Torulaspora delbrueckii May Help Manage Total and Volatile Acidity of Santorini-Assyrtiko Wine in View of Global Warming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Small-Scale Laboratory Fermentations in Sterile Must
2.3. Microvinifications
2.4. Pilot-Scale Fermentations
2.5. Microbiological Analysis
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
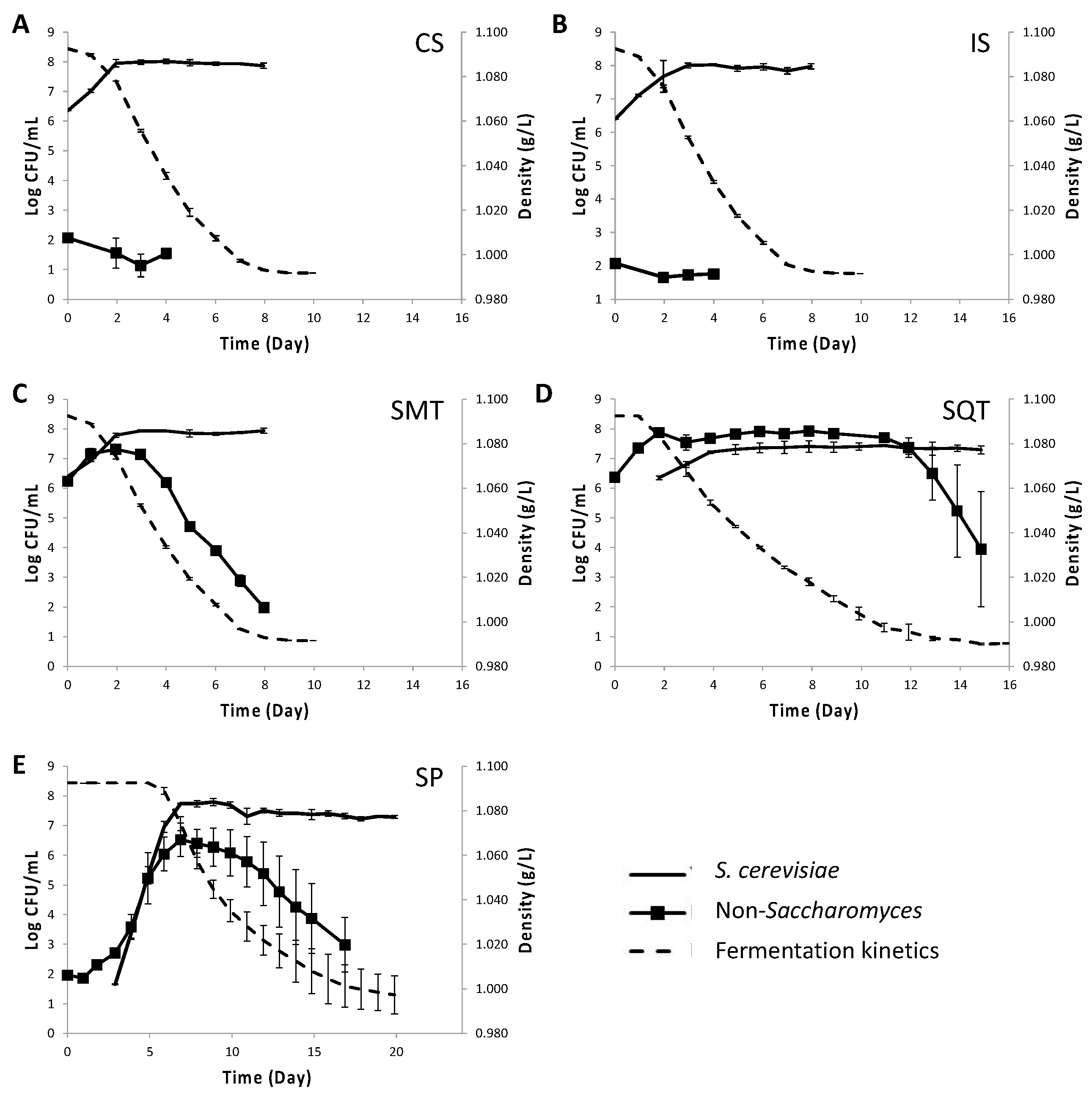
3.1. Fermentation Kinetics and Yeast Population Dynamics in Mixed Lab-Scale Fermentations
3.2. Chemical Profile of Laboratory-Scale Ferments
3.3. Fermentation Kinetics and Yeast Population Dynamics in Mixed Microvinifications
3.4. Chemical Profile of Wines Produced in Microvinifications
3.5. Fermentation Kinetics and Yeast Population Dynamics in Mixed Pilot-Scale Fermentations
3.6. Chemical Profile of Wines Produced in Pilot-Scale Fermentations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Patterns of genetic diversity and the invasion of commercial starters in Saccharomyces cerevisiae vineyard populations of Santorini island. Foods 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Biogeographical regionalization of wine yeast communities in Greece and environmental drivers of species distribution at a local scale. Front. Microbiol. 2021, 12, 1576. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Pretorius, I.S. Selection and improvement of wine yeasts. Ann. Microbiol. 2000, 50, 15–31. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Pat. Food. Nutr. Agric. 2019, 11, 27–39. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous yeasts: Emerging trends and challenges in winemaking. Curr. Opin. Food Sci. 2020, 32, 133–143. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Fraga, H.; Nakas, C.T.; Koundouras, S. A study on the effects of climate change on viticulture on Santorini Island. OENO One 2022, 56, 259–273. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Dequin, S.; Escudier, J.L.; Bely, M.; Noble, J.; Albertin, W.; Masneuf-Pomarède, I.; Marullo, P.; Salmon, M.J.; Sablayrolles, J.M.; Ollat, N. How to adapt winemaking practices to modified grape composition under climate change conditions. J. Int. Sci. Vigne Vin 2017, 51, 205–214. [Google Scholar] [CrossRef]
- Sgouros, G.; Mallouchos, A.; Filippousi, M.E.; Banilas, G.; Nisiotou, A. Molecular characterization and enological potential of a high lactic acid-producing Lachancea thermotolerans vineyard strain. Foods 2020, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Sainz, F.; Pardo, J.; Ruiz, A.; Expósito, D.; Armero, R.; Querol, A.; Guillamón, J.M. Use of non-conventional yeasts to increase total acidity in the Cava base wines. LWT 2022, 158, 113183. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult-to-use tool for winemaking. Fermentation 2018, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Nisiotou, A.A.; Spiropoulos, A.E.; Nychas, G.J.E. Yeast community structures and dynamics in healthy and Botrytis-affected grape must fermentations. Appl. Environ. Microbiol. 2007, 73, 6705–6713. [Google Scholar] [CrossRef] [Green Version]
- Legras, J.; Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Barquet, M.; Martín, V.; Medina, K.; Pérez, G.; Carrau, F.; Gaggero, C. Tandem repeat-tRNA (TRtRNA) PCR method for the molecular typing of non-Saccharomyces subspecies. Appl. Microbiol. Biotechnol. 2012, 93, 807–814. [Google Scholar] [CrossRef]
- Martín, B.; Garriga, M.; Hugas, M.; Bover-Cid, S.; Veciana-Nogués, M.T.; Aymerich, T. Molecular, technological and safety characterization of Gram-positive catalase-positive cocci from slightly fermented sausages. Int. J. Food Microbiol. 2006, 107, 148–158. [Google Scholar] [CrossRef]
- OIV Compendium of International Methods of Wine and Must Analysis 2015. Available online: https://www.oiv.int/es/standards/annex-a-methods-of-analysis-of-wines-and-musts (accessed on 30 October 2022).
- Gump, B.H.; Zoecklein, B.W.; Fugelsang, K.C. Food Microbiology Protocols. In Methods in Biotechnology; Spencer, J.F.T., Ragout de Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 283–296. [Google Scholar]
- Nisiotou, A.; Sgouros, G.; Mallouchos, A.; Nisiotis, C.S.; Michaelidis, C.; Tassou, C.; Banilas, G. The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Res. Int. 2018, 111, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.K.; King, E.S.; Ebeler, S.E.; Heymann, H. Characterizing the Chemical and Sensory Profiles of United States Cabernet Sauvignon Wines and Blends. Am. J. Enol. Vitic. 2013, 64, 169–179. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Andorrà, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT 2012, 49, 8–13. [Google Scholar] [CrossRef]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Fernández, M.A.; Carafa, I.; Vrhovsek, U.; Arapitsas, P. Modulating wine aromatic amino acid catabolites by using Torulaspora delbrueckii in sequentially inoculated fermentations or Saccharomyces cerevisiae alone. Microorganisms 2020, 8, 1349. [Google Scholar] [CrossRef]
- Sgouros, G.; Chalvantzi, I.; Mallouchos, A.; Paraskevopoulos, Y.; Banilas, G.; Nisiotou, A. Biodiversity and enological potential of non-Saccharomyces yeasts from Nemean vineyards. Fermentation 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [Green Version]
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; de Celis, M.; de Toro, M.; Mendes-Ferreira, A.; Rauhut, D.; Santos, A.; Belda, I. Phenotypic and transcriptional analysis of Saccharomyces cerevisiae during wine fermentation in response to nitrogen nutrition and co-inoculation with Torulaspora delbrueckii. Food Res. Int. 2020, 137, 109663. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Silva-Sousa, F.; Pereira, F.; Rito, T.; Soares, P.; Franco-Duarte, R.; Sousa, M.J. Biotechnological importance of Torulaspora delbrueckii: From the obscurity to the spotlight. J. Fungi 2021, 7, 712. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An integrative view of the role of Lachancea thermotolerans in wine technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef] [PubMed]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolina, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Remize, F.; Andrieu, E.; Dequin, S. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: Role of the cytosolic Mg2+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl. Environ. Microbiol. 2000, 66, 3151–3159. [Google Scholar] [CrossRef] [Green Version]
- Oura, E. Reaction Products of Yeast Fermentations. Process Biochem. 1977, 12, 19–21. [Google Scholar]
- Coulter, A.; Godden, P.W.; Pretorius, I. Succinic acid-how is it formed, what is its effect on titratable acidity, and what factors influence its concentration in wine? Aust. N. Z. Wine Ind. J. 2004, 19, 16–25. [Google Scholar]
- Baroň, M.; Fiala, J. Chasing after minerality, relationship to yeast nutritional stress and succinic acid production. Czech J. Food Sci. 2012, 30, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Henschke, P. Yeasts and Wine Flavour. In Wine Chemistry and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 313–392. ISBN 978-0-387-74116-1. [Google Scholar]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.-C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Vararu, F.; Moreno-Garcia, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Analyzing the minor volatilome of Torulaspora delbrueckii in an alcoholic fermentation. Eur. Food Res. Technol. 2022, 248, 613–624. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Changes in the concentration of yeast-derived volatile compounds of red wine during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2005, 53, 10134–10139. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef]

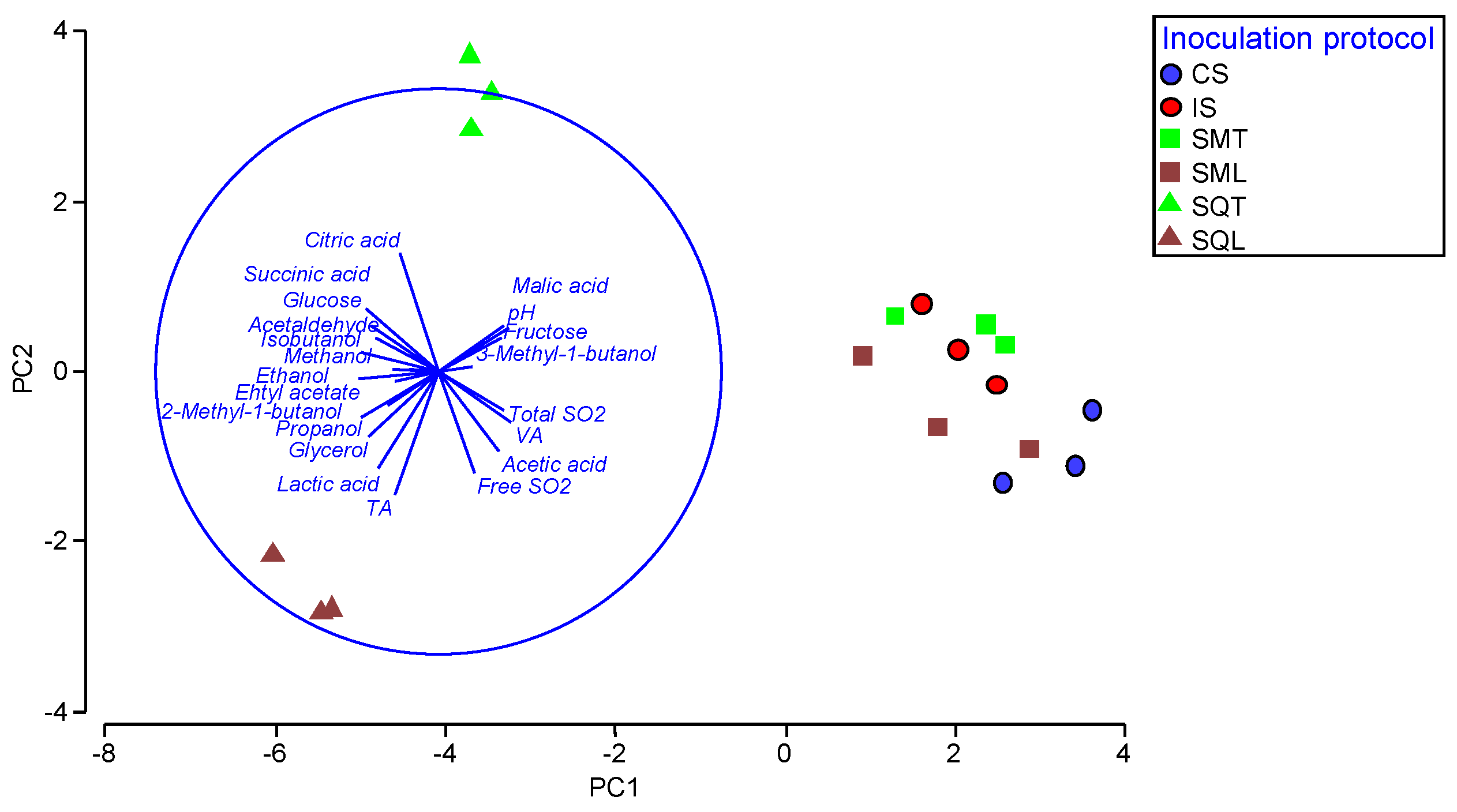


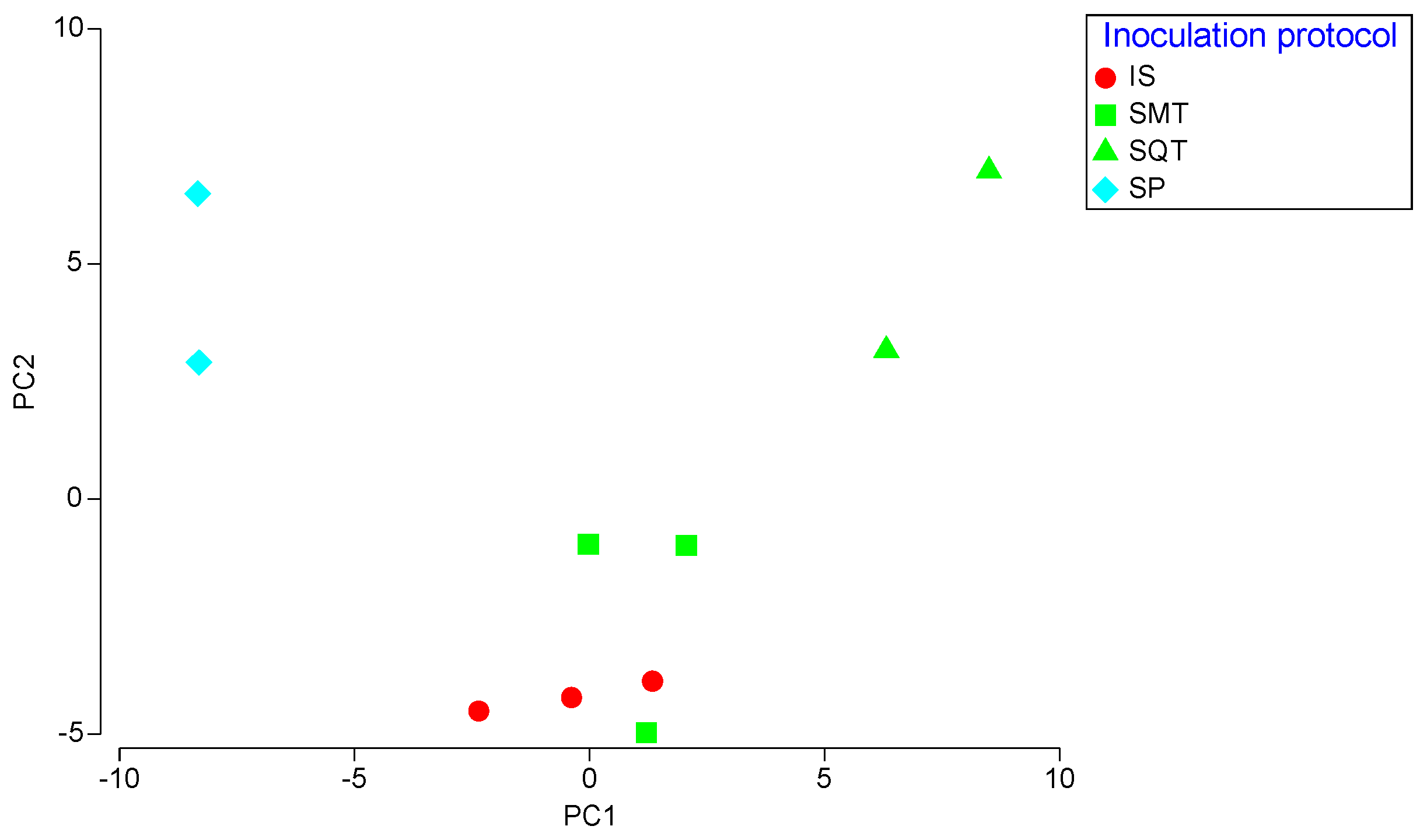
| Chemical Parameter | Inoculation Protocol 1 | |||||
|---|---|---|---|---|---|---|
| CS | IS | SML | SQL | SMT | SQT | |
| Total acidity (as tartaric acid g/L) | 9.9 ± 0.1 b | 9.9 ± 0.1 b | 10.0 ± 0.0 b | 11.6 ± 0.1 a | 9.9 ± 0.0 b | 9.4 ± 0.1 c |
| pH | 3.23 ± 0.01 b | 3.25 ± 0.01 a | 3.20 ± 0.01 c | 3.16 ± 0.01 d | 3.26 ± 0.01 a | 3.20 ± 0.01 c |
| Volatile acidity (as acetic acid g/L) | 0.43 ± 0.01 a | 0.34 ± 0.01 b | 0.39 ± 0.02 c | 0.18 ± 0.01 d | 0.30 ± 0.01 e | 0.12 ± 0.01 f |
| Free SO2 (mg/L) | 11.5 ± 2.5 a | 9.7 ± 1.9 ab | 11.4 ± 1.4 a | 9.8 ± 0.7 ab | 9.0 ± 0.0 ab | 6.8 ± 2.0 b |
| Total SO2 (mg/L) | 24.7 ± 1.5 a | 19.2 ± 0.0 cd | 23.9 ± 0.8 ab | 17.1 ± 0.7 d | 21.3 ± 1.4 bc | 17.1 ± 0.8 d |
| Citric acid (mg/L) | 359 ± 6 a | 365 ± 1 a | 363 ± 5 a | 361 ± 1 a | 359 ± 6 a | 397 ± 3 b |
| Tartaric acid (g/L) | 4.7 ± 0.1 c | 4.8 ± 0.0 ab | 4.8 ± 0.0 ab | 4.9 ± 0.0 a | 4.8 ± 0.0 bc | 4.8 ± 0.0 abc |
| Malic acid (g/L) | 2.0 ± 0.0 c | 2.1 ± 0.0 a | 2.0 ± 0.0 bc | 1.8 ± 0.0 e | 2.0 ± 0.0 ab | 1.9 ± 0.0 d |
| Glucose (g/L) | <0.6 | <0.6 | <0.6 | <0.6 | <0.6 | <0.6 |
| Fructose (g/L) | 1.1 ± 0.0 c | 1.6 ± 0.1 a | 1.4 ± 0.1 b | 0.8 ± 0.1 d | 1.6 ± 0.1 a | 1.0 ± 0.1 c |
| Succinic acid (g/L) | 0.7 ± 0.0 d | 0.7 ± 0.0 cd | 0.7 ± 0.0 c | 0.8 ± 0.0 b | 0.7 ± 0.0 c | 0.8 ± 0.0 a |
| Lactic acid (g/L) | <0.6 | <0.6 | <0.6 | 3.1 ± 0.1 | <0.6 | <0.6 |
| Glycerol (g/L) | 6.0 ± 0.1 c | 6.1 ± 0.0 bc | 6.2 ± 0.1 bc | 6.7 ± 0.0 a | 6.1 ± 0.1 bc | 6.2 ± 0.1 b |
| Acetic acid (mg/L) | 298 ± 18 a | 241 ± 8 b | 182 ± 33 c | 156 ± 5 c | 187 ± 4 c | 69 ± 6 d |
| Ethanol (g/L) | 105.7 ± 1.8 a | 107.0 ± 0.6 a | 106.5 ± 1.7 a | 106.8 ± 0.1 a | 105.6 ± 1.3 a | 106.7 ± 1.6 a |
| Chemical Parameter | Inoculation Protocol 1 | |||||
|---|---|---|---|---|---|---|
| CS | IS | SML | SQL | SMT | SQT | |
| Acetaldehyde | 7.0 ± 0.8 ab | 6.5 ± 0.4 b | 7.4 ± 1.7 ab | 8.4 ± 0.5 ab | 6.7 ± 0.3 ab | 8.9 ± 0.2 a |
| Ethyl acetate | 58.1 ± 2.3 c | 59.4 ± 1.7 c | 62.7 ± 2.7 c | 159.0 ± 3.1 a | 62.0 ± 1.1 c | 131.4 ± 4.5 b |
| Methanol | 46.4 ± 1.3 a | 47.5 ± 1.4 a | 46.3 ± 1.1 a | 48.8 ± 1.3 a | 47.7 ± 1.3 a | 47.8 ± 0.7 a |
| Propanol | 31.7 ± 0.7 c | 30.5 ± 0.6 c | 30.9 ± 0.5 c | 97.6 ± 2.2 a | 30.9 ± 0.4 c | 59.3 ± 1.9 b |
| Isobutanol | 25.2 ± 1.4 b | 25.0 ± 0.6 b | 24.4 ± 0.7 b | 35.4 ± 0.3 a | 23.3 ± 0.5 b | 35.7 ± 1.8 a |
| 2-Methyl-1-butanol | 21.4 ± 2.4 b | 23.4 ± 0.7 ab | 24.6 ± 0.3 a | 25.4 ± 0.4 a | 24.6 ± 0.7 a | 24.1 ± 1.0 ab |
| 3-Methyl-1-butanol | 122.4 ± 1.3 c | 134.4 ± 5.1 ab | 141.5 ± 0.7 a | 125.6 ± 2.3 bc | 140.7 ± 3.9 a | 123.8 ± 7.3 bc |
| Chemical Parameter | Inoculation Protocol 1 | ||||
|---|---|---|---|---|---|
| CS | IS | SMT | SQT | SP | |
| Total acidity (as tartaric acid g/L) | 9.6 ± 0.1 b | 9.3 ± 0.1 b | 9.2 ± 0.1 b | 10.4 ± 0.2 a | 10.6 ± 0.4 a |
| pH | 2.95 ± 0.01 c | 2.97 ± 0.01 bc | 2.97 ± 0.00 b | 3.03 ± 0.01 a | 2.90 ± 0.01 d |
| Volatile acidity (as acetic acid g/L) | 0.96 ± 0.04 ab | 0.86 ± 0.04 bc | 0.64 ± 0.03 cd | 0.37 ± 0.12 d | 1.22 ± 0.21 a |
| Ethanol (vol%) | 13.08 ± 0.02 a | 12.93 ± 0.07 ab | 12.90 ± 0.04 ab | 12.84 ± 0.05 ab | 12.47 ± 0.41 b |
| Glucose (g/L) | <0.6 | <0.6 | <0.6 | <0.6 | 0.8 ± 1.0 |
| Fructose (g/L | 0.9 ± 0.1 a | 0.8 ± 0.1 a | 0.7 ± 0.1 a | <0.6 | 7.4 ± 7.4 a |
| Citric acid (mg/L) | 460 ± 9 d | 470 ± 4 d | 497 ± 2 c | 632 ± 13 a | 569 ± 10 b |
| Tartaric acid (g/L) | 5.8 ± 0.1 ab | 5.7 ± 0.2 b | 5.7 ± 0.1 b | 6.0 ± 0.1 a | 6.0 ± 0.0 a |
| Malic acid (g/L) | 1.7 ± 0.0 a | 1.7 ± 0.0 a | 1.7 ± 0.0 a | 1.7 ± 0.1 a | 1.7 ± 0.1 a |
| Succinic acid (g/L) | <0.6 | <0.6 | <0.6 | 1.0 ± 0.0 a | 0.6 ± 0.1 b |
| Lactic acid (g/L) | <0.6 | <0.6 | <0.6 | <0.6 | <0.6 |
| Glycerol (g/L) | 7.4 ± 0.1 a | 7.2 ± 0.0 a | 7.0 ± 0.0 a | 6.2 ± 0.1 b | 6.7 ± 0.6 ab |
| Ethanol (g/L) | 105.5 ± 0.2 a | 105.7 ± 0.2 a | 106.0 ± 0.0 a | 105.8 ± 0.3 a | 101.0 ± 2.8 b |
| Chemical Component | Inoculation Protocol 2 | ||||
|---|---|---|---|---|---|
| CS | IS | SMT | SQT | SP | |
| Esters | |||||
| Methyl acetate | 3 ± 1 a | 3 ± 1 ab | 3 ± 1 ab | 1 ± 0 b | 4 ± 1 a |
| Ethyl acetate | 92,310 ± 9364 a | 98,640 ± 16,693 a | 84,633 ± 12,904 a | 40,757 ± 1513 b | 67,993 ± 16,135 ab |
| Propyl acetate | 429 ± 91 b | 655 ± 111 a | 537 ± 35 ab | 105 ± 52 c | 126 ± 64 c |
| Butyl acetate | 95 ± 21 a | 104 ± 14 a | 95 ± 7 a | 7 ± 12 b | 8 ± 14 b |
| Isobutyl acetate | 76,403 ± 17,864 ab | 91,863 ± 14,120 a | 75,240 ± 4668 ab | 32,517 ± 9707 c | 49,240 ± 16,881 bc |
| Isoamyl acetate | 3,156,060 ± 794,922 a | 3,959,940 ± 513,096 a | 3,085,093 ± 159,870 a | 683,323 ± 341,193 b | 1,013,410 ± 315,672 b |
| Hexyl acetate | 176,437 ± 27,004 a | 193,663 ± 19,067 a | 153,960 ± 1892 a | 76,237 ± 5435 b | 99,857 ± 15,010 b |
| (3E)-3-Hexenyl acetate | 158 ± 33 ab | 192 ± 30 a | 132 ± 8 b | 14 ± 7 c | 31 ± 10 c |
| (3Z)-3-Hexenyl acetate | 70 ± 17 ab | 86 ± 14 a | 51 ± 2 b | 2 ± 4 c | 22 ± 6 c |
| Octyl acetate | 51 ± 11 a | 44 ± 10 ab | 29 ± 2 b | 0 ± 0 c | 0 ± 0 c |
| 2-Phenylethyl acetate | 586,283 ± 17,186 ab | 896,080 ± 24,956 a | 861,183 ± 129,492 a | 277,427 ± 64,550 b | 728,957 ± 216,781 a |
| Ethyl butanoate | 382,167 ± 56,878 a | 415,743 ± 43,924 a | 365,343 ± 16,545 a | 231,670 ± 47,209 b | 24,2107 ± 36,357 b |
| Ethyl 2-methylbutanoate | 4 ± 4 b | 14 ± 7 ab | 13 ± 7 ab | 23 ± 7 a | 11 ± 6 ab |
| Ethyl 3-methylbutanoate | 27 ± 8 a | 34 ± 6 a | 25 ± 1 a | 20 ± 8 a | 17 ± 7 a |
| Ethyl isobutyrate | 89,027 ± 2772 b | 91,033 ± 2407 b | 90,987 ± 774 b | 109,867 ± 12,331 a | 95,800 ± 7802 ab |
| Methyl hexanoate | 188 ± 44 ab | 197 ± 16 a | 154 ± 13 abc | 95 ± 19 c | 121 ± 31 bc |
| Ethyl hexanoate | 719,013 ± 164,912 a | 661,823 ± 103,961 a | 498,310 ± 15,558 ab | 142,603 ± 40,088 c | 312,660 ± 90,314 bc |
| Methyl heptanoate | 6 ± 1 b | 5 ± 0 b | 5 ± 1 b | 5 ± 0 b | 10 ± 2 a |
| Ethyl lactate | 3037 ± 267 b | 3623 ± 556 b | 3570 ± 167 b | 5890 ± 1536 a | 4317 ± 775 ab |
| Methyl octanoate | 24 ± 5 b | 20 ± 1 b | 19 ± 5 b | 19 ± 1 b | 40 ± 7 a |
| Ethyl octanoate | 1,022,913 ± 250,174 a | 904,203 ± 233,561 ab | 715,090 ± 39,805 ab | 156,007 ± 42,462 c | 490,823 ± 139,919 bc |
| Isoamyl hexanoate | 82 ± 20 a | 70 ± 25 ab | 67 ± 4 ab | 5 ± 8 c | 31 ± 11 bc |
| Methyl nonanoate | 19 ± 3 ab | 18 ± 1 b | 18 ± 3 b | 19 ± 0 b | 26 ± 5 a |
| Methyl decanoate | 11 ± 1 b | 10 ± 0 b | 11 ± 1 b | 10 ± 1 b | 14 ± 1 a |
| Ethyl decanoate | 464,663 ± 120,974 a | 395,470 ± 105,413 a | 469,690 ± 6,2521 a | 44,420 ± 14,272 b | 262,493 ± 124,231 ab |
| 3-Methylbutyl octanoate | 446 ± 91 a | 382 ± 113 a | 502 ± 106 a | 60 ± 22 b | 329 ± 173 ab |
| Diethyl butanedioate | 22,930 ± 871 c | 25,420 ± 859 bc | 25,917 ± 1690 bc | 40,740 ± 2002 b | 69,097 ± 12,591 a |
| Ethyl 9-decenoate | 17,769 ± 3519 a | 16,565 ± 4162 a | 13,494 ± 622 a | 2064 ± 531 b | 19,278 ± 7671 a |
| Ethyl dodecanoate | 2829 ± 263 bc | 2756 ± 505 bc | 6360 ± 1374 a | 628 ± 199 c | 4923 ± 2294 ab |
| Alcohols | |||||
| 1-Propanol | 58,650 ± 4675 ab | 65,740 ± 11,472 a | 67,137 ± 5766 a | 41,390 ± 11,069 bc | 33,770 ± 9909 c |
| 2-Methyl-1-propanol | 26,320 ± 1508 a | 25,087 ± 3592 a | 26,327 ± 1007 a | 24,047 ± 6316 a | 32,677 ± 6100 a |
| 3-(Methylthio)-1-propanol | 1 ± 0 c | 2 ± 0 c | 2 ± 0 bc | 6 ± 1 a | 4 ± 1 b |
| 1-Butanol | 39 ± 2 a | 37 ± 5 a | 41 ± 1 a | 28 ± 8 a | 35 ± 8 a |
| 3-Methyl-1-butanol | 166,333 ± 27,191 a | 142,990 ± 23,921 ab | 132,873 ± 19,068 ab | 107,833 ± 40,643 ab | 76,623 ± 16,431 b |
| 4-Methyl-1-pentanol | 3 ± 0 b | 5 ± 0 a | 5 ± 0 a | 3 ± 1 b | 3 ± 0 b |
| 3-Methyl-1-pentanol | 7 ± 0 bc | 9 ± 0 ab | 10 ± 1 a | 6 ± 1 bc | 8 ± 1 bc |
| 1-Hexanol | 397,970 ± 13,441 b | 341,490 ± 13,450 b | 359,817 ± 34,917 b | 536,787 ± 17,438 a | 488,857 ± 20,199 a |
| (E)-3-Hexen-1-ol | 2 ± 0 a | 2 ± 0 a | 2 ± 0 a | 3 ± 0 a | 1 ± 0 b |
| 2-Ethyl-1-hexanol | 52,530 ± 684 a | 52,003 ± 208 a | 52,033 ± 81 a | 52,990 ± 936 a | 52,287 ± 499 a |
| 2,3-Butanediol (isomer 1) | 311 ± 17 a | 254 ± 38 a | 225 ± 48 a | 87 ± 3 b | 237 ± 41 a |
| 2,3-Butanediol (isomer 2) | 71 ± 6 a | 57 ± 9 ab | 53 ± 10 ab | 29 ± 2 c | 49 ± 8 bc |
| 1-Heptanol | 11 ± 0 b | 10 ± 0 b | 10 ± 0 b | 11 ± 3 b | 28 ± 6 a |
| 1-Octanol | 1437 ± 76 a | 1257 ± 32 ab | 1207 ± 85 ab | 827 ± 81 c | 1040 ± 135 bc |
| 1-Octen-3-ol | 0 ± 1 a | 1 ± 1 a | 1 ± 0 a | 1 ± 1 a | 1 ± 0 a |
| 1-Decanol | 2 ± 0 ab | 2 ± 0 ab | 2 ± 0 b | 2 ± 0 a | 2 ± 0 ab |
| 1-Nonanol | 91 ± 25 a | 87 ± 21 a | 64 ± 2 a | 79 ± 18 a | 80 ± 34 a |
| Phenylethyl Alcohol | 23,130 ± 1898 a | 26,287 ± 3234 a | 26,723 ± 1466 a | 29,847 ± 6030 a | 20,823 ± 2383 a |
| Acids | |||||
| Acetic acid | 38,684 ± 5425 a | 35,160 ± 8404 a | 27,271 ± 2660 ab | 12,116 ± 785 b | 33,209 ± 10,457 a |
| 2-Methylpropanoic acid | 313 ± 27 b | 315 ± 46 b | 376 ± 50 b | 1376 ± 214 a | 619 ± 144 b |
| Butanoic acid | 401 ± 41 a | 408 ± 67 a | 374 ± 96 a | 374 ± 39 a | 334 ± 69 a |
| 2-Methylbutanoic acid | 114 ± 8 b | 140 ± 20 b | 138 ± 18 b | 282 ± 82 a | 101 ± 25 b |
| 3-Methylbutanoic acid | 372 ± 27 a | 335 ± 76 a | 319 ± 16 a | 374 ± 71 a | 270 ± 85 a |
| Hexanoic acid | 1,790,940 ± 108,791 a | 1,537,050 ± 209,891 ab | 1,332,070 ± 128,762 b | 395,363 ± 12,067 c | 1,264,727 ± 165,953 b |
| Octanoic acid | 10,728 ± 1484 a | 10,341 ± 884 a | 8993 ± 1302 ab | 2318 ± 271 c | 6418 ± 284 b |
| n-Decanoic acid | 2271 ± 523 a | 2500 ± 293 a | 2278 ± 372 a | 491 ± 118 b | 895 ± 119 b |
| Carbonyl compounds | |||||
| Acetaldehyde | 47 ± 7 a | 67 ± 17 a | 87 ± 28 a | 47 ± 7 a | 89 ± 39 a |
| 2-Octanone | 906 ± 191 a | 957 ± 118 a | 736 ± 37 ab | 541 ± 105 b | 528 ± 104 b |
| 2-Nonanone | 6 ± 1 a | 6 ± 1 a | 5 ± 0 a | 0 ± 1 b | 0 ± 0 b |
| 2,3-Butanedione | 27 ± 4 ab | 34 ± 7 a | 28 ± 1 a | 13 ± 5 c | 15 ± 5 bc |
| Terpenes | |||||
| b-Citronellol | 5957 ± 520 a | 4957 ± 150 b | 4717 ± 166 b | 6130 ± 148 a | 3747 ± 176 c |
| Linalool | 63,233 ± 940 ab | 62,843 ± 456 ab | 62,953 ± 1140 ab | 65,083 ± 1168 a | 61,017 ± 762 b |
| Eucalyptol | 13 ± 2 a | 15 ± 4 a | 12 ± 1 a | 10 ± 2 a | 10 ± 1 a |
| a-Terpineol | 30,970 ± 2905 a | 36,530 ± 7206 a | 28,957 ± 9618 a | 22,430 ± 2737 ab | 10,193 ± 880 b |
| b-Damascenone | 200 ± 6 a | 192 ± 1 a | 177 ± 20 a | 111 ± 5 b | 97 ± 15 b |
| a-Pinene | 39,243 ± 860 ab | 40,053 ± 851 a | 39,270 ± 949 ab | 37,943 ± 447 b | 37,453 ± 310 b |
| Terpene_1095 | 157 ± 40 ab | 206 ± 46 a | 160 ± 44 ab | 105 ± 24 b | 71 ± 24 b |
| α- Phellandrene | 241 ± 59 ab | 296 ± 56 a | 228 ± 65 ab | 157 ± 27 bc | 89 ± 13 c |
| b-Myrcene | 186 ± 46 ab | 220 ± 40 a | 169 ± 48 ab | 122 ± 21 bc | 65 ± 12 c |
| D-Limonene | 38,513 ± 9672 ab | 45,867 ± 8264 a | 34,487 ± 9112 ab | 25,373 ± 4260 bc | 13,853 ± 2179 c |
| gamma-Terpinene | 24,660 ± 1324 ab | 25,523 ± 1010 a | 23,977 ± 1149 ab | 22,763 ± 519 bc | 21,403 ± 191 c |
| p-Cymene | 15,857 ± 1058 a | 15,823 ± 596 a | 15,380 ± 1099 a | 14,087 ± 279 ab | 13,330 ± 135 b |
| Other compounds | |||||
| 1,1-Diethoxy ethane | 42 ± 6 ab | 47 ± 6 ab | 40 ± 6 b | 49 ± 2 ab | 93 ± 42 a |
| Naphthalene | 161 ± 18 a | 179 ± 19 a | 142 ± 10 ab | 108 ± 11 bc | 91 ± 16 c |
| Chemical Parameter | Inoculation Protocol 1 | |||
|---|---|---|---|---|
| IS | SMT | SQT | SP | |
| Total acidity (as tartaric acid g/L) | 6.6 ± 0.1 ab | 6.7 ± 0.3 ab | 7.0 ± 0.2 a | 6.4 ± 0.2 b |
| pH | 3.14 ± 0.03 a | 3.11 ± 0.01 ab | 3.12 ± 0.00 ab | 3.00 ± 0.08 b |
| Volatile acidity (as acetic acid g/L) | 0.36 ± 0.05 b | 0.32 ± 0.02 b | 0.28 ± 0.00 b | 0.57 ± 0.02 a |
| Total SO2 (mg/L) | 99.6 ± 4.8 a | 103.7 ± 3.4 a | 87.3 ± 6.7 a | 96.6 ± 15.4 a |
| Glucose (g/L) | <0.6 | <0.6 | <0.6 | <0.6 |
| Fructose (g/L) | <0.6 | <0.6 | 0.9 ± 0.3 a | 0.7 ± 0.1 a |
| Citric acid (mg/L) | 504 ± 14 a | 503 ± 40 a | 504 ± 9 a | 493 ± 25 a |
| Tartaric acid (g/L) | 3.2 ± 0.3 a | 3.3 ± 0.2 a | 3.7 ± 0.4 a | 3.2 ± 0.5 a |
| Malic acid (g/L) | 1.7 ± 0.0 ab | 1.7 ± 0.1 a | 1.6 ± 0.1 b | 1.7 ± 0.0 ab |
| Succinic acid (g/L) | 0.7 ± 0.1 bc | 0.7 ± 0.1 ab | 0.9 ± 0.1 a | 0.6 ± 0.0 c |
| Lactic acid (g/L) | <0.6 | <0.6 | <0.6 | <0.6 |
| Glycerol (g/L) | 6.2 ± 0.2 b | 6.1 ± 0.5 ab | 6.6 ± 0.1 ab | 6.8 ± 0.0 a |
| Acetic acid (mg/L) | 273 ± 47 b | 208 ± 4 b | 188 ± 8 b | 509 ± 23 a |
| Ethanol (g/L) | 105.6 ± 0.8 a | 104.2 ± 8.0 a | 100.1 ± 4.5 a | 108.9 ± 1.6 a |
| Chemical Parameter | Inoculation Protocol 1 | |||
|---|---|---|---|---|
| IS | SMT | SQT | SP | |
| Acetaldehyde | 14.0 ± 1.0 a | 12.5 ± 1.4 a | 15.0 ± 3.1 a | 16.0 ± 1.7 a |
| Ethyl acetate | 51.0 ± 1.2 a | 55.3 ± 3.8 a | 46.5 ± 7.3 a | 77.9 ± 4.3 b |
| Methanol | 39.6 ± 3.0 a | 43.5 ± 1.8 a | 42.9 ± 1.3 a | 45.1 ± 1.6 a |
| Propanol | 21.3 ± 1.5 b | 24.4 ± 1.4 ab | 26.1 ± 0.4 a | 22.2 ± 0.9 ab |
| Isobutanol | 13.9 ± 0.6 b | 17.5 ± 1.4 b | 23.6 ± 2.4 a | 17.3 ± 0.1 b |
| 2-Methyl-1-butanol | 21.4 ± 0.9 b | 26.6 ± 1.8 a | 30.0 ± 0.1 a | 20.4 ± 1.2 b |
| 3-Methyl-1-butanol | 144.0 ± 5.4 b | 169.8 ± 10.3 a | 169.2 ± 2.1 a | 129.2 ± 5.6 b |
| Chemical Component | Inoculation Protocol 2 | |||
|---|---|---|---|---|
| IS | SMT | SQT | SP | |
| Esters | ||||
| Methyl acetate | 16 ± 0 b | 16 ± 2 b | 10 ± 2 c | 23 ± 2 a |
| Ethyl acetate | 4417 ± 247 b | 4466 ± 154 b | 3919 ± 412 b | 6108 ± 399 a |
| Propyl acetate | 18 ± 2 ab | 20 ± 1 ab | 13 ± 0 b | 22 ± 3 a |
| Isobutyl acetate | 36 ± 3 b | 42 ± 5 ab | 28 ± 2 b | 52 ± 6 a |
| Isoamyl acetate | 3663 ± 422 a | 3922 ± 798 a | 2427 ± 348 a | 3940 ± 915 a |
| Hexyl acetate | 2386 ± 577 ab | 1889 ± 354 ab | 1484 ± 272 b | 3419 ± 312 a |
| Phenylethyl acetate | 936 ± 205 a | 1009 ± 104 a | 737 ± 154 a | 920 ± 33 a |
| 3-Hexen-1-ol acetate, (E)- | 16 ± 2 ab | 16 ± 3 ab | 9 ± 0 b | 20 ± 1 a |
| 3-Hexen-1-ol acetate, (Z)- | 16 ± 2 ab | 16 ± 3 ab | 11 ± 0 b | 20 ± 2 a |
| Heptyl acetate | 6 ± 1 a | 4 ± 0 a | 9 ± 2 a | 8 ± 1 a |
| Ethyl propanoate | 33 ± 2 b | 38 ± 3 b | 54 ± 8 a | 29 ± 2 b |
| Ethyl isobutyrate | 9 ± 2 bc | 12 ± 2 ab | 16 ± 1 a | 6 ± 0 c |
| Ethyl butanoate | 140 ± 12 a | 156 ± 19 a | 120 ± 13 a | 182 ± 21 a |
| Ethyl 2-methylbutanoate | 4 ± 1 ab | 4 ± 1 ab | 6 ± 0 a | 1 ± 0 b |
| Ethyl isovalerate | 7 ± 2 a | 7 ± 2 a | 8 ± 1 a | 3 ± 0 a |
| Ethyl hexanoate | 3153 ± 729 a | 2971 ± 609 a | 2765 ± 390 a | 4106 ± 635 a |
| Ethyl heptanoate | 4 ± 0 ab | 3 ± 1 b | 7 ± 1 a | 3 ± 0 b |
| Ethyl lactate | 34 ± 5 a | 40 ± 6 a | 49 ± 5 a | 49 ± 22 a |
| Methyl octanoate | 15 ± 6 a | 9 ± 3 a | 8 ± 2 a | 14 ± 4 a |
| Ethyl octanoate | 11,702 ± 5101 a | 6261 ± 2239 a | 5542 ± 2371 a | 9264 ± 3095 a |
| Isoamyl hexanoate | 23 ± 9 a | 14 ± 4 a | 13 ± 5 a | 15 ± 4 a |
| Ethyl decanoate | 6518 ± 2963 a | 2804 ± 2017 a | 1988 ± 1588 a | 2196 ± 1149 a |
| 3-Methylbutyl octanoate | 115 ± 48 a | 60 ± 36 a | 25 ± 23 a | 41 ± 17 a |
| Butanedioic acid, diethyl ester | 10 ± 1 a | 11 ± 2 a | 13 ± 4 a | 6 ± 0 a |
| Ethyl 9-decenoate | 591 ± 268 a | 243 ± 152 a | 353 ± 275 a | 280 ± 63 a |
| Ethyl dodecanoate | 1063 ± 415 a | 705 ± 442 a | 206 ± 152 a | 528 ± 29 a |
| Ester_1862 3 | 91 ± 21 a | 71 ± 41 a | 30 ± 30 a | 40 ± 0 a |
| Ethyl tetradecanoate | 13 ± 3 a | 13 ± 3 a | 2 ± 3 b | 6 ± 0 ab |
| Ethyl hexadecanoate | 10 ± 4 a | 8 ± 2 a | 2 ± 3 a | 6 ± 2 a |
| Ethyl 9-hexedecenoate | 9 ± 2 a | 9 ± 2 a | 3 ± 4 a | 8 ± 2 a |
| Ethyl furoate | 7 ± 1 a | 7 ± 1 a | 8 ± 2 a | 10 ± 1 a |
| Alcohols | ||||
| 1-Propanol | 24 ± 8 a | 26 ± 4 a | 28 ± 4 a | 33 ± 5 a |
| 2-Methyl-1-propanol | 116 ± 25 a | 128 ± 18 a | 165 ± 5 a | 130 ± 21 a |
| 1-Butanol | 6 ± 1 a | 7 ± 1 a | 7 ± 1 a | 8 ± 1 a |
| Isoamyl alcohol | 2468 ± 334 a | 2622 ± 160 a | 2703 ± 50 a | 2034 ± 191 a |
| 4-Methyl-1-pentanol | 5 ± 0 a | 4 ± 1 a | 3 ± 0 a | 3 ± 0 a |
| 3-Methyl-1-pentanol | 11 ± 1 a | 11 ± 1 a | 8 ± 0 a | 8 ± 2 a |
| 1-Hexanol | 198 ± 6 b | 189 ± 5 b | 266 ± 29 a | 231 ± 15 ab |
| 3-Hexen-1-ol, (E)- | 2 ± 0 a | 2 ± 0 a | 2 ± 0 a | 2 ± 0 a |
| 3-Hexen-1-ol, (Z)- | 3 ± 0 a | 3 ± 0 a | 3 ± 2 a | 3 ± 0 a |
| 1-Heptanol | 11 ± 2 a | 13 ± 1 a | 12 ± 1 a | 14 ± 6 a |
| 2-Ethyl-1-hexanol | 5 ± 1 a | 7 ± 1 a | 5 ± 1 a | 2 ± 3 a |
| 2,3-Butanediol (isomer 1) | 60 ± 36 a | 73 ± 6 a | 102 ± 7 a | 307 ± 246 a |
| 2,3-Butanediol (isomer 2) | 16 ± 6 a | 19 ± 4 a | 26 ± 1 a | 74 ± 56 a |
| 1-Octanol | 5 ± 0 a | 5 ± 1 a | 4 ± 1 a | 6 ± 1 a |
| 1-Nonanol | 2 ± 2 a | 2 ± 2 a | 1 ± 1 a | 3 ± 0 a |
| 1-Decanol | 3 ± 0 a | 3 ± 0 a | 3 ± 1 a | 1 ± 2 a |
| Phenylethyl Alcohol | 990 ± 187 a | 1072 ± 118 a | 1032 ± 91 a | 596 ± 35 b |
| 1-Dodecanol | 10 ± 5 a | 7 ± 12 a | 5 ± 7 a | 0 ± 0 a |
| Acids | ||||
| Acetic acid | 94 ± 32 a | 87 ± 5 a | 47 ± 66 a | 353 ± 230 a |
| Hexanoic acid | 80 ± 5 b | 104 ± 24 a | 78 ± 9 b | 113 ± 0 a |
| Octanoic acid | 219 ± 38 a | 244 ± 22 a | 201 ± 22 a | 261 ± 6 a |
| Decanoic acid | 45 ± 8 a | 41 ± 36 a | 83 ± 4 a | 61 ± 4 a |
| Carbonyl compounds | ||||
| Acetaldehyde | 24 ± 2 a | 22 ± 2 a | 34 ± 5 a | 37 ± 9 a |
| Benzaldehyde | 6 ± 3 a | 4 ± 3 a | 4 ± 2 a | 1 ± 0 a |
| 2-Nonanone | 11 ± 3 ab | 9 ± 1 b | 9 ± 1 b | 16 ± 0 a |
| Decanal | 0 ± 0 b | 5 ± 5 a | 5 ± 2 ab | 2 ± 3 ab |
| 4-Methylbenzaldehyde | 2 ± 2 a | 1 ± 3 a | 2 ± 1 a | 0 ± 0 a |
| Terpenes | ||||
| D-Limonene | 4 ± 0 a | 6 ± 3 a | 7 ± 1 a | 2 ± 3 a |
| Linalool | 3 ± 0 a | 3 ± 0 a | 4 ± 1 a | 2 ± 0 a |
| b-Citronellol | 3 ± 0 a | 2 ± 0 a | 2 ± 1 a | 0 ± 0 b |
| b-Damascenone | 9 ± 2 a | 9 ± 1 a | 10 ± 0 a | 11 ± 1 a |
| Geraniol | 8 ± 2 a | 7 ± 3 a | 2 ± 1 a | 3 ± 0 a |
| Nerolidol | 5 ± 1 a | 6 ± 1 a | 13 ± 1 b | 5 ± 1 a |
| Other compounds | ||||
| 1,1-Diethoxy ethane | 64 ± 10 a | 63 ± 5 a | 56 ± 6 a | 71 ± 2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgouros, G.; Mallouchos, A.; Dourou, D.; Banilas, G.; Chalvantzi, I.; Kourkoutas, Y.; Nisiotou, A. Torulaspora delbrueckii May Help Manage Total and Volatile Acidity of Santorini-Assyrtiko Wine in View of Global Warming. Foods 2023, 12, 191. https://doi.org/10.3390/foods12010191
Sgouros G, Mallouchos A, Dourou D, Banilas G, Chalvantzi I, Kourkoutas Y, Nisiotou A. Torulaspora delbrueckii May Help Manage Total and Volatile Acidity of Santorini-Assyrtiko Wine in View of Global Warming. Foods. 2023; 12(1):191. https://doi.org/10.3390/foods12010191
Chicago/Turabian StyleSgouros, Georgios, Athanasios Mallouchos, Dimitra Dourou, Georgios Banilas, Ioanna Chalvantzi, Yiannis Kourkoutas, and Aspasia Nisiotou. 2023. "Torulaspora delbrueckii May Help Manage Total and Volatile Acidity of Santorini-Assyrtiko Wine in View of Global Warming" Foods 12, no. 1: 191. https://doi.org/10.3390/foods12010191






