A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta domesticus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals and Reagents
2.3. NMR Analysis
2.4. FT-ICR MS Analysis
2.5. SPME-GC-MS Analysis of Volatile Compounds
2.6. GC-MS Analysis of Hexane Extract
2.7. GC-MS Determination of Fatty Acid Content
3. Results
3.1. NMR Analysis
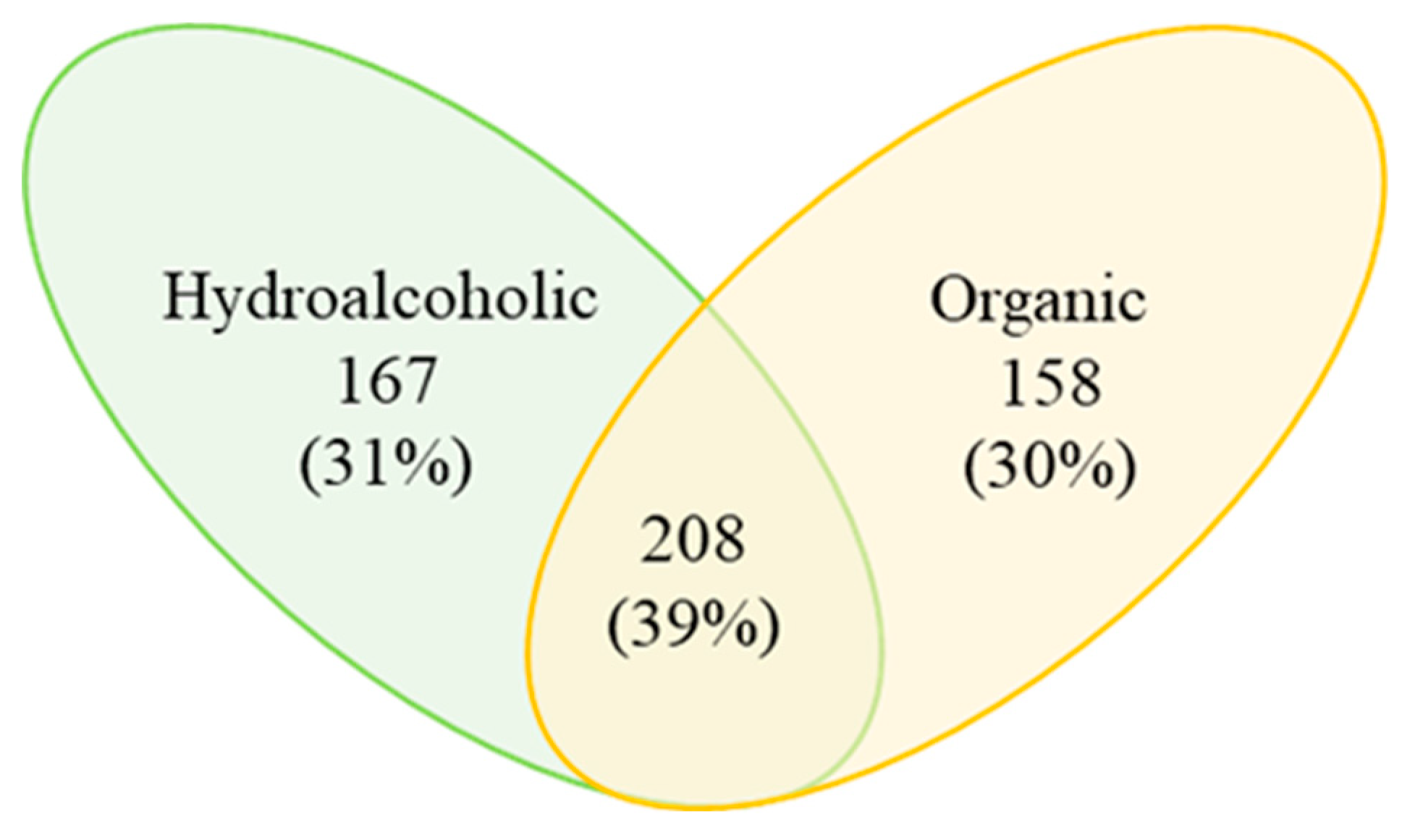
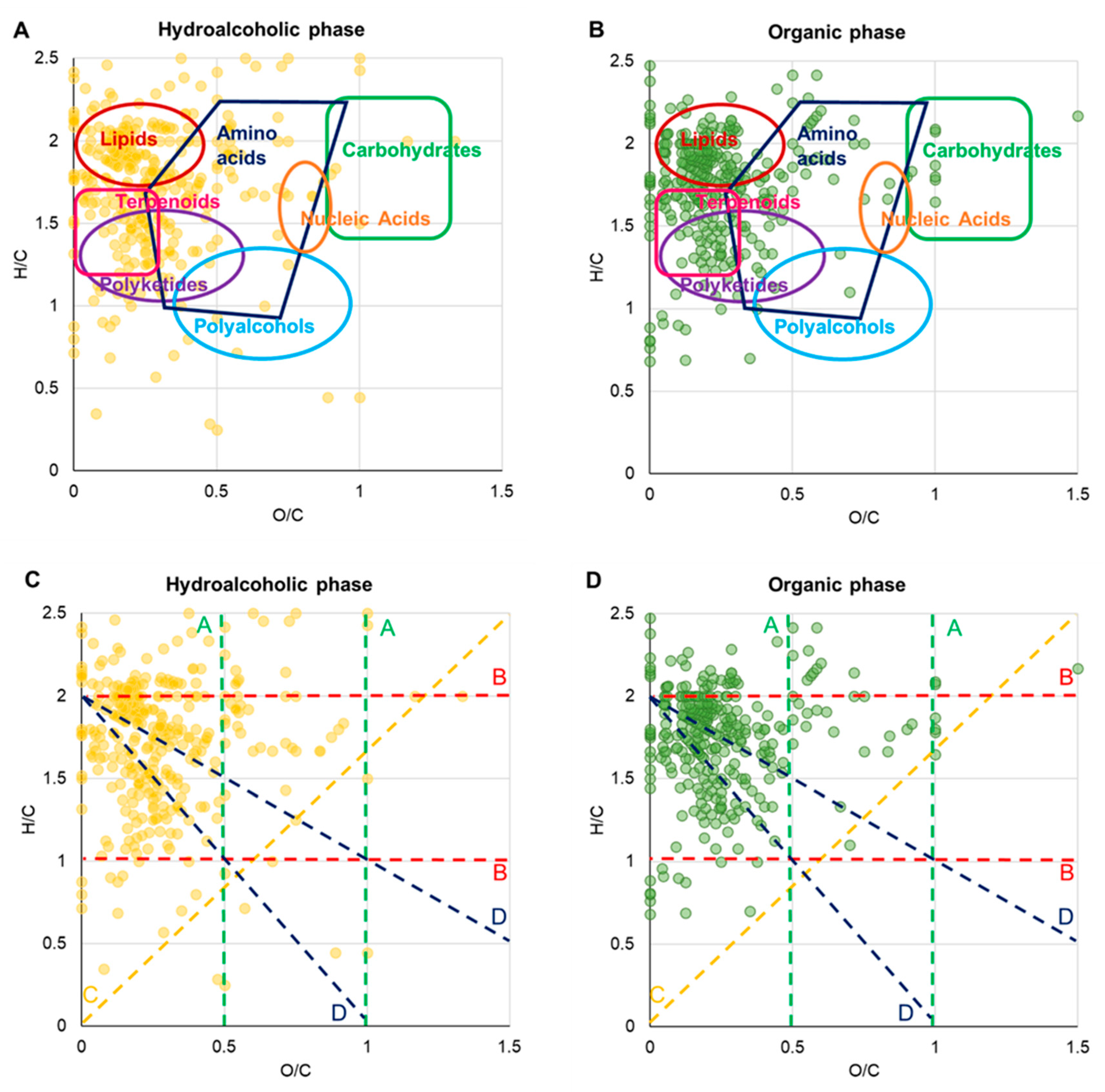
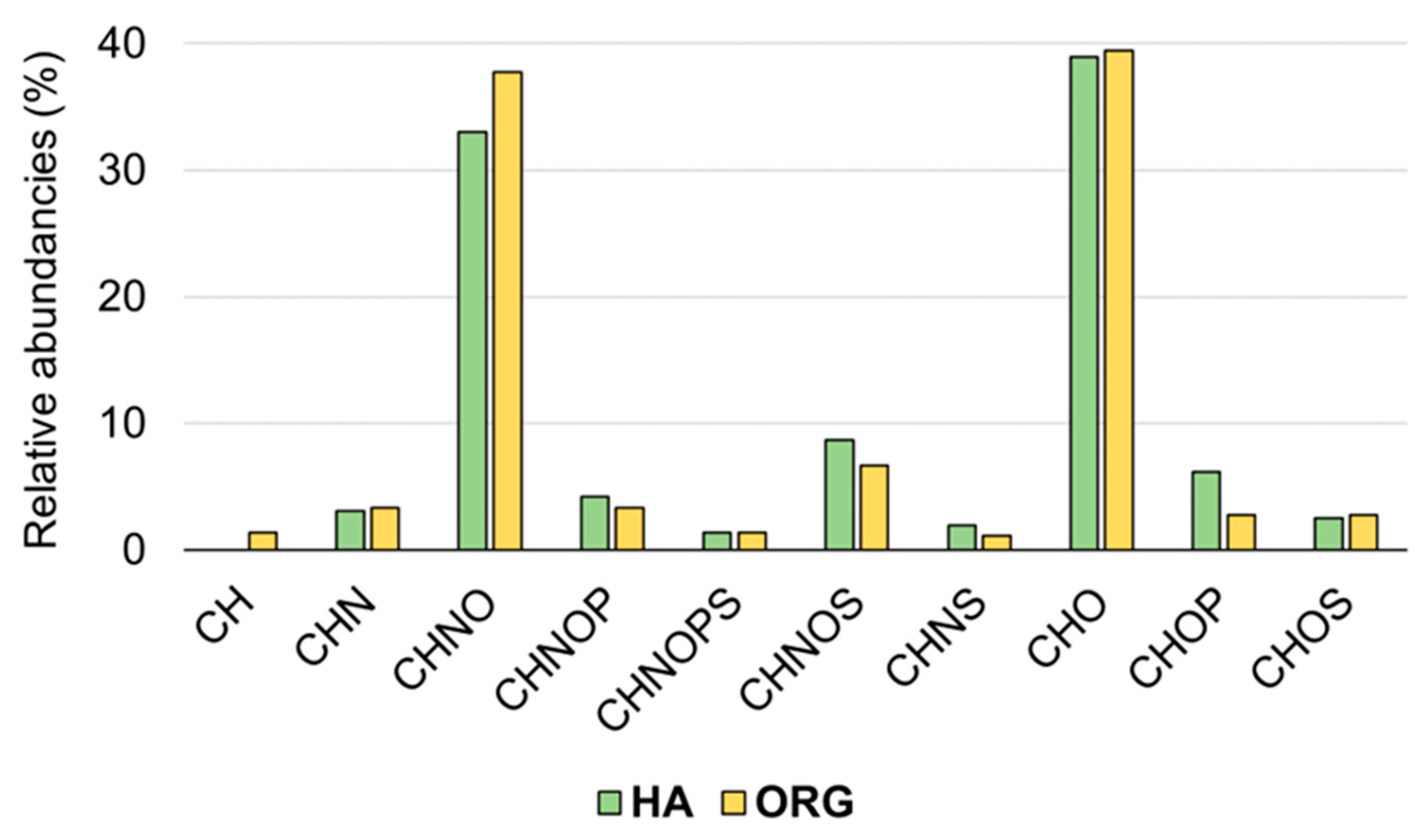
3.2. FT-ICR MS Analysis
3.3. GC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariutti, L.R.B.; Rebelo, K.S.; Bisconsin-Junior, A.; de Morais, J.S.; Magnani, M.; Maldonade, I.R.; Madeira, N.R.; Tiengo, A.; Maróstica, M.R.; Cazarin, C.B.B. The use of alternative food sources to improve health and guarantee access and food intake. Food Res. Int. 2021, 149, 110709. [Google Scholar] [CrossRef] [PubMed]
- United Nations Sustainable Development GOALS. Available online: https://www.un.org/sustainabledevelopment/development-agenda/ (accessed on 15 October 2022).
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2020, 6, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- FAO. Edible Insects—Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013. [Google Scholar]
- Union, E. EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022R0188 (accessed on 15 October 2022).
- Khatun, H.; Claes, J.; Smets, R.; De Winne, A.; Akhtaruzzaman, M.; Van Der Borght, M. Characterization of freeze-dried, oven-dried and blanched house crickets (Acheta domesticus) and Jamaican field crickets (Gryllus assimilis) by means of their physicochemical properties and volatile compounds. Eur. Food Res. Technol. 2021, 247, 13793. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Grapes, M.; Whiting, P.; Dinan, L. Fatty acid and lipid analysis of the house cricket, Acheta domesticus. Insect Biochem. 1989, 19, 767–774. [Google Scholar] [CrossRef]
- Beldean, B.V.; Chiș, M.S.; Alexa, E.; Pop, C.; Păucean, A.; Man, S.; Igual, M.; Haydee, K.M.; Dalma, K.E.; Stănilă, S.; et al. The Impact of Insect Flour on Sourdough Fermentation-Fatty Acids, Amino-Acids, Minerals and Volatile Profile. Insects 2022, 13, 576. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted phenolic characterization and antioxidant bioactivity of extracts from edible Acheta domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A multimethodological characterization of cannabis sativa l. Inflorescences from seven dioecious cultivars grown in Italy: The effect of different harvesting stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of Local Products for Their Industrial Use: The Case of Italian Potato Cultivars Analyzed by Untargeted and Targeted Methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Maccelli, A.; Di Matteo, G.; Ingallina, C.; Biava, M.; Crestoni, M.E.; Bardaud, J.X.; Giusti, A.M.; Mariano, A.; D’abusco, A.S.; et al. Metabolomic profiling of fresh goji (Lycium barbarum L.) berries from two cultivars grown in central Italy: A multi-methodological approach. Molecules 2021, 26, 5412. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Bianchi, E.; Vigani, B.; Sánchez-Espejo, R.; Spano, M.; Totaro Fila, C.; Mannina, L.; Viseras, C.; Rossi, S.; Sandri, G. Nutritional and Functional Properties of Novel Italian Spray-Dried Cricket Powder. Antioxidants 2023, 12, 112. [Google Scholar] [CrossRef]
- Dossey, A.T.; Tatum, J.T.; McGill, W.L. Modern Insect-Based Food Industry: Current Status, Insect Processing Technology, and Recommendations Moving Forward. In Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Academic Press: Cambridge, MA, USA, 2016; pp. 113–152. ISBN 9780128028568. [Google Scholar]
- Di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. Nmr characterization of ten apple cultivars from the piedmont region. Foods 2021, 10, 289. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Nascimento, R.; Monteiro, F.; Traquete, F.; Marques, A.P.; Cunha, J.; Eiras-Dias, J.E.; Cordeiro, C.; Figueiredo, A.; et al. Integrating metabolomics and targeted gene expression to uncover potential biomarkers of fungal/oomycetes-associated disease susceptibility in grapevine. Sci. Rep. 2020, 10, 15688. [Google Scholar] [CrossRef]
- Farinon, B.; Costantini, L.; Molinari, R.; Di Matteo, G.; Garzoli, S.; Ferri, S.; Ceccantoni, B.; Mannina, L.; Merendino, N. Effect of malting on nutritional and antioxidant properties of the seeds of two industrial hemp (Cannabis sativa L.) cultivars. Food Chem. 2022, 370, 131348. [Google Scholar] [CrossRef]
- Wägele, B.; Witting, M.; Schmitt-Kopplin, P.; Suhre, K. Masstrix reloaded: Combined analysis and visualization of transcriptome and metabolome data. PLoS ONE 2012, 7, e39860. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical Method for Analysis of Ultrahigh-Resolution Broadband Mass Spectra of Natural Organic Matter, the Van Krevelen Diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Fuda, H.; Tsukui, T.; Chiba, H.; Hui, S.P. Discovery of eicosapentaenoic acid esters of hydroxy fatty acids as potent nrf2 activators. Antioxidants 2020, 9, 397. [Google Scholar] [CrossRef]
- Chen, J.; Green, K.B.; Nichols, K.K. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5730–5753. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Bello, J.E.; McElfresh, J.S.; Millar, J.G. Isolation and determination of absolute configurations of insect-produced methyl-branched hydrocarbons. Proc. Natl. Acad. Sci. USA 2015, 112, 1077–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Kim, T.K.; Cha, J.Y.; Yong, H.I.; Kang, M.C.; Jang, H.W.; Choi, Y.S. Physicochemical characteristics and aroma patterns of oils prepared from edible insects. LWT 2022, 167, 113888. [Google Scholar] [CrossRef]
- Jardine, K.J.; de O. Piva, L.R.; Rodrigues, T.B.; Spanner, G.C.; Rodrigues, J.R.; Menezes, V.S.; Sampaio, I.; Oliveira, D.C.; Gimenez, B.O.; Higuchi, N.; et al. Volatiles defenses of Amazon Azteca ants (repellent ants). bioRxiv 2020, 2020.04.15.043547. [Google Scholar]
- Hamilton, R.J.; Raie, M.Y.; Weatherston, I.; Brooks, C.J.; Borthwick, J.H. Crustacean surface waxes. Part I. The hydrocarbons from the surface of Ligia oceanica. J. Chem. Soc. Perkin Trans. 1975, 354–357. [Google Scholar] [CrossRef]
- Izumiyama, S.; Suzuki, K.; Miya, K. Glycerol in the Eggs of the Two-Spotted Cricket, Gryllus bimaculatus de Geer. Appl. Entomol. Zool. 1983, 18, 295–300. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | Assignment | 1H (ppm) | Multiplicity [J(Hz)] | 13C (ppm) | mg/100 g of Sample |
|---|---|---|---|---|---|
| Amino acids and derivatives | |||||
| Alanine | COO- | 176.8 | 931.43 ± 18.06 | ||
| α-CH | 3.80 | q [7.3] | 51.5 | ||
| β-CH3 | 1.49 * | d [7.3] | 17.2 | ||
| Aspartate | α-CH | 3.92 | 52.4 | 78.00 ± 2.69 | |
| β, β’-CH2 | 2.70; 2.82 * | dd [17.4; 3.8] | 37.7 | ||
| Betaine | N(CH3)3+ | 3.27 * | s | 54.6 | 100.16 ± 1.44 |
| α-CH2 | 67.7 | ||||
| Citrulline | α-CH | 3.14 * | m | 397.96 ± 8.38 | |
| β-CH2 | 1.89 | ||||
| γ-CH2 | 1.60 | ||||
| Glycine | COO- | 175.0 | 631.41 ± 35.29 | ||
| α-CH2 | 3.57 * | s | 42.6 | ||
| Glutamate | α-CH | 3.78 | 55.6 | 464.30 ± 19.24 | |
| β, β’-CH2 | 2.07; 2.14 | 28.0 | |||
| γ-CH2 | 2.36 * | m | 34.8 | ||
| δ-COO- | 182.6 | ||||
| Glutamine | α-CH | 3.81 | 265.74 ± 11.73 | ||
| β, β’-CH2 | 2.15 | ||||
| γ-CH2 | 2.46 * | m | 31.8 | ||
| Histidine | CH-3, ring | 8.04 * | s | 120.36 ± 6.88 | |
| CH-5, ring | 7.15 | s | |||
| Isoleucine | α-CH | 3.69 | 60.7 | 189.50 ± 9.24 | |
| β-CH | 1.99 | 37.0 | |||
| γ-CH3 | 1.02 * | d [7.1] | 15.8 | ||
| γ’-CH | 1.29 | 25.6 | |||
| δ-CH3 | 0.94 | t [7.4] | 12.3 | ||
| Leucine | α-CH | 3.74 | 54.4 | 417.98 ± 3.21 | |
| β-CH2 | 1.72 | 40.8 | |||
| δ, δ’-CH3 | 0.96; 097 * | d [6.2] | 23.0 | ||
| Lysine | α-CH | 3.74 | 54.5 | 351.05 ± 9.41 | |
| β-CH2 | 1.91 | 30.9 | |||
| γ -CH2 | 1.48 | 21.2 | |||
| δ-CH2 | 1.75 | 27.5 | |||
| ε-CH2 | 3.02 * | t [7.3] | 40.2 | ||
| Methionine | α-CH | 3.74 | 55.0 | 23.69 ± 1.58 | |
| β-CH2 | 2.25 | m | 30.5 | ||
| γ-CH2 | 2.65 * | t [7.4] | 30.0 | ||
| S-CH3 | 2.16 | s | 14.9 | ||
| Phenylalanine | CH-2,6 ring | 7.32 | m | 130.5 | 167.43 ± 5.08 |
| CH-3,5 ring | 7.42 * | m | 130.1 | ||
| CH-4 ring | 7.37 | m | 128.7 | ||
| Proline | α-CH | 4.15 | 977.76 ± 17.27 | ||
| β, β’-CH2 | 2.06; 2.35 | m | |||
| γ-CH2 | 2.01 * | m | 25.3 | ||
| δ, δ’-CH2 | 3.35; 3.43 | m | |||
| Taurine | S-CH2 | 3.28 | t [6.5] | 48.4 | 259.83 ± 13.73 |
| N-CH2 | 3.44 * | t [6.5] | 36.3 | ||
| Threonine | α-CH | 3.60 | 61.4 | 88.89 ± 6.86 | |
| β-CH | 4.27 | 67.1 | |||
| γ-CH3 | 1.34 * | d [6.6] | 21.2 | ||
| Tryptophan | CH-4, ring | 7.72 | d [8.1] | 73.10 ± 2.54 | |
| CH-5, ring | 7.20 | ||||
| CH-6, ring | 7.27 | ||||
| CH-7, ring | 7.51 * | d [8.1] | |||
| Tyrosine | CH-2,6 ring | 6.89 * | d [8.6] | 116.9 | 537.32 ± 4.82 |
| CH-3,5 ring | 7.20 | d [8.6] | 129.5 | ||
| C-4 ring | 155.5 | ||||
| Valine | α-CH | 3.63 | 61.6 | 390.18 ± 17.35 | |
| β-CH | 2.30 | 30.3 | |||
| γ-CH3 | 0.99 | d [7.06] | 18.0 | ||
| γ’-CH3 | 1.05 | d [7.06] | 19.1 | ||
| Organic acids | |||||
| Acetate | COO- | 182.5 | 359.64 ± 16.25 | ||
| CH3 | 1.92 * | s | 24.4 | ||
| Formate | HCOO- | 8.47 * | s | 22.80 ± 0.42 | |
| Fumarate | α, β-CH=CH | 6.53 * | s | 1.87 ± 0.09 | |
| Lactate | COO- | 183.5 | 730.94 ± 11.40 | ||
| α-CH | 4.12 | 69.6 | |||
| β-CH3 | 1.33 * | d [6.6] | 22.6 | ||
| Succinate | COO- | 183.7 | 411.96 ± 8.59 | ||
| α, β-CH2 | 2.41 * | s | 35.1 | ||
| Other metabolites | |||||
| Choline | N(CH3)3+ | 3.21 * | s | 55.2 | 153.21 ± 1.54 |
| α-CH2 | 68.9 | ||||
| Glycerol | CH-1,3 | 3.56 * | dd [11.7; 6.5] | 63.6 | 1175.31 ± 23.08 |
| CH2-2 | 3.79 | m | 55.4 | ||
| CH-1′,3′ | 3.66 | dd [11.7; 4.3] | 63.6 | ||
| N° | Component 1 | LRI 2 | LRI 3 | (%) |
|---|---|---|---|---|
| 1 | α-thujene | 820 | 823 | 0.9 ± 0.02 |
| 2 | β-thujene | 962 | 968 | 0.7 ± 0.03 |
| 3 | β-myrcene | 985 | 983 | 3.5 ± 0.08 |
| 4 | 4-carene | 1008 | 1001 | 1.5 ± 0.02 |
| 5 | p-cymene | 1015 | 1013 | 17.2 ± 0.02 |
| 6 | 1,2-dipropenyl-cyclobutane | 1021 | * | 1.5 ± 0.02 |
| 7 | γ-terpinene | 1051 | 1054 | 16.8 ± 0.03 |
| 8 | 6-ethyl-2-methyl-decane | 1351 | * | 2.5 ± 0.03 |
| 9 | linalyl butyrate | 1406 | 1402 | 0.8 ± 0.02 |
| 10 | hexadecanoic acid | 1968 | 1973 | 54.6 ± 0.02 |
| SUM | 100.0 |
| N° | Component 1 | LRI 2 | LRI 3 | (%) |
|---|---|---|---|---|
| 1 | methylcyclopentane | 635 | 629 | 62.9 ± 0.04 |
| 2 | 4-methyl-heptane | 771 | 768 | 0.2 ± 0.02 |
| 3 | octane | 810 | * | 0.1 ± 0.02 |
| 4 | 2,4-dimethyl-1-heptane | 822 | 821 | 0.2 ± 0.02 |
| 5 | 2,3,4-trimethyl-hexane | 854 | 850 | 0.1 ± 0.02 |
| 6 | 3,3-dimethyl-octane | 930 | 935 | 0.2 ± 0.01 |
| 7 | 2,3,6,7-tetramethyl-octane | 928 | 935.5 | 0.3 ± 0.02 |
| 8 | decane | 1010 | * | 0.3 ± 0.01 |
| 9 | 4,7-dimethyl-undecane | 1121 | * | 0.2 ± 0.02 |
| 10 | 4-methyl-undecane | 1167 | 1160 | 0.6 ± 0.02 |
| 11 | 2,5-dimethyl-benzaldehyde | 1215 | 1208 | 0.2 ± 0.02 |
| 12 | 4,6-dimethyl-dodecane | 1335 | 1325* | 0.7 ± 0.02 |
| 13 | 2,4-di-tert-butylphenol | 1529 | 1521 | 0.7 ± 0.02 |
| 14 | hexadecanoic acid | 1980 | 1973 | 32.8 ± 0.04 |
| SUM | 99.5 |
| N° | Component 1 | LRI 2 | LRI 3 | (%) |
|---|---|---|---|---|
| 1 | pentanoic acid | 1758 | 1762 | 0.2 ± 0.03 |
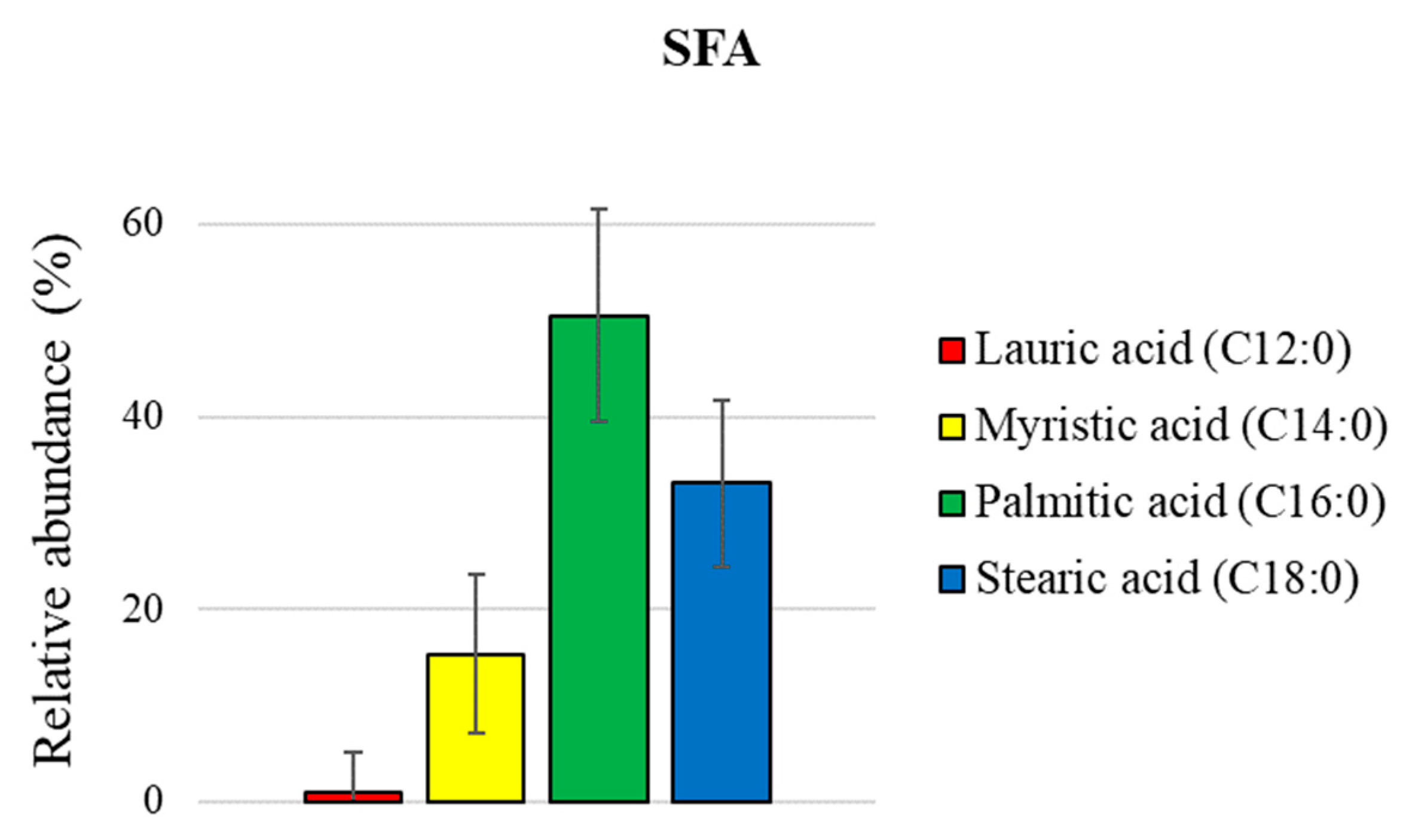
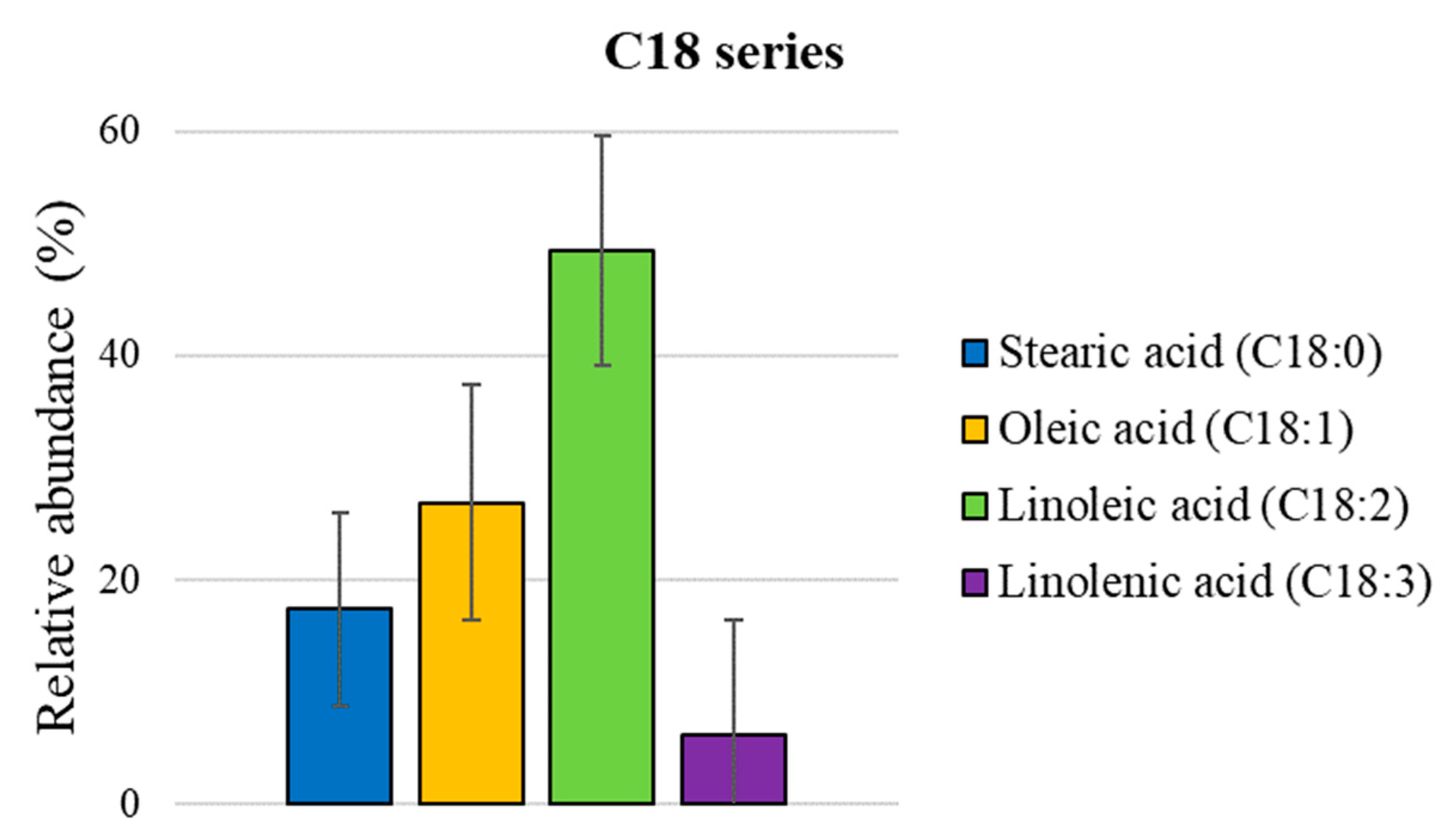
| 2 | palmitic acid | 2941 | 2946 | 27.8 ± 0.05 |
| 3 | stearic acid | 3183 | 3181 | 10.4 ± 0.03 |
| 4 | oleic acid | 3190 | 3184 | 21.0 ± 0.07 |
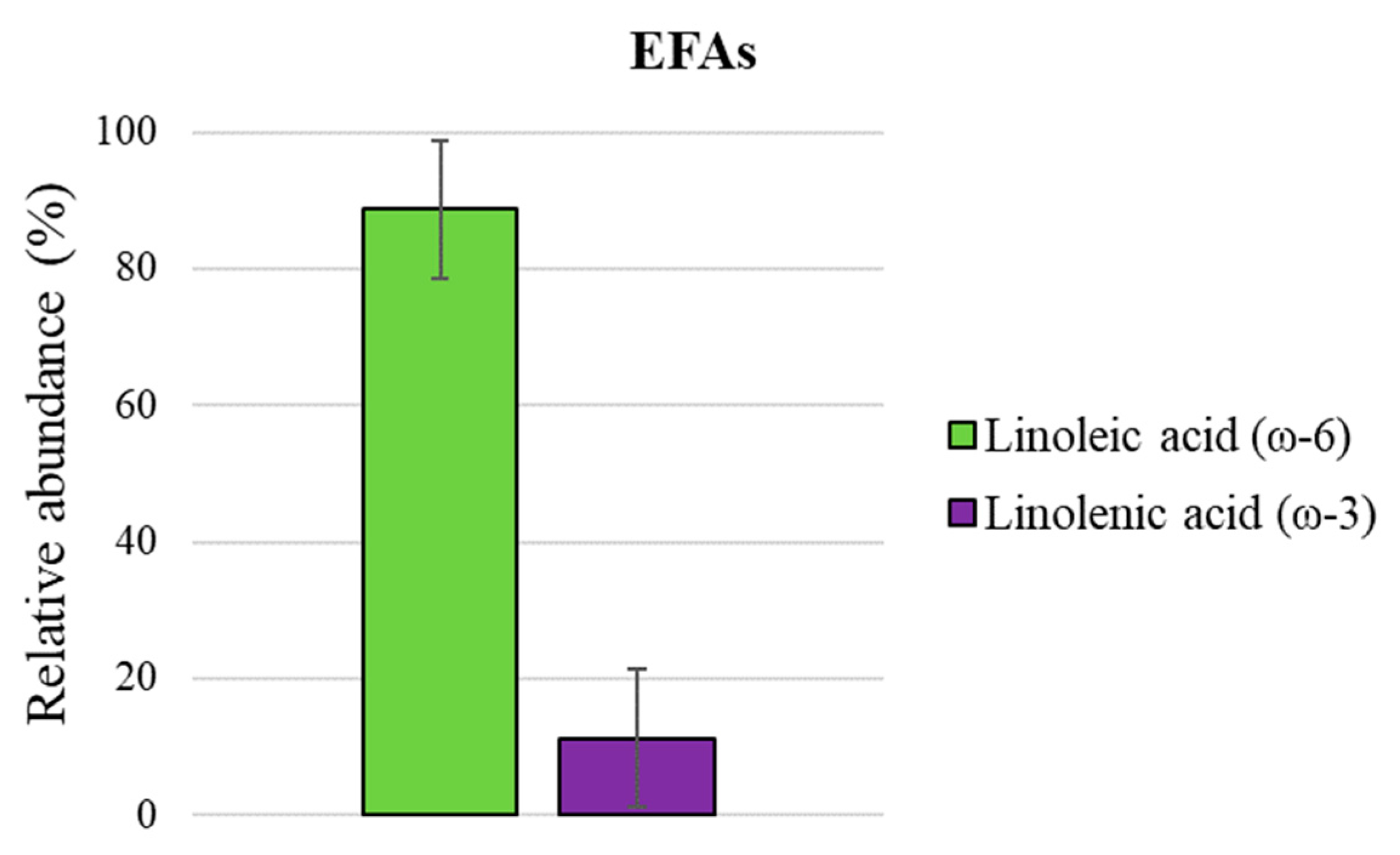
| 5 | linoleic acid | 3217 | * | 38.1 ± 0.06 |
| 6 | linolenic acid | 3289 | 3292 | 2.5 ± 0.02 |
| SUM | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spano, M.; Di Matteo, G.; Fernandez Retamozo, C.A.; Lasalvia, A.; Ruggeri, M.; Sandri, G.; Cordeiro, C.; Sousa Silva, M.; Totaro Fila, C.; Garzoli, S.; et al. A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta domesticus. Foods 2023, 12, 2331. https://doi.org/10.3390/foods12122331
Spano M, Di Matteo G, Fernandez Retamozo CA, Lasalvia A, Ruggeri M, Sandri G, Cordeiro C, Sousa Silva M, Totaro Fila C, Garzoli S, et al. A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta domesticus. Foods. 2023; 12(12):2331. https://doi.org/10.3390/foods12122331
Chicago/Turabian StyleSpano, Mattia, Giacomo Di Matteo, Carlos Alberto Fernandez Retamozo, Alba Lasalvia, Marco Ruggeri, Giuseppina Sandri, Carlos Cordeiro, Marta Sousa Silva, Carlotta Totaro Fila, Stefania Garzoli, and et al. 2023. "A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta domesticus" Foods 12, no. 12: 2331. https://doi.org/10.3390/foods12122331