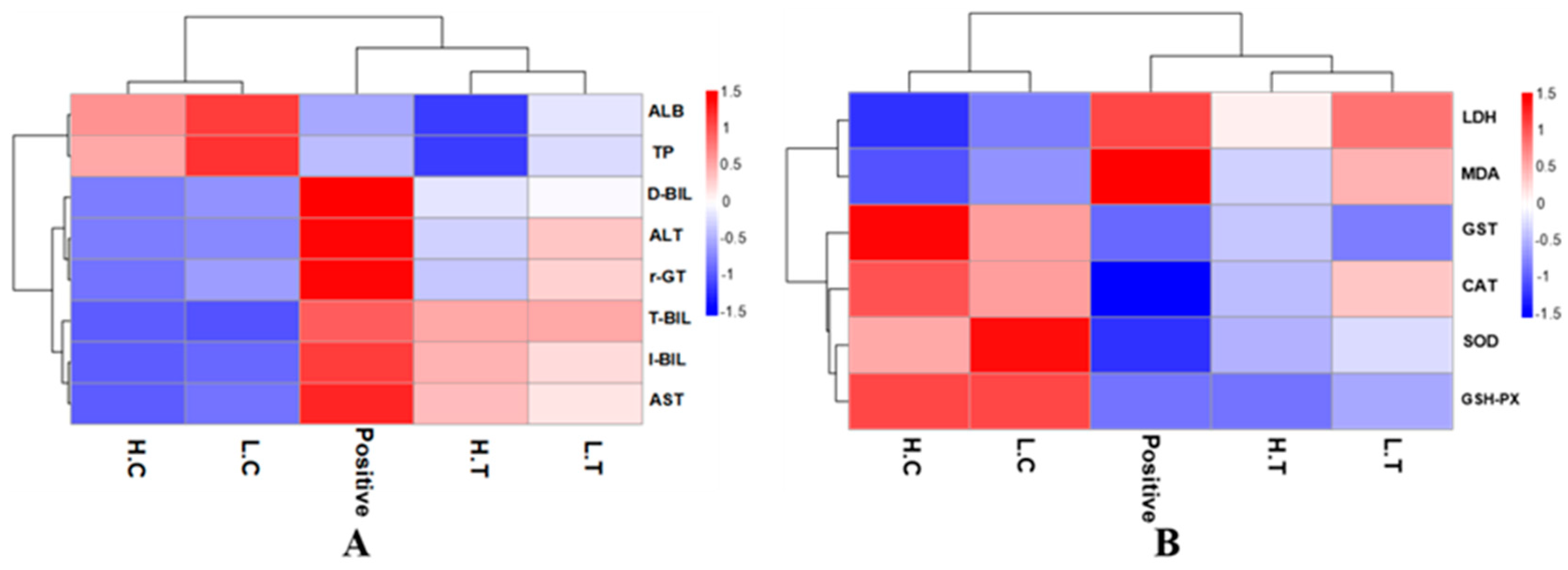
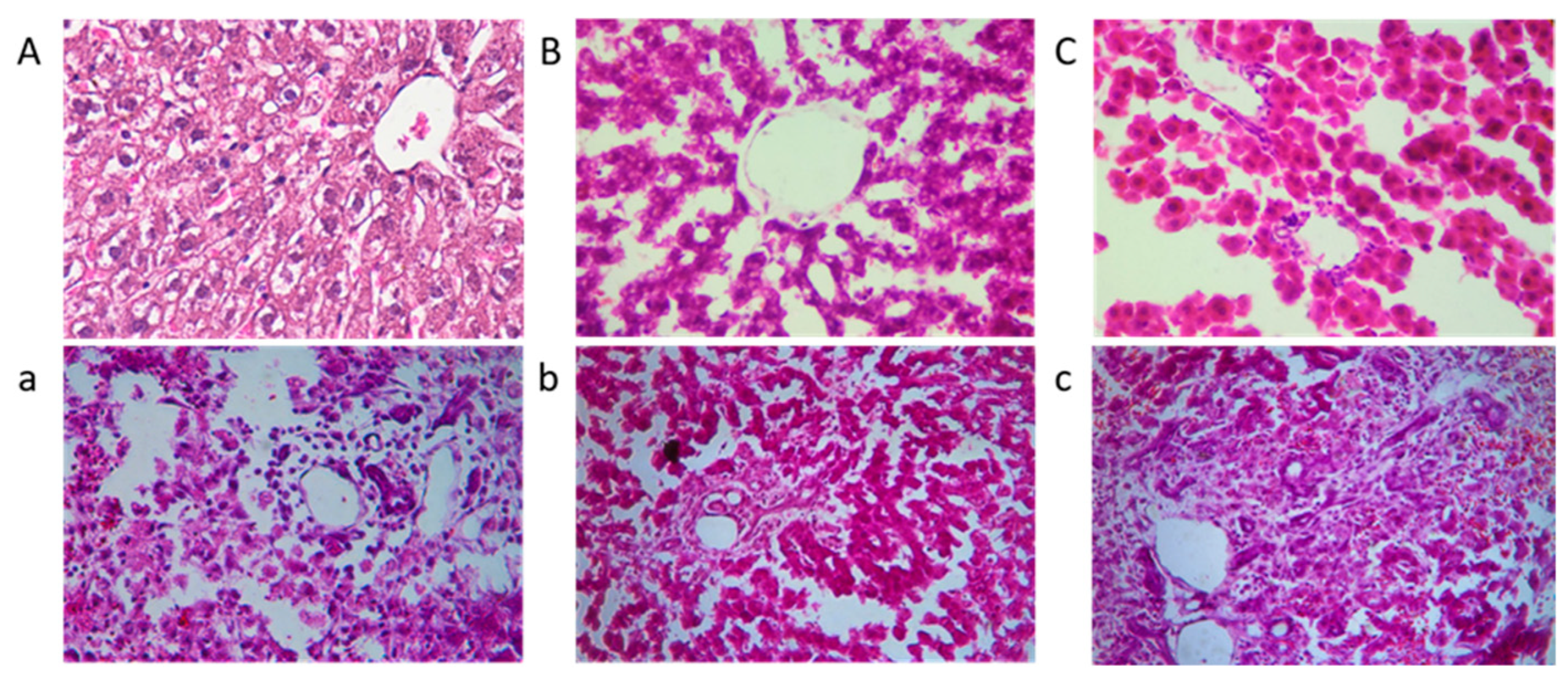
3.3. Evaluation of the Intervention Effect of A. auricular on AFB1-Induced Liver Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, P.; Venâncio, A.; Lima, N. Aflatoxigenic fungi and aflatoxins in portuguese almonds. Sci. World J. 2012, 2012, 471926. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kew, M.C. Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointestin. Liver Dis. 2013, 22, 305–310. [Google Scholar] [PubMed]
- Santos, V.O.; Pelegrini, P.B.; Mulinari, F.; Lacerda, A.F.; Moura, R.S.; Cardoso, L.P.V.; Buhrer-Sekula, S.; Miller, R.N.G.; Grossi-de-Sa, M.F. Development and validation of a novel lateral flow immunoassay device for detection of aflatoxins in soy-based foods. Anal. Methods 2017, 9, 2715–2722. [Google Scholar] [CrossRef]
- Massey, T.E.; Smith, G.B.; Tam, A.S. Mechanisms of aflatoxin B1 lung tumorigenesis. Exp. Lung Res. 2000, 26, 673–683. [Google Scholar]
- Autrup, H.; Jorgensen, E.C.; Jensen, O. Aflatoxin B1 induced lacI mutation in liver and kidney of transgenic mice C57BL/6N: Effect of phorone. Mutagenesis 1996, 11, 69–73. [Google Scholar] [CrossRef]
- Moon, E.Y.; Rhee, D.K.; Pyo, S. Inhibition of various functions in murine peritoneal macrophages by aflatoxin B1 exposure in vivo. Int. J. Immunopharmacol. 1999, 21, 47–58. [Google Scholar] [CrossRef]
- Raisuddin, S.; Singh, K.; Zaidi, S.; Paul, B.; Ray, P. Immunosuppressive effects of aflatoxin in growing rats. Mycopathologia 1993, 124, 189–194. [Google Scholar] [CrossRef]
- Hathout, A.S.; Aly, S.E. Biological detoxification of mycotoxins: A review. Ann. Microbiol. 2014, 64, 905–919. [Google Scholar] [CrossRef]
- Do, J.H.; Choi, D.-K. Aflatoxins: Detection, toxicity, and biosynthesis. Biotechnol. Bioprocess Eng. 2007, 12, 585–593. [Google Scholar] [CrossRef]
- Sara, D.; Watanya, J.; Flavia, G.; Consiglia, L.; Carlo, N.; Emanuela, A.; Chiara, L.; Sihem, D.; Giuseppina, A.; Achille, S.; et al. Curcumin supplementation protects broiler chickens against the renal oxidative stress induced by the dietary exposure to low levels of aflatoxin B1. Front. Vet. Sci. 2021, 8, 822227. [Google Scholar]
- Owumi, S.E.; Kazeem, A.I.; Wu, B.C.; Ishokare, L.O.; Arunsi, U.O.; Oyelere, A.K. Apigeninidin-rich Sorghum bicolor (L. Moench) extracts suppress A549 cells proliferation and ameliorate toxicity of aflatoxin B1-mediated liver and kidney derangement in rats. Sci. Rep. 2022, 12, 7438. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, D.; Belloir, C.; Suschetet, M.; Siess, M.-H.; Le Bon, A.-M. Mechanisms of protection against aflatoxin B1 genotoxicity in rats treated by organosulfur compounds from garlic. Carcinogenesis 2002, 23, 1335–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.S.; Coles, B.F.; Raney, K.D.; Thier, R.; Guengerich, F.P.; Harris, T.M. DNA adduction by the potent carcinogen aflatoxin B1 mechanistic studies. J. Am. Chem. Soc. 1994, 116, 1603–1609. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tang, L.; Wang, J.; Wang, J.S. Aflatoxin B1 disrupts gut-microbial metabolisms of short-chain fatty acids, long-chain fatty acids, and bile acids in male F344 rats. Toxicol. Sci. 2018, 164, 453–464. [Google Scholar] [CrossRef]
- Zychowski, K.E.; Hoffmann, A.R.; Ly, H.J.; Pohlenz, C.; Buentello, A.; Romoser, A.; Gatlin, D.M.; Phillips, T.D. The effect of aflatoxin B1 on red drum (Sciaenops ocellatus) and assessment of dietary supplementation of NovaSil for the prevention of aflatoxicosis. Toxins 2013, 5, 1555–1573. [Google Scholar] [CrossRef] [Green Version]
- Brana, M.T.; Cimmarusti, M.T.; Haidukowski, M.; Logrieco, A.F.; Altomare, C. Bioremediation of aflatoxin B1-contaminated maize by king oyster mushroom (Pleurotus eryngii). PLoS ONE 2017, 12, e0182574. [Google Scholar] [CrossRef] [Green Version]
- El-Shiekh, H.H.; Mahdy, H.M.; El-Aaser, M.M. Bioremediation of aflatoxins by some reference fungal strains. Pol. J. Microbiol. 2007, 56, 215–223. [Google Scholar]
- Farzaneh, M.; Shi, Z.-Q.; Ghassempour, A.; Sedaghat, N.; Ahmadzadeh, M.; Mirabolfathy, M.; Javan-Nikkhah, M. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control 2012, 23, 100–106. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Q.; Zhao, L.; Lei, Y.; Shan, Y.; Ji, C. Isolation of Bacillus subtilis: Screening for aflatoxins B-1, M-1, and G1 detoxification. Eur. Food Res. Technol. 2011, 232, 957–962. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Mao, Y.Z.; Opoku, J.; Meh, H.L. Enzymatic degradation is an effective means to reduce aflatoxin contamination in maize. BMC Biotechnol. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Ramy, S.Y. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz. J. Microbiol. 2014, 47, 127–133. [Google Scholar]
- Ali, H.S.; Badr, A.N.; Alsulami, T.; Shehata, M.G.; Youssef, M.M. Quality attributes of Sesame Butter (Tahini) fortified with lyophilized powder of edible mushroom (Agaricus blazei). Foods 2022, 11, 3691. [Google Scholar] [CrossRef]
- Choo, M.J.; Hong, S.Y.; Chuang, S.H.; Om, A.S. Removal of aflatoxin B1 by edible mushroom-forming fungi and its mechanism. Toxins 2021, 13, 668. [Google Scholar] [CrossRef]
- Igbiri, S.; Udowelle, N.A.; Ekhator, O.C.; Asomugha, R.N.; Igweze, Z.N.; Orisakwe, O.E. Edible mushrooms from niger delta, nigeria with heavy metal levels of public health concern: A human health risk assessment. Recent Pat. Food Nutr. Agric. 2018, 9, 31–41. [Google Scholar] [CrossRef]
- Dai, Y.J.; Li, J.J.; Shan, D.X. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 2020, 238, 124432. [Google Scholar] [CrossRef]
- Su, L.; Zhang, H.B.; Oh, K.; Liu, N.; Luo, Y.; Cheng, H.Y.; Zhang, G.S.; He, X.F. Activated biochar derived from spent Auricularia auricula substrate for the efficient adsorption of cationic azo dyes from single and binary adsorptive systems. Water Sci. Technol. 2021, 84, 101–121. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, X.P.; Yang, Q.H.; Zhang, C.; Song, X.L.; Wang, W.H.; Jia, L.; Zhang, J.J. Antioxidation, anti-hyperlipidaemia and hepatoprotection of polysaccharides from Auricularia auricular residue. Chem. Biol. Interact. 2021, 335, 109323. [Google Scholar] [CrossRef]
- Xing, X.B.; Chitrakar, B.; Hati, S.; Xie, S.Y.; Li, H.B.; Li, C.T.; Liu, Z.B.; Mo, H.Z. Development of black fungus-based 3D printed foods as dysphagia diet: Effect of gums incorporation. Food Hydrocoll. 2022, 123, 107173. [Google Scholar] [CrossRef]
- Xu, D.; Peng, S.R.; Guo, R.; Yao, L.S.; Mo, H.Z.; Li, H.B.; Song, H.X.; Hu, L.B. EGCG alleviates oxidative stress and inhibits aflatoxin B1 biosynthesis via MAPK signaling pathway. Toxins 2021, 13, 693. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Muhammad, I.; Li, W.; Sun, X.Q.; Cheng, P.; Zhang, X.Y. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-II enzymes and Nrf2. Environ. Toxicol. Phar. 2018, 59, 94–104. [Google Scholar] [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin removal by Lactobacillus spp. and their application in animal liquid feed. Toxins 2021, 13, 185. [Google Scholar] [CrossRef]
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Tech. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Qi, M.; Ge, L.; Tian, Z.; Li, J.; Zhang, M.; Wang, M.; Huang, L.; Tang, X. Anti-tumor effect of Rhaponticum uniflorum ethyl acetate extract by regulation of peroxiredoxin1 and epithelial-to-mesenchymal transition in oral cancer. Front. Pharmacol. 2017, 8, 870. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Kang, M.A.; Jeon, Y.K.; Nam, M.J. Auricularia auricula increases an apoptosis in human hepatocellular carcinoma cells via a regulation of the peroxiredoxin1. J. Food Biochem. 2020, 44, e13373. [Google Scholar] [CrossRef]
- Alberts, J.F.; Engelbrecht, Y.; Steyn, P.S.; Holzapfel, W.H.; van Zyl, W.H. Biological degradation of aflatoxin B1 by Rhodococcus erythropolis cultures. Int. J. Food Microbiol. 2006, 109, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, L.; Ma, Q.; Li, X.; Shi, H.; Zhou, T.; Zhang, J.; Ji, C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013, 59, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The quality of red bell pepper subjected to freeze-drying preceded by traditional and novel pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
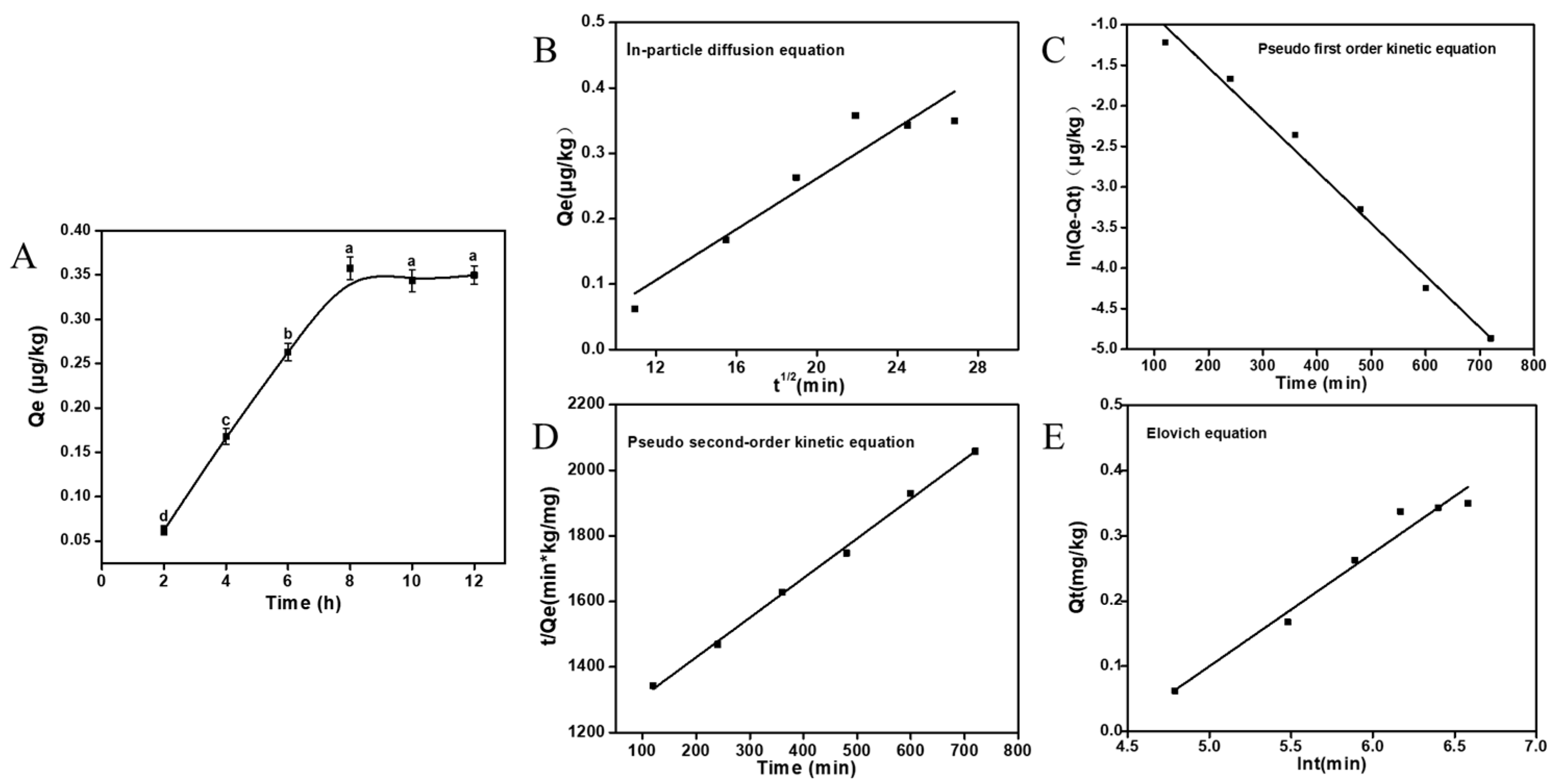
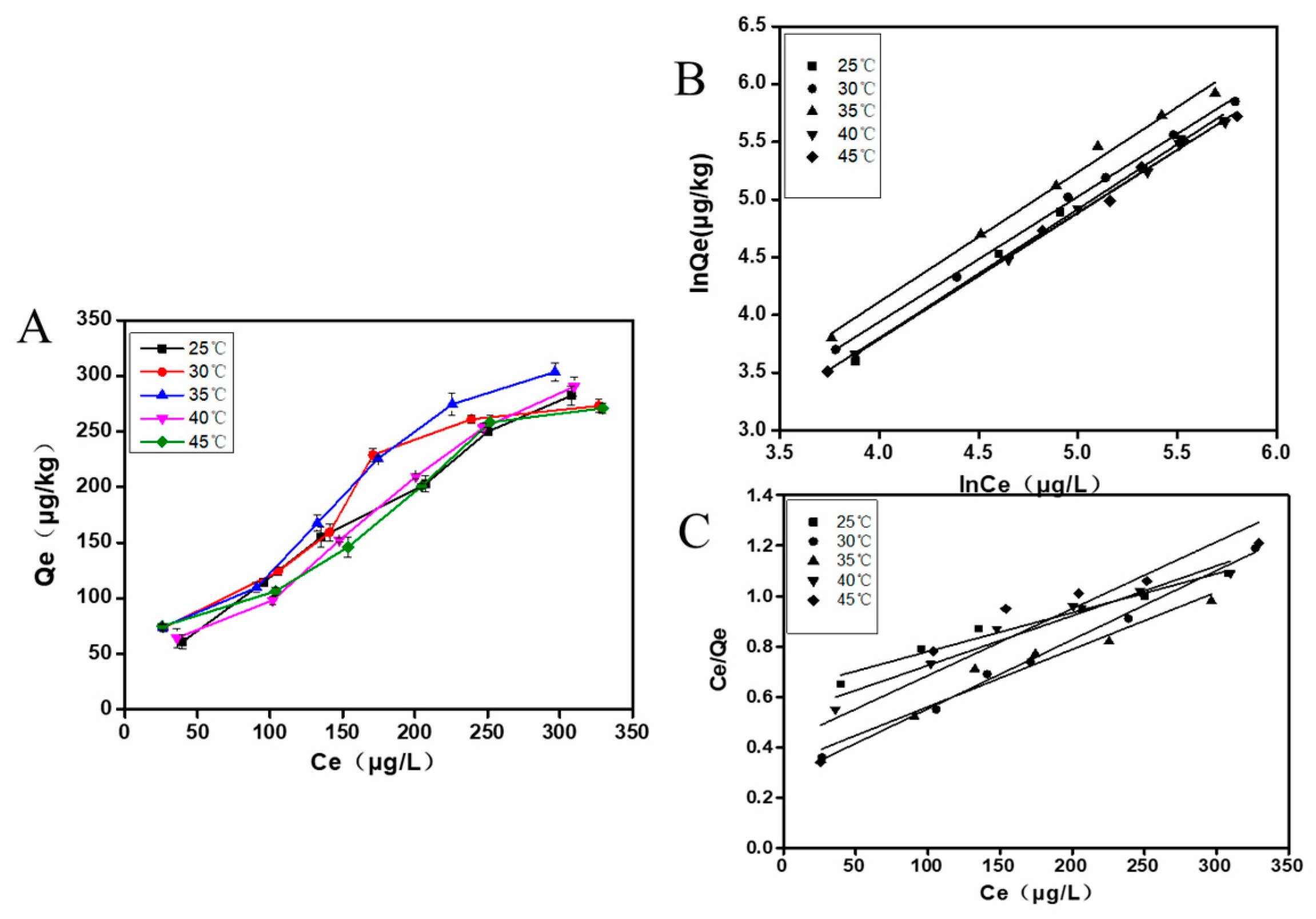
| Fitting Model | Formula | R2 |
|---|---|---|
| In-particle diffusion equation | Qt = 0.06 t0.5 | 0.8870 |
| Pseudo first-order kinetic equation | ln(qe − qt) = lnqe − 0.16 t | 0.9856 |
| Pseudo second-order kinetic equation | t/qt = 1/13.77 qe2 + 1/qe | 0.9976 |
| Elovich equation | Qe = 0.09 + 0.02 lnt | 0.9618 |
| Temperature (K) | Kf | 1/n | Freundlich Formula | R2 |
|---|---|---|---|---|
| 298 | 0.11 | 0.02 | Qe = 0.11 × Ce0.02 | 0.9981 |
| 303 | 0.22 | 0.04 | Qe = 0.22 × Ce0.04 | 0.9927 |
| 308 | 0.24 | 0.05 | Qe = 0.24 × Ce0.05 | 0.9901 |
| 313 | 0.17 | 0.03 | Qe = 0.17 × Ce0.03 | 0.9951 |
| 318 | 0.11 | 0.02 | Qe = 0.11 × Ce0.02 | 0.9979 |
| Temperature (K) | KL | Langmuir Formula | R2 |
|---|---|---|---|
| 298 | 0.01 | Qe = 0.278 × Ce/(1 + 0.01 Ce) | 0.9943 |
| 303 | 0.02 | Qe = 0.625 × Ce/(1 + 0.02 Ce) | 0.9680 |
| 308 | 0.04 | Qe = 0.333 × Ce/(1 + 0.04 Ce) | 0.9462 |
| 313 | 0.09 | Qe = 0.419 × Ce/(1 + 0.09 Ce) | 0.8551 |
| 318 | 0.04 | Qe = 0.527 × Ce/(1 + 0.04 Ce) | 0.9464 |
| Temperature (K) | Koc | ∆G (KJ/mol) | ∆H (KJ/mol) | ∆S (KJ/mol) |
|---|---|---|---|---|
| 298 | 10.59 | −22.43 | −6.31 | 0.05 |
| 303 | 21.59 | −21.35 | −6.31 | 0.07 |
| 308 | 24.38 | −36.85 | −6.31 | 0.10 |
| 313 | 17.27 | −50.39 | −6.31 | 0.11 |
| 318 | 11.13 | −43.82 | −6.31 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Huang, M.; Lei, J.; Song, H.; Hu, L.; Mo, H. Auricularia auricular Adsorbs Aflatoxin B1 and Ameliorates Aflatoxin B1-Induced Liver Damage in Sprague Dawley Rats. Foods 2023, 12, 2644. https://doi.org/10.3390/foods12142644
Xu D, Huang M, Lei J, Song H, Hu L, Mo H. Auricularia auricular Adsorbs Aflatoxin B1 and Ameliorates Aflatoxin B1-Induced Liver Damage in Sprague Dawley Rats. Foods. 2023; 12(14):2644. https://doi.org/10.3390/foods12142644
Chicago/Turabian StyleXu, Dan, Minmin Huang, Jiao Lei, Hongxin Song, Liangbin Hu, and Haizhen Mo. 2023. "Auricularia auricular Adsorbs Aflatoxin B1 and Ameliorates Aflatoxin B1-Induced Liver Damage in Sprague Dawley Rats" Foods 12, no. 14: 2644. https://doi.org/10.3390/foods12142644






