Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying
Abstract
1. Introduction
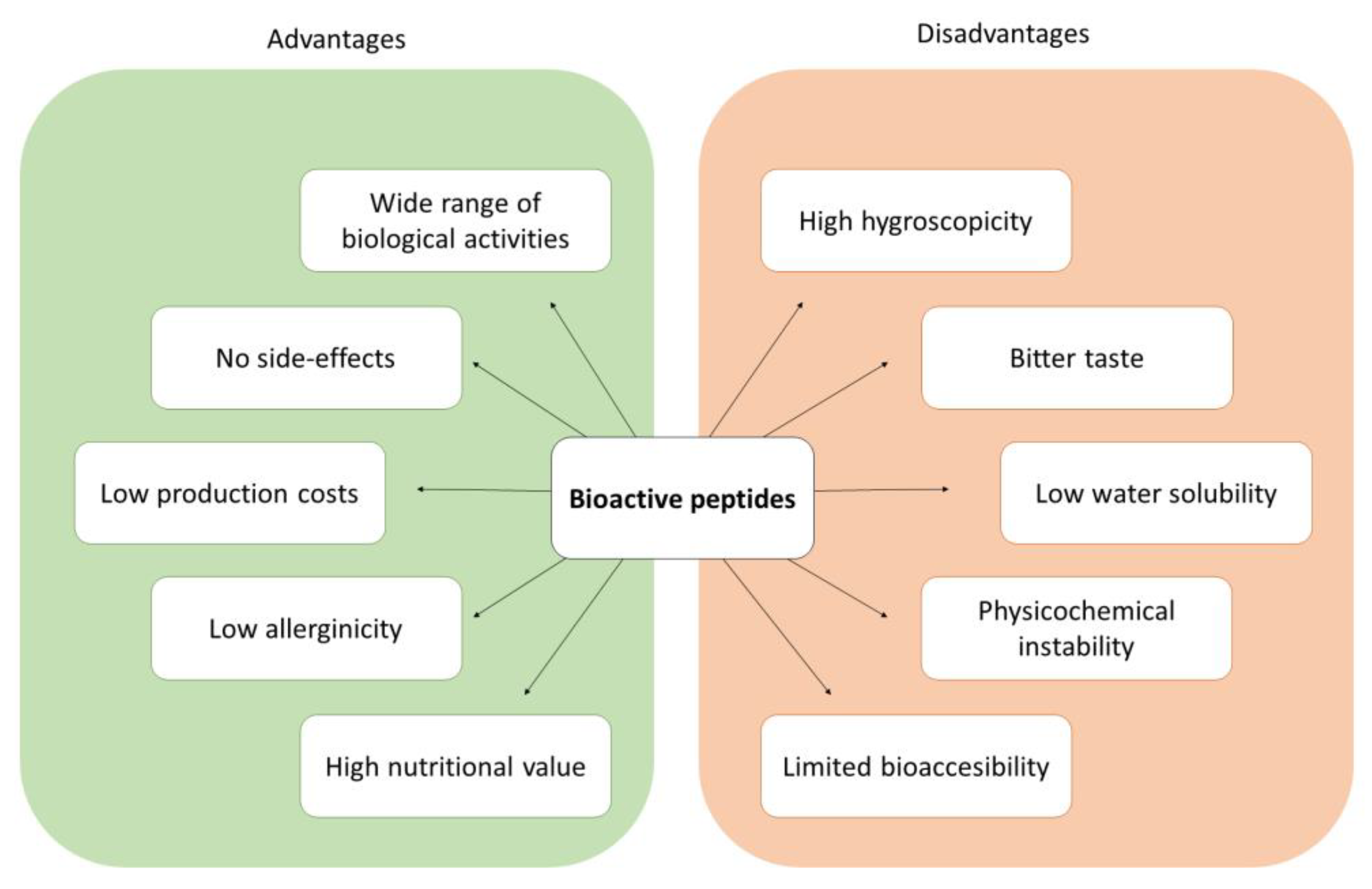
- High hygroscopicity, which may result in physicochemical instability and loss of bioactivity [9].
- Bitterness due to the exposure to taste receptors of hydrophobic amino acid residues generated from hydrolysis. It has a negative impact on consumer’s acceptance [10].
- Low water-solubility, limiting the introduction of hydrolysates or peptides into food matrices, which requires generating dispersed systems [11].
- Physicochemical instability during storage, processing, and digestion, due to the exposure of peptides to environmental conditions (e.g., oxygen, heat) or their interaction with the digestive proteases and other compounds present in the food matrix [12].
- Limited bioaccessibility. Once ingested, peptides must be able to remain intact until they are absorbed in the intestine in order to exert their bioactivity. This is challenging due to the harsh conditions found in the gastrointestinal tract, such as the strongly acidic pH in the stomach and the enzymatically active gastric and intestinal fluids [13].
2. Literature Search
3. Encapsulation of Protein-Based Bioactives by Spray-Drying
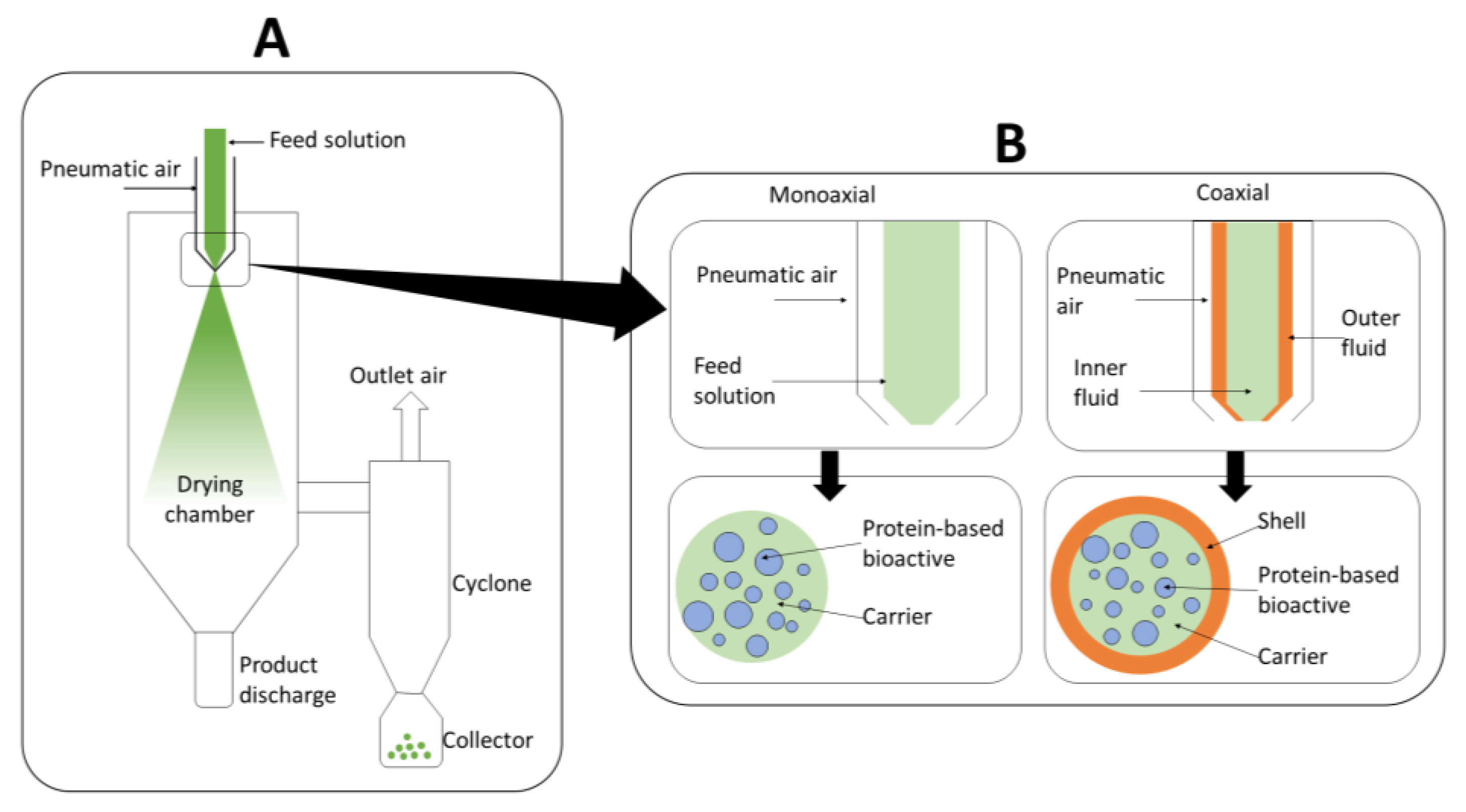
3.1. Fundamentals of Spray-Drying
- Inlet gas temperature is a key parameter that provides the driving force for the solvent evaporation. The temperature should be set at a level that is sufficiently high to promote water evaporation and ensure complete drying, without leading to agglomeration or deposition of wet particles on the chamber wall. Additionally, an increase in the temperature of the inlet air leads to a decrease in its relative humidity, which promotes water transport. However, excessively high inlet temperatures can lead to prompt crust formation, which limits water diffusion and subsequent evaporation. Therefore, careful control of the temperature of the inlet gas is necessary to ensure effective microencapsulation [29].
- Feed flow rate determines the size of the atomized droplets as well as the amount of water to be evaporated, which influences the temperature of the outlet gas and of the resulting particles.
- Drying-gas flow rate determines the amount of water evaporated and the residence time of the particles in the drying chamber. Too low a flow rate results in higher water condensation, as well as agglomeration or deposition of particles in the drying chamber. On the other hand, too high a flow rate could lead to degradation of the particles by shear stress.
3.2. Encapsulation by Monoaxial Spray-Drying
3.2.1. Formulation of the Feed Stream
3.2.2. Processing Conditions
3.3. Encapsulation by Coaxial Spray-Drying
4. Encapsulation of Protein-Based Bioactives by Electrospraying
4.1. Fundamentals of Electrospraying
4.2. Encapsulation by Monoaxial Electrospraying
4.2.1. Formulation of the Feed Stream
4.2.2. Processing Conditions
4.3. Encapsulation by Coaxial Electrospraying
5. Activity Retention and Release of the Encapsulated Protein-Based Bioactives
6. Bioaccessibility of Encapsulated Protein-Based Bioactives and Enrichment of Food Matrices
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jakubczyk, A.; Karas, M.; Rybczynska-Tkaczyk, K.; Zielinska, E.; Zielinski, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-Drying Encapsulation of Protein Hydrolysates and Bioactive Peptides: Opportunities and Challenges. Dry. Technol. 2019, 38, 577–595. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic Food-Derived Peptides for Functional Feeding: Production, Functionality and In Vivo Evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive Peptides: A Review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef]
- Moreira, A.; Lawson, D.; Onyekuru, L.; Dziemidowicz, K.; Angkawinitwong, U.; Costa, P.F.; Radacsi, N.; Williams, G.R. Protein Encapsulation by Electrospinning and Electrospraying. J. Control. Release 2021, 329, 1172–1197. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano- Zaragoza, M.L. Encapsulation of Bioactive Peptides: A Strategy to Improve the Stability, Protect the Nutraceutical Bioactivity and Support Their Food Applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Iwaniak, A.; Hrynkiewicz, M.; Bucholska, J.; Minkiewicz, P.; Darewicz, M. Understanding the Nature of Bitter-Taste Di- and Tripeptides Derived from Food Proteins Based on Chemometric Analysis. J. Food Biochem. 2019, 43, e12500. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-adergani, B. Bioactive Food Derived Peptides: A Review on Correlation between Structure of Bioactive Peptides and Their Functional Properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the Stability Challenges of Bioactive Peptides and Improvement Strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides Using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Rajendran, S.R.C.K.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of Food Protein Hydrolysates and Peptides: A Review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Sun, X.; Okagu, O.D.; Udenigwe, C.C. Encapsulation Technology for Protection and Delivery of Bioactive Peptides. Biol. Act. Pept. Basic Sci. Appl. Hum. Health 2021, 331–356. [Google Scholar] [CrossRef]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to Microencapsulation and Controlled Delivery in Foods. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; pp. 3–12. [Google Scholar] [CrossRef]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M.; Akbarbaglu, Z. Spray Drying Encapsulation of Proteins and Bioactive Peptides. In Spray Drying Encapsulation of Bioactive Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 241–270. [Google Scholar] [CrossRef]
- Rahmani-Manglano, N.E.; Jones, N.C.; Hoffmann, S.V.; Guadix, E.M.; Pérez-Gálvez, R.; Guadix, A.; García-Moreno, P.J. Structure of Whey Protein Hydrolysate Used as Emulsifier in Wet and Dried Oil Delivery Systems: Effect of PH and Drying Processing. Food Chem. 2022, 390, 133169. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of Electrohydrodynamic Processing for Encapsulation of Sensitive Bioactive Compounds and Applications in Food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Maltesen, M.J.; Andersen, S.K.; Bjerregaard, S.; Foged, C.; Rantanen, J.; Yang, M. One-Step Production of Protein-Loaded PLGA Microparticles via Spray Drying Using 3-Fluid Nozzle. Pharm. Res. 2014, 31, 1967–1977. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Jacobs, I.C. Atomization and Spray-Drying Processes. In Microencapsulation in the Food Industry: A Practical Implementation Guide; Academic Press: Cambridge, MA, USA, 2014; pp. 47–56. [Google Scholar] [CrossRef]
- Woo, M.W.; Bhandari, B. Spray Drying for Food Powder Production. In Handbook of Food Powders: Processes and Properties; Woodhead Publishing: Sawston, UK, 2013; pp. 29–56. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid. Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Pinto, J.T.; Faulhammer, E.; Dieplinger, J.; Dekner, M.; Makert, C.; Nieder, M.; Paudel, A. Progress in Spray-Drying of Protein Pharmaceuticals: Literature Analysis of Trends in Formulation and Process Attributes. Dry. Technol. 2021, 39, 1415–1446. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray Drying Encapsulation of Bioactive Compounds within Protein-Based Carriers; Different Options and Applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef]
- Wang, Y.; Selomulya, C. Spray Drying Strategy for Encapsulation of Bioactive Peptide Powders for Food Applications. Adv. Powder Technol. 2020, 31, 409–415. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Schwarz, K.; Amft, J. Spray-Dried Capsules and Extrudates as Omega-3 Lipids Delivery Systems. In Omega-3 Delivery Systems; Academic Press: Cambridge, MA, USA, 2021; pp. 321–343. [Google Scholar] [CrossRef]
- Rahmani-Manglano, N.E.; Guadix, E.M.; Jacobsen, C.; García-Moreno, P.J. Comparative Study on the Oxidative Stability of Encapsulated Fish Oil by Monoaxial or Coaxial Electrospraying and Spray-Drying. Antioxidants 2023, 12, 266. [Google Scholar] [CrossRef]
- Giroldi, M.; Grambusch, I.M.; Schlabitz, C.; Kuhn, D.; Lehn, D.N.; Volken de Souza, C.F. Encapsulation of Protein Hydrolysates by Spray Drying: Feasibility of Using Buffalo Whey Proteins. Int. J. Food Sci. Technol. 2022, 57, 3419–3427. [Google Scholar] [CrossRef]
- Peres, I.; Rocha, S.; Loureiro, J.A.; do Carmo Pereira, M.; Ivanova, G.; Coelho, M. Carbohydrate Particles as Protein Carriers and Scaffolds: Physico-Chemical Characterization and Collagen Stability. J. Nanoparticle Res. 2012, 14, 1144. [Google Scholar] [CrossRef]
- Salleh, N.H.; Jusoh, Y.M.M.; Zaidel, D.N.A.; Hashim, Z. The Scavenging Activity of Encapsulated EBN Hydrolysates Using Different Combinations of Polysaccharides as Wall Material by Spray Drying. Biocatal. Agric. Biotechnol. 2022, 45, 102503. [Google Scholar] [CrossRef]
- Palamutoğlu, R.; Sariçoban, C. Physico-Chemical Investigation and Antioxidant Activity of Encapsulated Fish Collagen Hydrolyzates with Maltodextrin. Ann. Univ. Dunarea de Jos Galati Fascicle VI Food Technol. 2019, 43, 94–105. [Google Scholar] [CrossRef]
- Camargo, T.R.; Khelissa, S.; Chihib, N.E.; Dumas, E.; Wang, J.; Valenti, W.C.; Gharsallaoui, A. Preparation and Characterization of Microcapsules Containing Antioxidant Fish Protein Hydrolysates: A New Use of Bycatch in Brazil. Mar. Biotechnol. 2021, 23, 321–330. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M. Improving the Antioxidant Stability of Flaxseed Peptide Fractions during Spray Drying Encapsulation by Surfactants: Physicochemical and Morphological Features. J. Food Eng. 2020, 286, 110131. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of Spray Drying Encapsulation on the Retention of Antioxidant Properties and Microstructure of Flaxseed Protein Hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mohammadi, M.; Akbarbaglu, Z.; Pezeshki, A.; Khakbaz Heshmati, M. Production of Reconstitutable Nanoliposomes Loaded with Flaxseed Protein Hydrolysates: Stability and Characterization. Food Hydrocoll. 2019, 96, 442–450. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M. Effect of Chitosan Coating on the Properties of Nanoliposomes Loaded with Flaxseed-Peptide Fractions: Stability during Spray-Drying. Food Chem. 2020, 310, 125951. [Google Scholar] [CrossRef]
- Lotfy, S.N.; Fadel, H.H.M.; El-Ghorab, A.H.; Shaheen, M.S. Stability of Encapsulated Beef-like Flavourings Prepared from Enzymatically Hydrolysed Mushroom Proteins with Other Precursors under Conventional and Microwave Heating. Food Chem. 2015, 187, 7–13. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, J.; Jiang, S.; Zeng, M. Effect of Chitosan Coating on the Properties of Nanoliposomes Loaded with Oyster Protein Hydrolysates: Stability during Spray-Drying and Freeze-Drying. Food Chem. 2022, 385, 132603. [Google Scholar] [CrossRef]
- Cian, R.E.; Campos-Soldini, A.; Chel-Guerrero, L.; Drago, S.R.; Betancur-Ancona, D. Bioactive Phaseolus Lunatus Peptides Release from Maltodextrin/Gum Arabic Microcapsules Obtained by Spray Drying after Simulated Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2019, 54, 2002–2009. [Google Scholar] [CrossRef]
- Sepúlveda, C.T.; Alemán, A.; Zapata, J.E.; Montero, M.P.; Gómez-Guillén, M.C. Characterization and Storage Stability of Spray Dried Soy-Rapeseed Lecithin/Trehalose Liposomes Loaded with a Tilapia Viscera Hydrolysate. Innov. Food Sci. Emerg. Technol. 2021, 71, 102708. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, X.; Zhang, C.; Ding, Z.; Sun, F.; Zhao, C. Effect of Inlet Temperature on the Physicochemical Properties of Spray-Dried Seed-Watermelon Seed Protein Powder. J. Food Sci. 2020, 85, 3442–3449. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Xie, C.; Zhang, X.; Yan, S.; Xu, J.; et al. Effect of Spray-Drying and Freeze-Drying on the Properties of Soybean Hydrolysates. J. Chem. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Aquino, R.P.; Auriemma, G.; Conte, G.M.; Esposito, T.; Sommella, E.; Campiglia, P.; Sansone, F. Development of Chitosan/Mannitol Microparticles as Delivery System for the Oral Administration of a Spirulina Bioactive Peptide Extract. Molecules 2020, 25, 2086. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hamishehkar, H.; Ghorbani, M.; Shahvalizadeh, R.; Pateiro, M.; Lorenzo, J.M. Engineering of Liposome Structure to Enhance Physicochemical Properties of Spirulina Plantensis Protein Hydrolysate: Stability during Spray-Drying. Antioxidants 2021, 10, 1953. [Google Scholar] [CrossRef]
- Lima, K.O.; da Rocha, M.; Alemán, A.; López-Caballero, M.E.; Tovar, C.A.; Gómez-Guillén, M.C.; Montero, P.; Prentice, C. Yogurt Fortification by the Addition of Microencapsulated Stripped Weakfish (Cynoscion Guatucupa) Protein Hydrolysate. Antioxidants 2021, 10, 1567. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Miralles, B.; Recio, I.; López-Rubio, A. Microencapsulation of a Whey Protein Hydrolysate within Micro-Hydrogels: Impact on Gastrointestinal Stability and Potential for Functional Yoghurt Development. J. Funct. Foods 2016, 26, 290–300. [Google Scholar] [CrossRef]
- Yang, S.; Mao, X.Y.; Li, F.F.; Zhang, D.; Leng, X.J.; Ren, F.Z.; Teng, G.X. The Improving Effect of Spray-Drying Encapsulation Process on the Bitter Taste and Stability of Whey Protein Hydrolysate. Eur. Food Res. Technol. 2012, 235, 91–97. [Google Scholar] [CrossRef]
- de Figueiredo Furtado, G.; da Silva Carvalho, A.G.; Hubinger, M.D. Model Infant Formulas: Influence of Types of Whey Proteins and Oil Composition on Emulsion and Powder Properties. J. Food Eng. 2021, 292, 110256. [Google Scholar] [CrossRef]
- Murthy, L.N.; Phadke, G.G.; Mohan, C.O.; Chandra, M.V.; Annamalai, J.; Visnuvinayagam, S.; Unnikrishnan, P.; Ravishankar, C.N. Characterization of Spray-Dried Hydrolyzed Proteins from Pink Perch Meat Added with Maltodextrin and Gum Arabic. J. Aquat. Food Prod. Technol. 2017, 26, 913–928. [Google Scholar] [CrossRef]
- Cian, R.E.; Salgado, P.R.; Mauri, A.N.; Drago, S.R. Pyropia Columbina Phycocolloids as Microencapsulating Material Improve Bioaccessibility of Brewers’ Spent Grain Peptides with ACE-I Inhibitory Activity. Int. J. Food Sci. Technol. 2020, 55, 1311–1317. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, Y.; Zhang, X.; Mei, L.; Pan, X.; Li, G.; Wu, C. Comparative Studies on Exenatide-Loaded Poly (d,l-Lactic-Co-Glycolic Acid) Microparticles Prepared by a Novel Ultra-Fine Particle Processing System and Spray Drying. Colloids Surf. B Biointerfaces 2015, 132, 103–110. [Google Scholar] [CrossRef]
- Webber, V.; de Siqueira Ferreira, D.; Barreto, P.L.M.; Weiss-Angeli, V.; Vanderlinde, R. Preparation and Characterization of Microparticles of β-Cyclodextrin/Glutathione and Chitosan/Glutathione Obtained by Spray-Drying. Food Res. Int. 2018, 105, 432–439. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Pedroza-Islas, R.; Escalona-Buendía, H.B.; Pedraza-Chaverri, J.; Ponce-Alquicira, E. Comparative Study of the Microencapsulation by Complex Coacervation of Nisin in Combination with an Avocado Antioxidant Extract. Food Hydrocoll. 2017, 62, 49–57. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, X.; He, R.; Yuan, J.; Aluko, R.E. Effect of High Pressure Treatment on Rapeseed Protein Microparticle Properties and Gastrointestinal Release Behavior of the Encapsulated Peptides. Food Res. Int. 2015, 77, 549–555. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, X.; He, R.; Yuan, J.; Wang, L. The Effect of Rapeseed Protein Structural Modification on Microstructural Properties of Peptide Microcapsules. Food Bioproc. Tech. 2015, 8, 1305–1318. [Google Scholar] [CrossRef]
- Ying, X.; Gao, J.; Lu, J.; Ma, C.; Lv, J.; Adhikari, B.; Wang, B. Preparation and Drying of Water-in-Oil-in-Water (W/O/W) Double Emulsion to Encapsulate Soy Peptides. Food Res. Int. 2021, 141, 110148. [Google Scholar] [CrossRef]
- De Koker, S.; Fierens, K.; Dierendonck, M.; De Rycke, R.; Lambrecht, B.N.; Grooten, J.; Remon, J.P.; De Geest, B.G. Nanoporous Polyelectrolyte Vaccine Microcarriers. A Formulation Platform for Enhancing Humoral and Cellular Immune Responses. J. Control. Release 2014, 195, 99–109. [Google Scholar] [CrossRef]
- Cambronero-Rojas, A.; Torres-Vergara, P.; Godoy, R.; von Plessing, C.; Sepúlveda, J.; Gómez-Gaete, C. Capreomycin Oleate Microparticles for Intramuscular Administration: Preparation, in Vitro Release and Preliminary in Vivo Evaluation. J. Control. Release 2015, 209, 229–237. [Google Scholar] [CrossRef]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; Pho, T.V.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of Vitamin C Stability in Vitamin Gummies by Encapsulation in Casein Gel. Food Hydrocoll. 2021, 113, 106414. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Spray- or Freeze-Drying of Casein Micelles Loaded with Vitamin D2: Studies on Storage Stability and in Vitro Digestibility. LWT 2018, 97, 87–93. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of Eugenol by Spray-Drying Using Whey Protein Isolate or Lecithin: Release Kinetics, Antioxidant and Antimicrobial Properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
- Locali Pereira, A.R.; Gonçalves Cattelan, M.; Nicoletti, V.R. Microencapsulation of Pink Pepper Essential Oil: Properties of Spray-Dried Pectin/SPI Double-Layer versus SPI Single-Layer Stabilized Emulsions. Colloids. Surf. A Phys. Eng. Asp. 2019, 581, 123806. [Google Scholar] [CrossRef]
- Molina Ortiz, S.E.; Mauri, A.; Monterrey-Quintero, E.S.; Trindade, M.A.; Santana, A.S.; Favaro-Trindade, C.S. Production and Properties of Casein Hydrolysate Microencapsulated by Spray Drying with Soybean Protein Isolate. LWT Food Sci. Technol. 2009, 42, 919–923. [Google Scholar] [CrossRef]
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-Based Biomaterials for Protein Delivery. Med. Drug Discov. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Largo Avila, E.; Cortes Rodríguez, M.; Ciro Velásquez, H.J. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Rev. Fac. Nac. Agron. Medellin. 2015, 68, 7509–7520. [Google Scholar] [CrossRef]
- Zarrabi, A.; Abadi, M.A.A.; Khorasani, S.; Reza Mohammadabadi, M.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The Effect of Spray-Drying and Freeze-Drying on Encapsulation Efficiency, in Vitro Bioaccessibility and Oxidative Stability of Krill Oil Nanoemulsion System. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Park, C.W.; Bastian, E.; Farkas, B.; Drake, M. The Effect of Feed Solids Concentration and Inlet Temperature on the Flavor of Spray Dried Whey Protein Concentrate. J. Food Sci. 2014, 79, C19–C24. [Google Scholar] [CrossRef]
- Chegini, G.R.; Ghobadian, B. Effect of Spray-Drying Conditions on Physical Properties of Orange Juice Powder. Dry. Technol. 2005, 23, 657–668. [Google Scholar] [CrossRef]
- Wang, W.; Dufour, C.; Zhou, W. CyTA-Journal of Food Impacts of Spray-Drying Conditions on the Physicochemical Properties of Soy Sauce Powders Using Maltodextrin as Auxiliary Drying Carrier. CyTA -J. Food 2015, 13, 548–555. [Google Scholar] [CrossRef]
- Keogh, K.; Murray, C.; Kelly, J.; O’kennedy, B. Effect of the Particle Size of Spray-Dried Milk Powder on Some Properties of Chocolate. Lait 2004, 84, 375–384. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Sunderland, T.; Kelly, J.G.; Ramtoola, Z. Application of a Novel 3-Fluid Nozzle Spray Drying Process for the Microencapsulation of Therapeutic Agents Using Incompatible Drug-Polymer Solutions. Arch Pharm. Res. 2015, 38, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Microencapsulation Technology Using a Three Fluid Nozzle and Spray Drying; University of Illinois: Urbana, IL, USA, 2022. [Google Scholar]
- Pérez-Masiá, R.; Lagaron, J.M.; Lopez-Rubio, A. Morphology and Stability of Edible Lycopene-Containing Micro- and Nanocapsules Produced Through Electrospraying and Spray Drying. Food Bioproc. Tech. 2015, 8, 459–470. [Google Scholar] [CrossRef]
- Shi, X.; Lee, Y. Encapsulation of Tributyrin with Whey Protein Isolate (WPI) by Spray-Drying with a Three-Fluid Nozzle. J. Food Eng. 2020, 281, 109992. [Google Scholar] [CrossRef]
- Tavares, L.; Zapata Noreña, C.P. Encapsulation of Garlic Extract Using Complex Coacervation with Whey Protein Isolate and Chitosan as Wall Materials Followed by Spray Drying. Food Hydrocoll. 2019, 89, 360–369. [Google Scholar] [CrossRef]
- Ghorani, B.; Tucker, N. Fundamentals of Electrospinning as a Novel Delivery Vehicle for Bioactive Compounds in Food Nanotechnology. Food Hydrocoll 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Lim, L.T.; Mendes, A.C.; Chronakis, I.S. Electrospinning and Electrospraying Technologies for Food Applications. Adv. Food Nutr. Res. 2019, 88, 167–234. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Mendes, A.C.; Jacobsen, C.; Chronakis, I.S. Biopolymers for the Nano-microencapsulation of Bioactive Ingredients by Electrohydrodynamic Processing. In Polymers for Food Applications; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 447–479. [Google Scholar]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Gañán-Calvo, A.M. Micro/Nano Encapsulation via Electrified Coaxial Liquid Jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- López-Herrera, J.M.; Barrero, A.; López, A.; Loscertales, I.G.; Márquez, M. Coaxial Jets Generated from Electrified Taylor Cones. Scaling Laws. J. Aerosol. Sci. 2003, 34, 535–552. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Rahmani-Manglano, N.E.; Chronakis, I.S.; Guadix, E.M.; Yesiltas, B.; Sørensen, A.D.M.; Jacobsen, C. Omega-3 Nano-Microencapsulates Produced by Electrohydrodynamic Processing. In Omega-3 Delivery Systems; Academic Press: Cambridge, MA, USA, 2021; pp. 345–370. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Si, T.; Xu, R.X. Coaxial Electrospray of Microparticles and Nanoparticles for Biomedical Applications. Expert Rev. Med. Devices 2014, 9, 595–612. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Félix, F.; Katouzian, I. Nanocapsule Formation by Electrospraying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; pp. 320–345. [Google Scholar] [CrossRef]
- Parhizkar, M.; Reardon, P.J.T.; Knowles, J.C.; Browning, R.J.; Stride, E.; Pedley, R.B.; Grego, T.; Edirisinghe, M. Performance of Novel High Throughput Multi Electrospray Systems for Forming of Polymeric Micro/Nanoparticles. Mater Des. 2017, 126, 73–84. [Google Scholar] [CrossRef]
- Kang, J.S.; Park, I.; Jung, J.H.; Kim, S.S. Free-Surface Electrospray Technique Using a Multi-Hole Array. J. Aerosol. Sci. 2013, 55, 25–30. [Google Scholar] [CrossRef]
- Busolo, M.A.; Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Electrospraying Assisted by Pressurized Gas as an Innovative High-Throughput Process for the Microencapsulation and Stabilization of Docosahexaenoic Acid-Enriched Fish Oil in Zein Prolamine. Innov. Food Sci. Emerg. Technol. 2019, 51, 12–19. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Controlling Microencapsulation and Release of Micronized Proteins Using Poly(Ethylene Glycol) and Electrospraying. Eur. J. Pharm. Biopharm. 2014, 87, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Musaei, M.; Mokhtari, J.; Nouri, M.; Rad, Z.P. Fabrication and Characterization of Nanocapsules of PLGA Containing BSA Using Electrospray Technique. Nanomed. Res. J. 2017, 2, 158–164. [Google Scholar] [CrossRef]
- Rasekh, M.; Young, C.; Roldo, M.; Lancien, F.; Le Mével, J.C.; Hafizi, S.; Ahmad, Z.; Barbu, E.; Gorecki, D. Hollow-Layered Nanoparticles for Therapeutic Delivery of Peptide Prepared Using Electrospraying. J. Mater. Sci. Mater. Med. 2015, 26, 256. [Google Scholar] [CrossRef]
- Onyekuru, L.C.; Moreira, A.; Zhang, J.; Angkawinitwong, U.; Costa, P.F.; Brocchini, S.; Williams, G.R. An Investigation of Alkaline Phosphatase Enzymatic Activity after Electrospinning and Electrospraying. J. Drug Deliv. Sci. Technol. 2021, 64, 102592. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, N.; Faridi Majidi, R.; Faramarzi, M.A.; Baharifar, H.; Amani, A. Preparation, Optimization and Activity Evaluation of PLGA/Streptokinase Nanoparticles Using Electrospray. Adv. Pharm. Bull. 2017, 7, 131–139. [Google Scholar] [CrossRef]
- Kumar, A.; Belhaj, M.; DiPette, D.J.; Potts, J.D. A Novel Alginate-Based Delivery System for the Prevention and Treatment of Pressure-Overload Induced Heart Failure. Front Pharm. 2021, 11, 2227. [Google Scholar] [CrossRef]
- Furtmann, B.; Tang, J.; Kramer, S.; Eickner, T.; Luderer, F.; Fricker, G.; Gomez, A.; Heemskerk, B.; Jähn, P.S. Electrospray Synthesis of Poly(Lactide-Co-Glycolide) Nanoparticles Encapsulating Peptides to Enhance Proliferation of Antigen-Specific CD8+ T Cells. J. Pharm. Sci. 2017, 106, 3316–3327. [Google Scholar] [CrossRef]
- Yao, S.; Liu, H.; Yu, S.; Li, Y.; Wang, X.; Wang, L. Drug-Nanoencapsulated PLGA Microspheres Prepared by Emulsion Electrospray with Controlled Release Behavior. Regen. Biomater. 2016, 3, 309–317. [Google Scholar] [CrossRef]
- Ho, W.X.; Chen, W.T.; Lien, C.H.; Yang, H.Y.; Chen, K.H.; Wei, Y.F.; Wang, M.H.; Ko, I.T.; Tseng, F.G.; Yin, H.S. Physical, Chemical, and Biological Properties of Chitosan-Coated Alginate Microparticles Loaded with Porcine Interleukin-1β: A Potential Protein Adjuvant Delivery System. Int. J. Mol. Sci. 2022, 23, 9959. [Google Scholar] [CrossRef]
- Aragón, J.; Salerno, S.; de Bartolo, L.; Irusta, S.; Mendoza, G. Polymeric Electrospun Scaffolds for Bone Morphogenetic Protein 2 Delivery in Bone Tissue Engineering. J. Colloid. Interface Sci. 2018, 531, 126–137. [Google Scholar] [CrossRef]
- Song, Y.; Chan, Y.K.; Ma, Q.; Liu, Z.; Shum, H.C. All-Aqueous Electrosprayed Emulsion for Templated Fabrication of Cytocompatible Microcapsules. ACS Appl. Mater. Interfaces 2015, 7, 13925–13933. [Google Scholar] [CrossRef] [PubMed]
- Van, V.; Lee, T.-C. Electrospray Technique for Preparation of Core-Shell Materials: A Mini-Review. Online Part. Aerosol. Res. Part. Aerosol. Res. 2018, 14, 49–63. [Google Scholar] [CrossRef]
- Romano, L.; Camposeo, A.; Manco, R.; Moffa, M.; Pisignano, D. Core-Shell Electrospun Fibers Encapsulating Chromophores or Luminescent Proteins for Microscopically Controlled Molecular Release. Mol. Pharm. 2016, 13, 729–736. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Chen, M.; Ren, Z.; Chen, L.; Fu, C.; Ma, Z.; Li, Z. Advancement of Protein- and Polysaccharide-Based Biopolymers for Anthocyanin Encapsulation. Front. Nutr. 2022, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Electrospraying of Polymers with Therapeutic Molecules: State of the Art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Thian, E.S.; Ramakrishna, S. Protein Encapsulated Core–Shell Structured Particles Prepared by Coaxial Electrospraying: Investigation on Material and Processing Variables. Int. J. Pharm. 2014, 473, 134–143. [Google Scholar] [CrossRef]
- Zhang, L.; Si, T.; Fischer, A.J.; Letson, A.; Yuan, S.; Roberts, C.J.; Xu, R.X. Coaxial Electrospray of Ranibizumab-Loaded Microparticles for Sustained Release of Anti-VEGF Therapies. PLoS ONE 2015, 10, e0135608. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and Their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Gallovic, M.D.; Schully, K.L.; Bell, M.G.; Elberson, M.A.; Palmer, J.R.; Darko, C.A.; Bachelder, E.M.; Wyslouzil, B.E.; Keane-Myers, A.M.; Ainslie, K.M.; et al. Acetalated Dextran Microparticulate Vaccine Formulated via Coaxial Electrospray Preserves Toxin Neutralization and Enhances Murine Survival Following Inhalational Bacillus Anthracis Exposure. Adv Heal. Mater 2016, 5, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jansman, M.M.T.; Thulstrup, P.W.; Mendes, A.C.; Chronakis, I.S.; Hosta-Rigau, L.; Liu, X.; Jansman, M.M.T.; Hosta-Rigau, L.; Thulstrup, P.W.; et al. Low-Fouling Electrosprayed Hemoglobin Nanoparticles with Antioxidant Protection as Promising Oxygen Carriers. Macromol. Biosci. 2020, 20, 1900293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, G.; Wang, H.; Zhao, Y.; Chai, R. Bio-Inspired Intestinal Scavenger from Microfluidic Electrospray for Detoxifying Lipopolysaccharide. Bioact. Mater 2021, 6, 1653–1662. [Google Scholar] [CrossRef]
- Paz-Samaniego, R.; Rascón-Chu, A.; Brown-Bojorquez, F.; Carvajal-Millan, E.; Pedroza-Montero, M.; Silva-Campa, E.; Sotelo-Cruz, N.; López-Franco, Y.L.; Lizardi-Mendoza, J. Electrospray-Assisted Fabrication of Core-Shell Arabinoxylan Gel Particles for Insulin and Probiotics Entrapment. J. Appl. Polym. Sci. 2018, 135, 46411. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef]
- Dai, C.; Wang, B.; Zhao, H. Microencapsulation Peptide and Protein Drugs Delivery System. Colloids Surf. B Biointerfaces 2005, 41, 117–120. [Google Scholar] [CrossRef]
- Ospina-Quiroga, J.L.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M.; Almécija-Rodríguez, M.d.C.; Pérez-Gálvez, R. Evaluation of Plant Protein Hydrolysates as Natural Antioxidants in Fish Oil-In-Water Emulsions. Antioxidants 2022, 11, 1612. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive Peptides as Natural Antioxidants in Food Products—A Review. Trends. Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Chen, H.; Deng, Y.; Wei, Z.; Zhang, Y.; Tang, X.; Li, P.; Zhao, Z.; Zhou, P.; et al. Rice Bran-Modified Wheat Gluten Nanoparticles Effectively Stabilized Pickering Emulsion: An Interfacial Antioxidant Inhibiting Lipid Oxidation. Food Chem. 2022, 387, 132874. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug Transport Mechanisms and in Vitro Release Kinetics of Vancomycin Encapsulated Chitosan-Alginate Polyelectrolyte Microparticles as a Controlled Drug Delivery System. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and Bioaccessibility of Food Bioactive Compounds; Overview and Assessment by in Vitro Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Fogliano, V. Food Matrix Interaction and Bioavailability of Bioactive Peptides: Two Faces of the Same Coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]


| Formulation | Process Variables | Capsule Characteristics | Ref. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrolysate/Peptide | Bioactivity | Carrier | Solvent | Peptide: Carrier Ratio (w/w) | Prep. Method | T Inlet (°C) | T Outlet (°C) | Drying Air Flow Rate (L/h) | Feed Flow Rate (mL/h) | Nozzle Diam. (μm) | Size (μm) | EE (%) | ||
| Hydrolysate | Buffalo whey proteins hydrolysate | – | GA and MD | Water | 1:10 and 1:15 | Blend | 105 | 90 | 9000 | 160 | 700 | 2.0/20.0 | >95% | [31] |
| Collagen hydrolysate | Organ formation | GA and MD | Water | 1:4 | Blend | 150–170 | 50–60 | – | – | – | 0.4–50 | 86 ± 7–85 ± 4% | [32] | |
| Edible bird’s nest hydrolysate | Antioxidant | MD/MD and CMC/MD and XG | Water | 1:3.33 | Blend | 180 ± 2 | 90 ± 2 | – | 600 | 700 | – | 85.97 (MD) 81.15 (MD + CMC) 86.58 (MD + XG) | [33] | |
| Fish collagen hydrolysate | Antioxidant | MD | Water | 1:9 and 1:4 | Blend | 140 ± 1 | 80 ± 0.5 | – | – | 700 | <425 µm | – | [34] | |
| Fish protein hydrolysates | Antioxidant | MD | Imidazole acetate buffer | 1:200 | Blend | 180 | 90 | – | 500 | 500 | 61.5 ± 1.7–183 ± 2.8 | – | [35] | |
| Flaxseed peptide fractions | Antioxidant | MD | Water | 1:3 | Blend | 110 ± 1 | 60 ± 2 | 540 | 300 | 500 | 9–11 | – | [36] | |
| Flaxseed protein hydrolysate | Antioxidant | MD | Water | 1:1, 2:1 and 3:1 | Blend | 130 ± 1 | 75 ± 2 | – | 300 | 700 | ~ 5 | – | [37] | |
| Flaxseed protein hydrolysate | Antioxidant | MD | Water | 1:1 | Nanoliposome | 130 ± 1 | 75 ± 2 | – | 300 | 500 | 0.326–0.353 | 72.12–84.99 | [38] | |
| Flaxseed protein hydrolysates | Antioxidant | MD | Water | 1:1 (v/v) | Nanoliposome | 130 ± 1 | 73 ± 2 | 540 | 300 | 500 | 0.132 ± 0.015–0.86 ± 0.012 | 84.0 ± 1.9–90.7 ± 1.6 | [39] | |
| Mushroom protein hydrolysate | Food flavoring | GA | Water | 1:2 | Blend | 150 | 95 | – | 300 g/h | 500 | – | – | [40] | |
| Oyster protein hydrolysate | Antioxidant | MD | Water | 1:1 | Nanoliposome | 170 | – | – | 300 | – | 0.392–0.719 | 71.43–82.36 | [41] | |
| P. lunatus hydrolysate | Antidiabetic | MD and GA | Water | 1:25 and 1:10 | Blend | 160 ± 4 | 88 ± 2 | 33.6 | 1380 | – | 3.3–6.8 | 59.9–82.0 | [42] | |
| Red tilapia viscera hydrolysate | Antioxidant and antihypertensive | Soy rapeseed lecithin | Phosphate buffer | 1:5 | Nanoliposome | 130 | 62 | – | 630.5 | 700 | 0.25–0.31 | 80–81 | [43] | |
| Watermelon seed hydrolyzed protein | antioxidant | MD and sucrose | Water | – | Blend | 150–180 | 80 | – | 200 | – | 10–12.9 | – | [44] | |
| Soy protein hydrolysates | Antioxidant | Soy protein isolate and MD | Water | 1:1.2 and 1:0.8 | Blend | 180 | 80–90 | – | 900 | 700 | – | – | [45] | |
| Spirulina platensis hydrolysate | Hepatoprotective, antioxidant, anticancer, etc. | Mannitol and CS | Water (mannitol) and acidified water (CS) | 1:10 (w/w) | Blend | 120 | 70 | 500/600 | 300 | 500 | 14.24 ± 2.66 | ~ 100 | [46] | |
| Spirulina platensis hydrolysate | Antioxidant | MD and CS | Water and acetic acid | 60:40 | Nanoliposome | 130 | 75 | – | – | – | 1–3 | 88.0–89.0 | [47] | |
| Stripped weakfish hydrolysate | Antioxidant and ACE inhibitor | MD | Water | 60:40 | Blend | 130 | 70 ± 2 | – | – | – | – | – | [48] | |
| Whey protein hydrolysate | – | CS/gelatin | Acetic acid 20% | 3:1 (gelatin) 15:1 (CS) | Blend | 90 | 50 ± 5 | 9000 | – | – | 0.603 ± 0.627 (G) 0.571 ± 0.440 (C) | – | [49] | |
| Whey protein hydrolysate | Physiological functionality | MD/MD and β-CD | Water | 30:70 | Blend | 200 | 90 ± 5 | - | 1000 | – | 2.47–3.26 | – | [50] | |
| Whey protein hydrolysate | Infant formulas | MD | HOSO (O) Water (W) | 1:2 and 1:4 | O/W emulsion | 170 | 95 ± 3 | 38000 | 360 | 700 | 10 | 84.8–97.2 | [51] | |
| Pink perch meat protein hydrolysate | Antioxidant | None/MD and GA | Water | Blend | 160 | 80 | – | 900–1200 | 500 | 4.05–17.3 (no carrier) 5.12–15 (with carrier) | – | [52] | ||
| Peptide | Brewers’ spent grain digested peptides | ACE-I inhibitor | LBG and MD/LBG, PG and MD/PG and MD | Water | 1:6 | Blend | 180 ± 2 | 96 ± 8 | 357 | 180 | 700 | 5–7 | >90% | [53] |
| Exenatide | Antidiabetic | Mannitol and PLGA | DCM and DMC (O) and water (W) | 20:1 (v/v) | W/O emulsion | 60 | – | – | 180 | – | 4.83 ± 1.79 | 84.65 ± 2.93 | [54] | |
| Glutathione | Antioxidant | CS/β-CD | Water (β-CD) Acetic acid and water (CS) | 1:3.5 (β-CD) 1:2.5 (CS) | Blend | 200 (β-C) 130 (C) | 72 (β-C) 47 (C) | – | 600 | – | (β-CD < chitosan) | 62.4 (β-CD) 25 (CS) | [55] | |
| Nisin | Antimicrobial | Pectin/alginate | Water (Blend)/Soybean oil (O) and water (W1, W2) | 1:2.5 (pectin) 1:125 (alginate) | Blend/W1/O/W2 emulsion | 140 ± 5 | 70 ± 5 | – | 900 | 700 | 17.91–18.67 (B) 44.87–66.59 (E) | 63.70 ± 1.31–69.88 ± 1.10 (B) 72.80 ± 1.98–84.66 ± 1.20 (E) | [56] | |
| Rapeseed peptides | Dietary protein source | Rapeseed protein isolates | Water | 1:2 | Blend | 135 ± 2 | 74 ± 2 | 450 | 350 | – | 6.2 ± 0.12–8.5 ± 0.21 | 87.1 ± 1.2–94.7 ± 1.8 | [57] | |
| Rapeseed peptides | Dietary protein source | Rapeseed protein isolates | Water | 1:1, 1:2 y 2:1 | Blend | 135 ± 2 | 74 ± 2 | 450 | 350 | – | 5.8 ± 0.01–16.3 ± 0.12 | 60 ± 0.9–72 ± 1.1 | [58] | |
| Soy peptides | Antihypertensive | Modified starch and MD (W2) | MCT oil (O) and water (W1, W2) | 1:1 (w/w) | W/O/W emulsion | 150 | – | – | 900–1200 | – | 1.44 ± 0.04–8.39 ± 0.21 | 29.51 ± 0.89–45.83 ± 0.47 | [59] | |
| Protein | Ovalbumin (OVA) | Vaccine antigen | Dextran sulfate, mannitol and PLARG | Water | 1:9 | Blend | 120 | – | – | 60–600 | 700 | 1–10 | 99–110 | [60] |
| Capreomycin oleate | Antituberculosis | HA and DPPC | Water (DPPC) Ethanol (HA) | 75:25 (v/v) | Blend | 110 | 60–65 | 500 | 1020 | 700 | 2.06–9.14 | 56 | [61] | |
| Formulation | Process Variables | Capsule Characteristics | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrolysate/Peptide | Bioactivity | Carrier | Solvent | Peptide: Carrier Ratio | Prep. Method | Voltage (kV) | Feed Flow Rate (mL/h) | Distance N-C (cm) | Nozzle Diam. (μm) | Size (μm) | EE (%) | ||
| Peptide | Alpha-calcitonin gene-related peptide | Vasodilator | Alginate | Water | 1:2 (w/v) | Blend | 6 | 60 | 7 × 10−3 | – | 194.23 ± 10.08 | – | [97] |
| Peptide pp65489-503 CMV 4–peptide mix | Immune stimulatory | PLGA | TFE/DMSO | 1:22 (w/w) | Blend | 10 | 0.01 | 14 | 30 | 200 | 84–85 | [98] | |
| Protein | SA | – | PCL and PEG/PLGA and PEG | Chloroform (PCL and PEG)/DCM (PLGA and PEG) | 10:90, 5:95, 15:85 | Blend | 10 | 0.5–3 | 15 or 25 | 450–800 | 5.6 ± 0.8 (PLGA) 12.0 ± 4.0 (PCL) | 20–40 | [92] |
| BSA | – | PLGA | Acetone (PLGA) Ethanol and acetic acid (BSA) | 1:1, 1:4, 1:9 | Blend | 10, 15 and 20 | 36 × 10−3 and 72 × 10−3 | 10, 15 and 20 | – | 0.085–0.26 | – | [93] | |
| BSA | – | PLGA | Chloroform (O) and water (W) | – | W/O emulsion | 6 | 1 | 20 | – | 9.6 ± 1.4–7.2 ± 2.4 | 92–80 | [99] | |
| BSA or porcine interleukin-1β | Vaccine adjuvant | Alginate | Water | 2:1, 4:1, 6:1 | Blend | 12 | 0.1 | 20 | 511 | 20 | 50 (BSA) | [100] | |
| Bone morphogenetic protein 2 | Bone regeneration | PLGA, BSA | DMF (PLGA) and water (BSA) | 1:100 (v/v) | Blend | 9–12 | 0.5 | 30 | 900 | 1.0 ± 0.6 | 39 | [101] | |
| Enzyme | Alkaline phosphatase | Enzyme- anti-inflammatory | PEO | Ethanol and water | 1:7 (v/v) | Blend | 9–15 | 0.5–1 | 12–22.5 | 610 | 0.73 ± 0.16 | 85.0 ± 4.0 | [95] |
| Amylase | Enzyme | Dextran and sodium alginate | Water | – | Water-in-water (w/w) emulsion | 2.67–2.85 | 0.5 | 1 × 10−3 | 40–320 | <1000 | 47 ± 3 | [102] | |
| Streptokinase | Thrombotic disease treatment | PLGA | Water and DCM | 1:100 (v/v) | Blend | 13 | 0.1 | 10 | 180 | 0.037 ± 0.012 | 90 | [96] | |
| Hormone | Angiotensin II | Antihypertensive | NOSC | DMSO/water/DCM | 1:1 (w/w) | Blend | 15–19 | 1.08 | 0.1 | 508 | 1.057 ± 4 × 10−3 | 70–90 | [94] |
| Formulation | Process Variables | Capsule Characteristics | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrolysate/Peptide | Bioactivity | Carrier | Solvent | Peptide: Carrier Ratio | Voltage (kV) | Feed Flow Rate (mL/h) | Distance N-C (cm) | Nozzle Diameter (μm) | Size (μm) | EE (%) | ||
| Protein | Bovine serum albumin (BSA) | – | PLGA (outer) | Water (inner) DCM and DMF (outer) | – | 9–11 | 0.1–0.2 (inner) 1–2 (outer) | – | 300 (inner) 840 (outer) | 5.4 ± 1.56–2.90 ± 0.76 | 46.7 ± 4.3–74.6 ± 2.9 | [107] |
| Bovine hemoglobin | Oxygen carrier | – | Ethanol | – | 20 | 0.08 (inner) 0.48 (outer) | 10 | – | 0.37 | – | [111] | |
| Anthrax protective antigen | Antibacterial | Acetylated dextran (outer) | Ethanol, ethyl acetate and n-butanol | 1:1.85 | 10–12 | 0.02 (inner) 0.85 (outer) | 14 | 210 (inner) 603 (outer) | ~1 | – | [110] | |
| Ranibizumab | Age-related macular degeneration | PLGA (outer) | Water and EG (inner) DCM and acetonitrile (outer) | 1:6 | 5 | 0.5 (inner) | 16.95 | – | 1–2 | 70 | [108] | |
| Enzyme | Alkaline phosphatase | Detoxifying | CMC (inner) Alginate and PEGDA (outer) | Water | – | 12.5 | 3.6 (inner) 18 (outer) | 5 | – | 440 | 84 | [112] |
| Alkaline phosphatase | Anti-inflammatory | PEO (outer) | PBS (inner) Ethanol and water (outer) | 1:2 | 22.5 | 0.02 (inner) 0.3 (outer) | 15–20 | 1000 (inner) 2000 (outer) | 1.29 ± 0.24 | 99 | [95] | |
| Hormone | Angiotensin II | Antihypertensive | NOSC and tristearin | Water (inner) DCM (outer) | 1:1 (inner) | 15–17.9 | 1.8 (inner) 3.96 (outer) | 5–2.5 | 900 (inner) 1900 (outer) | 0.17–0.26 | 92 | [94] |
| Insulin | Antidiabetic | MBA (inner) MWA (outer) | Water | 4:1 (inner) 6.67:1 (outer) | 16 | 1 | 5 | 184 (inner) 1194 (outer) | 290 | 72 | [113] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berraquero-García, C.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M.; García-Moreno, P.J. Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying. Foods 2023, 12, 2005. https://doi.org/10.3390/foods12102005
Berraquero-García C, Pérez-Gálvez R, Espejo-Carpio FJ, Guadix A, Guadix EM, García-Moreno PJ. Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying. Foods. 2023; 12(10):2005. https://doi.org/10.3390/foods12102005
Chicago/Turabian StyleBerraquero-García, Carmen, Raúl Pérez-Gálvez, F. Javier Espejo-Carpio, Antonio Guadix, Emilia M. Guadix, and Pedro J. García-Moreno. 2023. "Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying" Foods 12, no. 10: 2005. https://doi.org/10.3390/foods12102005
APA StyleBerraquero-García, C., Pérez-Gálvez, R., Espejo-Carpio, F. J., Guadix, A., Guadix, E. M., & García-Moreno, P. J. (2023). Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying. Foods, 12(10), 2005. https://doi.org/10.3390/foods12102005









