Rapid and Sensitive Detection of Polycyclic Aromatic Hydrocarbons in Tea Leaves Using Magnetic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Real Sample
2.2. Chemicals and Standards
2.3. Preparation and Characterization of C18/MNPs
2.3.1. Synthesis of C18/MNPs
2.3.2. Characterization of C18/MNPs
2.4. C18/MNPs-Based Extraction Procedure for the Preconcentration and Detection of Five Kinds of PAHs in Tea
2.4.1. Configuration of Standard Solution and Preparation of Spike Samples
2.4.2. Preconcentration Procedures
2.5. Recycling of C18/MNPs
2.6. UPLC Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterizing of C18/MNPs
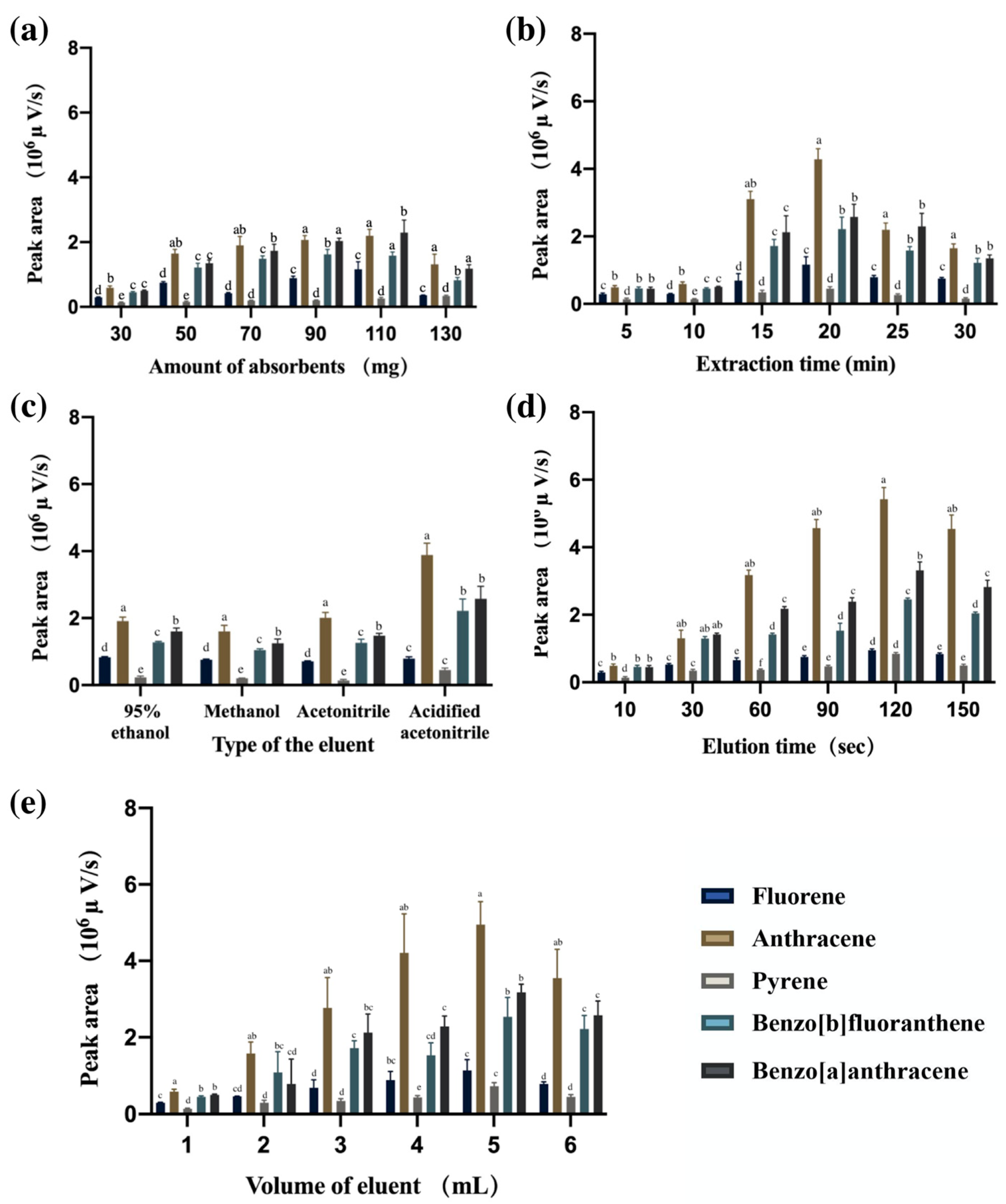
3.2. Optimizing MSPE Conditions
3.2.1. Amount of Absorbents
3.2.2. Extraction Time
3.2.3. Type of the Eluent
3.2.4. Elution Time
3.2.5. Volume of Eluent
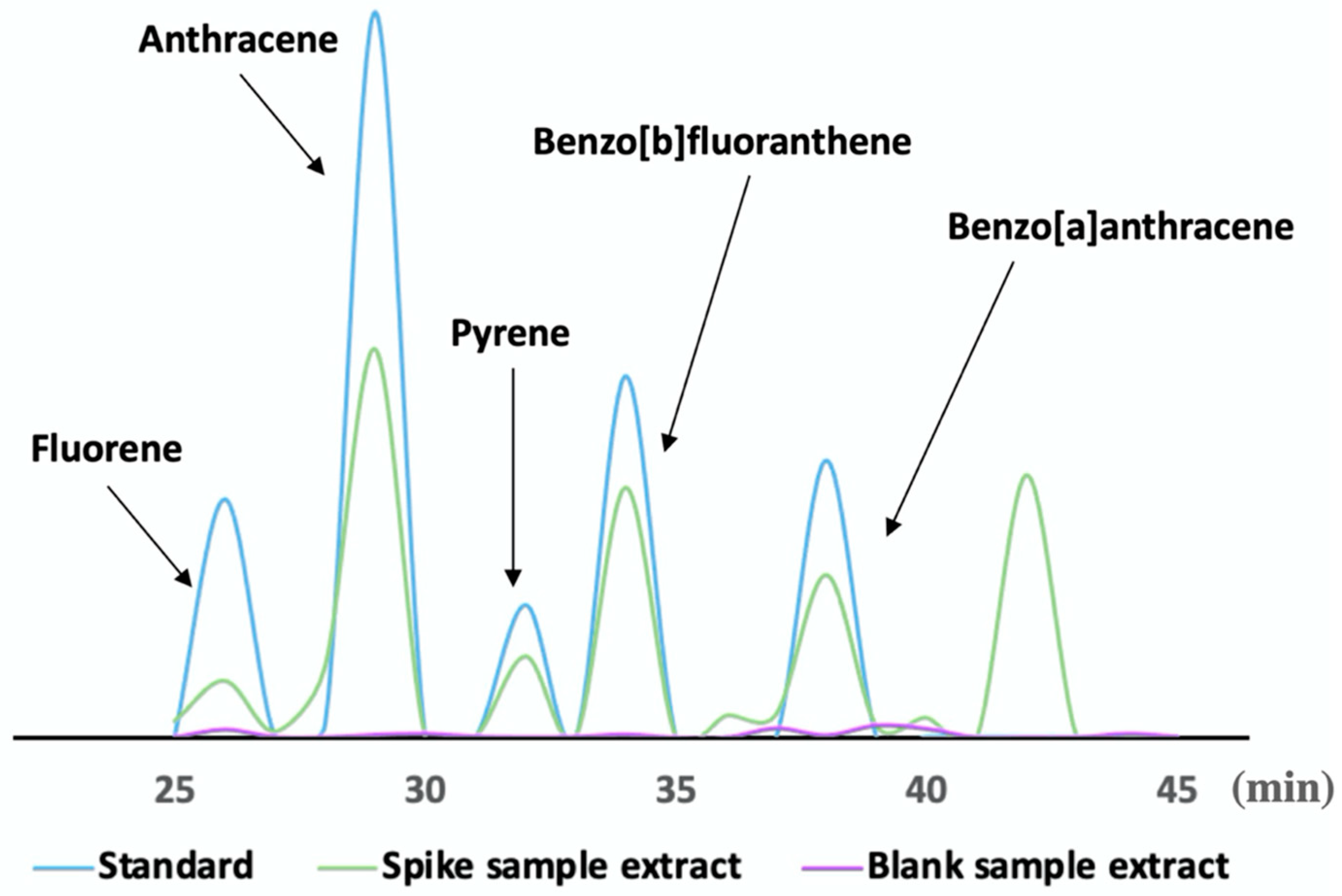
3.3. Evaluation of the Detection Method Performance
3.4. Reproducibility and Reusability of the C18/MNPs
3.5. Method Comparison
3.6. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sagar, B.; Anupam, M.; Anusha, M.; Avishek, B. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Li, P.; Zhang, Q.; Zhang, W.; Ding, X. Determination for major chemical contaminants in tea (Camellia sinensis) matrices: A review. Food Res. Int. 2013, 53, 649–658. [Google Scholar] [CrossRef]
- Tallei, T.E.; Niode, N.J.; Idroes, R.; Zidan, B.; Mitra, S.; Celik, I.; Nainu, F.; Ağagündüz, D.; Emran, T.B.; Capasso, R. A comprehensive review of the potential use of green tea polyphenols in the management of COVID-19. Evid.-Based Complement. Altern. Med. 2021, 2021, 7170736. [Google Scholar] [CrossRef] [PubMed]
- Tran-Lam, T.-T.; Hai Dao, Y.; Kim Thi Nguyen, L.; Kim Ma, H.; Nguyen Tran, H.; Truong Le, G. Simultaneous Determination of 18 Polycyclic Aromatic Hydrocarbons in Daily Foods (Hanoi Metropolitan Area) by Gas Chromatography–Tandem Mass Spectrometry. Foods 2018, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Roudbari, A.; Rafiei Nazari, R.; Shariatifar, N.; Moazzen, M.; Abdolshahi, A.; Mirzamohammadi, S.; Madani-Tonekaboni, M.; Delvarianzadeh, M.; Arabameri, M. Concentration and health risk assessment of polycyclic aromatic hydrocarbons in commercial tea and coffee samples marketed in Iran. Environ. Sci. Pollut. Res. 2021, 28, 4827–4839. [Google Scholar] [CrossRef]
- Plaza-Bolaños, P.; Frenich, A.G.; Vidal, J.L.M. Polycyclic aromatic hydrocarbons in food and beverages. Analytical methods and trends. J. Chromatogr. A 2010, 1217, 6303–6326. [Google Scholar] [CrossRef]
- Atirah Mohd Nazir, N.; Raoov, M.; Mohamad, S. Spent tea leaves as an adsorbent for micro-solid-phase extraction of polycyclic aromatic hydrocarbons (PAHs) from water and food samples prior to GC-FID analysis. Microchem. J. 2020, 159, 105581. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The Occurrence of 16 EPA PAHs in Food—A Review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [Green Version]
- Ziegenhals, K.; Jira, W.; Speer, K. Polycyclic aromatic hydrocarbons (PAH) in various types of tea. Eur. Food Res. Technol. 2008, 228, 83–91. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Navaratnam, M.A.; Jewula, J.; Jensen, A.H. PAH in Some Brands of Tea and Coffee. Polycycl. Aromat. Compd. 2015, 35, 74–90. [Google Scholar] [CrossRef]
- Li, X.-Y.; Li, N.; Luo, H.-D.; Lin, L.-R.; Zou, Z.-X.; Jia, Y.-Z.; Li, Y.-Q. A Novel Synchronous Fluorescence Spectroscopic Approach for the Rapid Determination of Three Polycyclic Aromatic Hydrocarbons in Tea with Simple Microwave-Assisted Pretreatment of Sample. J. Agric. Food Chem. 2011, 59, 5899–5905. [Google Scholar] [CrossRef] [PubMed]
- Adisa, A.; Jimenez, A.; Woodham, C.; Anthony, K.; Nguyen, T.; Saleh, M.A. Determination of polycyclic aromatic hydrocarbons in dry tea. J. Environ. Sci. Health Part B 2015, 50, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Khiadani, M.; Amin, M.; Beik, F.; Ebrahimi, A.; Farhadkhani, M.; Mohammadi-Moghadam, F. Determination of polycyclic aromatic hydrocarbons concentration in eight brands of black tea which are used more in Iran. Int. J. Environ. Health Eng. 2013, 2, 40. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M.; Cieślik, E. Comparison of different modifications on QuEChERS sample preparation method for PAHs determination in black, green, red and white tea. Environ. Sci. Pollut. Res. 2014, 21, 1326–1338. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Li, Z.; Weng, W.; Yang, H.; Li, B. Effect of heat-treated tea water-insoluble protein nanoparticles on the characteristics of Pickering emulsions. LWT 2021, 149, 111999. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Yang, H.; Li, B. Characteristics and application of fish oil-in-water pickering emulsions structured with tea water-insoluble proteins/κ-carrageenan complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Zhou, D.-B.; Sheng, X.; Han, F.; Hu, Y.-Y.; Ding, L.; Lv, Y.-L.; Song, W.; Zheng, P. Magnetic solid-phase extraction based on [60]fullerene functionalization of magnetic nanoparticles for the determination of sixteen polycyclic aromatic hydrocarbons in tea samples. J. Chromatogr. A 2018, 1578, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhong, T.; Zhang, Y.; Zhao, X.; Xiao, Y.; Wang, L.; Liu, X.; Zhang, X. Design, Preparation, and Application of Magnetic Nanoparticles for Food Safety Analysis: A Review of Recent Advances. J. Agric. Food Chem. 2022, 70, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Baber, O.; Jang, M.; Barber, D.; Powers, K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhal. Toxicol. 2011, 23, 532–543. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, H.; Wang, C.; Guo, X.; Du, J.; Du, L. Determination of polycyclic aromatic hydrocarbons in coffee and tea samples by magnetic solid-phase extraction coupled with HPLC–FLD. Food Chem. 2016, 199, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Chiesi, V.; Casoli, F.; Luches, P.; Nasi, L.; Careri, M.; Mangia, A. Magnetic solid-phase extraction based on diphenyl functionalization of Fe3O4 magnetic nanoparticles for the determination of polycyclic aromatic hydrocarbons in urine samples. J. Chromatogr. A 2012, 1231, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, F.; Chen, F.; Jiang, L.; Li, J.; Chen, L. C18-Functionalized Magnetic Silica Nanoparticles for Solid Phase Extraction of Microcystin-LR in Reservoir Water Samples Followed by HPLC-DAD Determination. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 655–661. [Google Scholar] [CrossRef]
- Guo, B.; Ji, S.; Zhang, F.; Yang, B.; Gu, J.; Liang, X. Preparation of C18-functionalized Fe3O4@SiO2 core–shell magnetic nanoparticles for extraction and determination of phthalic acid esters in Chinese herb preparations. J. Pharm. Biomed. Anal. 2014, 100, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, Y.; Yu, X.; Zhang, L.; Sun, X.; Gao, Y.; Zhang, H.; Song, D. Removal of sudan dyes from water with C18-functional ultrafine magnetic silica nanoparticles. Talanta 2012, 89, 38–46. [Google Scholar] [CrossRef]
- Noël, L.; Authier-martin, M.; Patry, G.; Marcoux, A.; Jean, P. Quantitative Analysis of PAH in Pitch by HPLC with Fluorescence Detector. Polycycl. Aromat. Compd. 1996, 9, 373–380. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H. Pyrethroid residue determination in organic and conventional vegetables using liquid-solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem. 2017, 217, 303–310. [Google Scholar] [CrossRef]
- Xiong, X.; Li, D.; Du, Z.; Xiong, C.; Jiang, H. Magnetic solid-phase extraction modified Quick, Easy, Cheap, Effective, Rugged and Safe method combined with pre-column derivatization and ultra-high performance liquid chromatography-tandem mass spectrometry for determination of estrogens and estrogen mimics in pork and chicken samples. J. Chromatogr. A 2020, 1622, 461137. [Google Scholar] [CrossRef]
- Singh, A.; Singh, M.; Pandey, A.; Ullas, A.V.; Mishra, S. Hydrophobicity of cotton fabric treated with plant extract, TiO2 nanoparticles and beeswax. Mater. Today Proc. 2023, 80, 1530–1533. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Li, M.; Zou, H.; Wang, Z.; Ran, H.; Zheng, Y.; Jian, J.; Zhou, Y.; Luo, Y.; et al. Multifunctional Nanoparticles for Multimodal Imaging-Guided Low-Intensity Focused Ultrasound/Immunosynergistic Retinoblastoma Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5642–5657. [Google Scholar] [CrossRef]
- Wang, J.; Meng, G.; Tao, K.; Feng, M.; Zhao, X.; Li, Z.; Xu, H.; Xia, D.; Lu, J.R. Immobilization of Lipases on Alkyl Silane Modified Magnetic Nanoparticles: Effect of Alkyl Chain Length on Enzyme Activity. PLoS ONE 2012, 7, e43478. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Rao, Y.S.; Balaji, T.; Prathima, B.; Jyothi, N.V.V. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zhao, M.; Lau, S.C.S.; Ru Tan, H.; Teh, W.J.; Yang, H.; Zheng, C.; Zhang, Y. Quantification of aflatoxin B1 in vegetable oils using low temperature clean-up followed by immuno-magnetic solid phase extraction. Food Chem. 2019, 275, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Shahriman, M.S.; Ramachandran, M.R.; Zain, N.N.M.; Mohamad, S.; Manan, N.S.A.; Yaman, S.M. Polyaniline-dicationic ionic liquid coated with magnetic nanoparticles composite for magnetic solid phase extraction of polycyclic aromatic hydrocarbons in environmental samples. Talanta 2018, 178, 211–221. [Google Scholar] [CrossRef]
- Srivastava, N.; Kumari, S.; Nair, K.; Alam, S.; Raza, S.K. Determination of Organophosphorous Pesticides in Environmental Water Samples Using Surface-Engineered C18 Functionalized Silica-Coated Core-Shell Magnetic Nanoparticles-Based Extraction Coupled with GC-MS/MS Analysis. J. AOAC Int. 2017, 100, 804–809. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, J.; He, Q.; Chen, X.; Jin, M. Fabrication of benzenesulfonic acid groups modified magnetic microspheres as an MSPE adsorbent for fast determination of paraquat and diquat in human urine combined with UPLC-HRMS. J. Chromatogr. B 2020, 1136, 121880. [Google Scholar] [CrossRef]
- Ma, L.; Nilghaz, A.; Choi, J.R.; Liu, X.; Lu, X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018, 246, 437–441. [Google Scholar] [CrossRef]
- Ciemniak, A.; Kuźmicz, K.; Rajkowska-Myśliwiec, M.; Cadena, M.F. Assessing the contamination levels of dried teas and their infusions by polycyclic aromatic hydrocarbons (PAHs). J. Consum. Prot. Food Saf. 2019, 14, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Stuppner, S.; Hussain, S.; Mark, B.; Gjerde, D.; Rainer, M.; Jakschitz, T.; Bonn, G.K. An automated preloaded pipet tip SPE method for the accurate quantification of carcinogenic polycyclic aromatic hydrocarbons from tea. Anal. Methods 2020, 12, 1827–1833. [Google Scholar] [CrossRef]
- Pissinatti, R.; Nunes, C.M.; de Souza, A.G.; Junqueira, R.G.; de Souza, S.V.C. Simultaneous analysis of 10 polycyclic aromatic hydrocarbons in roasted coffee by isotope dilution gas chromatography-mass spectrometry: Optimization, in-house method validation and application to an exploratory study. Food Control 2015, 51, 140–148. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Zhang, Y.; Zheng, L. Determination of 19 polyphenolic compounds in tea by ultra-high performance liquid chromatography combined with quadrupole-time of flight mass spectrometry. Food Sci. Hum. Wellness 2022, 11, 719–726. [Google Scholar] [CrossRef]
- Benson, N.U.; Fred-Ahmadu, O.H.; Olugbuyiro, J.A.O.; Anake, W.U.; Adedapo, A.E.; Olajire, A.A. Concentrations, sources and risk characterisation of polycyclic aromatic hydrocarbons (PAHs) in green, herbal and black tea products in Nigeria. J. Food Compos. Anal. 2018, 66, 13–22. [Google Scholar] [CrossRef]
- da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Pincemaille, J.; Schummer, C.; Heinen, E.; Moris, G. Determination of polycyclic aromatic hydrocarbons in smoked and non-smoked black teas and tea infusions. Food Chem. 2014, 145, 807–813. [Google Scholar] [CrossRef]
- Rowshan, V.; Bahmanzadegan, A.; Saharkhiz, M.J. Influence of storage conditions on the essential oil composition of Thymus daenensis Celak. Ind. Crop. Prod. 2013, 49, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Roszko, M.; Kamińska, M.; Szymczyk, K.; Jędrzejczak, R. Dietary risk evaluation for 28 polycyclic aromatic hydrocarbons (PAHs) in tea preparations made of teas available on the Polish retail market. J. Environ. Sci. Health Part B 2018, 53, 25–34. [Google Scholar] [CrossRef]



| Chemical Name | Abbreviation | Molecular Structure | Molecular Formula | Molecular Weight | Ring | Type |
|---|---|---|---|---|---|---|
| Fluorene | FLU |  | C13H10 | 166.22 | 3 | Light PAHs |
| Anthracene | ANT |  | C14H10 | 178.23 | 3 | Light PAHs |
| Pyrene | PYR |  | C16H10 | 202.25 | 4 | Light PAHs |
| Benzo[a]anthracene | BaA | 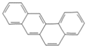 | C18H12 | 228.3 | 4 | Light PAHs |
| Benzo[b]fluoranthene | BbF | 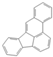 | C20H12 | 252.3 | 5 | Heavy PAHs |
| Analyte | Equation of Calibration Curve | R2 | LOD (ng g−1) | LOQ (ng g−1) |
|---|---|---|---|---|
| Fluorene | y = 48627x + 71642 | 0.998 | 7.93 | 24.03 |
| Anthracene | y = 106034x + 4318855 | 0.999 | 5.32 | 16.12 |
| Pyrene | y = 22396x + 216848 | 0.997 | 9.97 | 30.21 |
| Benzo[a]anthracene | y = 56517x + 357848 | 0.998 | 1.69 | 5.12 |
| Benzo[b]fluoranthene | y = 51622x + 661315 | 0.998 | 5.43 | 16.45 |
| Analyte | Concentration (ng g−1) | Intra-Day Precision | Inter-Day Precision | ||
|---|---|---|---|---|---|
| Recovery ± SD (%) | RSD (%) | Recovery ± SD (%) | RSD (%) | ||
| Fluorene | 50 | 94.8 ± 2.8 | 3.4 | 84.8 ± 2.9 | 6.8 |
| 100 | 95.2 ± 0.7 | 1.3 | 91.6 ± 0.3 | 3.1 | |
| 200 | 88.9 ± 1.9 | 1.4 | 93.6 ± 6.1 | 6.6 | |
| Anthracene | 50 | 91.7 ± 0.8 | 2.9 | 88.1 ± 0.9 | 5.9 |
| 100 | 100.2 ± 1.6 | 1.3 | 94.6 ± 0.7 | 3.7 | |
| 200 | 93.0 ± 0.2 | 6.7 | 99.0 ± 2.3 | 2.3 | |
| Pyrene | 50 | 89.9 ± 1.2 | 5.9 | 99.3 ± 0.7 | 2.8 |
| 100 | 95.8 ± 1.4 | 2.3 | 100.5 ± 1.7 | 4.7 | |
| 200 | 102.1 ± 0.7 | 11.9 | 105.4 ± 3.0 | 2.9 | |
| Benzo[a] anthracene | 50 | 99.1 ± 1.1 | 2.1 | 93.2 ± 1.8 | 5.1 |
| 100 | 97.4 ± 0.9 | 2.4 | 96.7 ± 0.6 | 6.2 | |
| 200 | 81.1 ± 2.2 | 2.2 | 86.3 ± 3.6 | 4.2 | |
| Benzo[b] fluoranthene | 50 | 102.1 ± 3.1 | 7.1 | 96.2 ± 2.1 | 4.9 |
| 100 | 98.2 ± 1.7 | 2.9 | 98.5 ± 5.2 | 5.2 | |
| 200 | 93.4 ± 3.5 | 5.2 | 91.4 ± 3.6 | 4.0 | |
| Method | Detection Technique | Sample | Linear Range (ng g−1) | LOD (ng g−1) | Recovery (%) | RSD (%) | References |
|---|---|---|---|---|---|---|---|
| SPE | GC-MS | Dry tea | - | 0.09–0.32 | 37.00–96.10 | - | Ciemniak et al. (2019) [38] |
| SPE | HPLC-FLD | Tea | - | 16.00 | 90.00–95.00 | <10.00 | Stuppner et al. (2020) [39] |
| LLE | GC-MS | Roasted coffee | 0.25–4.00 | 0.04–0.18 | 87.08–111.28 | 3.26–23.75 | Pissinatti et al. (2015) [40] |
| GE | UPLC-Q-TOF-MS | Tea | - | 2.00–100.00 | 78.40–109.20 | 1.33–9.72 | Li et al. (2022) [41] |
| UAE | GC-FID | Dry tea | - | 0.30 | 90.24–108.92 | 77.02–100.60 | Benson et al. (2018) [42] |
| MSPE | UPLC-UV | Tea | 0.50–300.00 | 1.69–9.97 | 84.80–105.40 | 2.30–6.80 | This work |
| Tea Type | Fluorene (ng g−1) | Anthracene (ng g−1) | Pyrene (ng g−1) | Benzo[a]anthracene (ng g−1) | Benzo[b]fluoranthene (ng g−1) | |
|---|---|---|---|---|---|---|
| Green (10 tea brands) | Mean | 132.3 | 41.9 | 109.5 | N.D. | N.D. |
| Min | 31.2 | N.D. | N.D. | N.D. | N.D. | |
| Max | N.D. | 196.5 | N.D. | 8.4 | N.D. | |
| Black (3 tea brands) | Mean | 109.8 | 21.7 | 94.6 | N.D. | N.D. |
| Min | 81.9 | N.D. | 70.3 | N.D. | N.D. | |
| Max | 141.5 | 31.2 | 113.4 | 5.3 | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Feng, X.; Chen, J.; Zhang, L.; Zhai, L.; Lv, S.; Ye, Y.; Chen, Y.; Zhong, T.; Yu, X.; et al. Rapid and Sensitive Detection of Polycyclic Aromatic Hydrocarbons in Tea Leaves Using Magnetic Approach. Foods 2023, 12, 2270. https://doi.org/10.3390/foods12112270
Zhao X, Feng X, Chen J, Zhang L, Zhai L, Lv S, Ye Y, Chen Y, Zhong T, Yu X, et al. Rapid and Sensitive Detection of Polycyclic Aromatic Hydrocarbons in Tea Leaves Using Magnetic Approach. Foods. 2023; 12(11):2270. https://doi.org/10.3390/foods12112270
Chicago/Turabian StyleZhao, Xiaohan, Xiao Feng, Jingwen Chen, Lanxin Zhang, Lingzi Zhai, Sizhe Lv, Yonghao Ye, Yongqi Chen, Tian Zhong, Xi Yu, and et al. 2023. "Rapid and Sensitive Detection of Polycyclic Aromatic Hydrocarbons in Tea Leaves Using Magnetic Approach" Foods 12, no. 11: 2270. https://doi.org/10.3390/foods12112270






