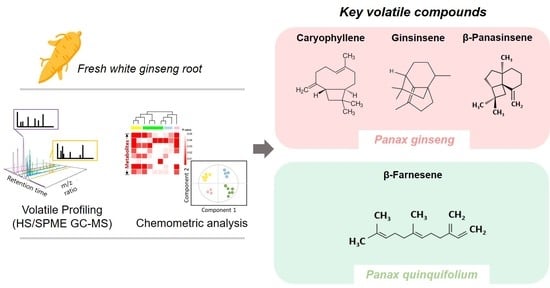
Volatile Compositions of Panax ginseng and Panax quinquifolium Grown for Different Cultivation Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. White Ginseng Root Samples
2.3. Volatile Compound Analysis
2.3.1. HS-SPME Conditions
2.3.2. GC-MS Analysis
2.4. Statistical Analyses
3. Results
3.1. Volatile Compounds of Four Different White Ginseng Roots Grown for 3–6 Years
3.2. Chemometric Analysis
3.3. Major Discriminant Volatile Composition of Four Different White Ginseng Roots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wang, S.; Liu, M.; Yang, F.; Zhang, H.; Liu, S.; Wang, Q.; Zhao, Y. De novo sequencing and analysis of the transcriptome of Panax ginseng in the leaf-expansion period. Mol. Med. Rep. 2016, 14, 1404–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, S.; Fan, Y.; Chen, Y.; Liu, D.; Cheng, H.; Gao, X.; Zhou, Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. J. Ethnopharmacol. 2010, 130, 421–423. [Google Scholar] [CrossRef]
- Scaglione, F.; Ferrara, F.; Dugnani, S.; Falchi, M.; Santoro, G.; Fraschini, F. Immunomodulatory effects of two extracts of Panax ginseng CA Meyer. Drugs Under Exp. Clin. Res. 1990, 16, 537–542. [Google Scholar]
- Shishtar, E.; Sievenpiper, J.L.; Djedovic, V.; Cozma, A.I.; Ha, V.; Jayalath, V.H.; Jenkins, D.J.A.; Meija, S.B.; Souza, R.J.; Jovanovski, E.; et al. The effect of ginseng (the genus panax) on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE 2014, 9, e107391. [Google Scholar] [CrossRef] [Green Version]
- Sangsukiam, T.; Duangmal, K. A comparative study of physico-chemical properties and antioxidant activity of freeze-dried mung bean (Vigna radiata) and adzuki bean (Vigna angularis) sprout hydrolysate powders. Int. J. Food Sci. Technol. 2017, 52, 1971–1982. [Google Scholar] [CrossRef]
- Cho, I.H. Volatile compounds of ginseng (Panax sp.): A review. J. Appl. Biol. Chem. 2015, 58, 67–75. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 3, 585–599. [Google Scholar] [CrossRef]
- Cho, I.H.; Lee, H.J.; Kim, Y.S. Differences in the volatile compositions of ginseng species (Panax sp.). J. Agric. Food Chem. 2012, 60, 7616–7622. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lu, X.; Pang, T.; Ma, C.; Li, X.; Xu, G. Determination of radix ginseng volatile oils at different ages by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. J. Sep. Sci. 2008, 31, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, J.; Feng, Z.; Yan, M.; Piao, X.; Yu, Y.; Hou, W.; Jin, Y.; Ying-Ping, W. Simultaneous determination and difference evaluation of volatile components generated from ginseng fruit by HS-SPME Coupled with GC-MS according to fruit color. LWT-Food Sci. Technol. 2020, 40, 532–536. [Google Scholar] [CrossRef]
- Lee, J.; Shibamoto, T.; Ha, J.; Jang, H.W. Identification of volatile markers for the detection of adulterants in red ginseng (Panax ginseng) juice using headspace stir-bar sorptive extraction coupled with gas chromatography and mass spectrometry. J. Sep. Sci. 2018, 41, 2903–2912. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Bang, K.; Kim, J.; Hyun, D.; Lee, S.; Kang, S.; Cha, S.; Kim, K.; Choi, J.; et al. high yielding and salt resistance ginseng variety ‘Cheonryang’. Korean J. Breed. Sci. 2013, 45, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Kwon, W.S.; Lee, J.H.; Park, C.S.; Yang, D.C. Breeding Process and Characteristics of Gopoong, a New Variety of Panax ginseng CA Meyer. J. Ginseng. Res. 2003, 27, 86–91. [Google Scholar]
- Lee, J.H.; Lee, J.S.; Kwon, W.S.; Kang, J.Y.; Lee, D.Y.; In, J.G.; Kim, Y.S.; Seo, J.; In, H.B.; Chang, I.M.; et al. Characteristics of Korean ginseng varieties of gumpoong, sunun, sunpoong, sunone, cheongsun, and sunhyang. J. Ginseng. Res. 2015, 39, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Pu, Z.J.; Zhang, S.; Tang, Y.P.; Shi, X.Q.; Tao, H.J.; Yan, H.; Chen, J.Q.; Yue, S.J.; Chen, Y.Y.; Zhu, Z.H.; et al. Study on changes in pigment composition during the blooming period of safflower based on plant metabolomics and semi-quantitative analysis. J. Sep. Sci. 2021, 44, 4082–4091. [Google Scholar] [CrossRef]
- Sung, J.; Suh, J.H.; Chambers, A.H.; Crane, J.; Wang, Y. Relationship between sensory attributes and chemical composition of different mango cultivars. J. Agric. Food Chem. 2019, 67, 5177–5188. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, Y.; Shen, L.; Xu, D.; Wang, B.; Ruan, K.; Cong, W. Effect of metformin on the urinary metabolites of diet-induced-obese mice studied by ultra performance liquid chromatography coupled to time-of-flight mass spectrometry (UPLC-TOF/MS). J. Chromatogr. B 2013, 925, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, Y. Biotechnology for the production of essential oils, flavours and volatile isolates. A review. Flavour Fragr. J. 2010, 25, 367–386. [Google Scholar] [CrossRef]
- Hurkova, K.; Uttl, L.; Rubert, J.; Navratilova, K.; Kocourek, V.; Stranska-Zachariasova, M.; Paprstein, F.; Hajslova, J. Cranberries versus lingonberries: A challenging authentication of similar Vaccinium fruit. Food Chem. 2019, 284, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, S.H.; Ahn, H.M.; Lim, S.R.; Oh, J.; Choi, S.; Lee, H.J.; Auh, J.H.; Choi, H.K. Differentiation of highbush blueberry (Vaccinium corymbosum L.) fruit cultivars by GC–MS-based metabolic profiling. J. Appl. Biol. Chem. 2015, 58, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Zhai, X.; Huang, X.; Li, Z.; Shi, J.; Li, Q.; Zou, X.; Battino, M. Rapid discrimination of beer based on quantitative aroma determination using colorimetric sensor array. Food Chem. 2021, 363, 30297. [Google Scholar] [CrossRef]
- Belmonte-Sánchez, J.R.; Romero-Gonzále, R.; Arrebol, F.J.; Vidal, J.L.M.; Garrido Frenich, A. An innovative metabolomic approach for golden rum classification combining ultrahigh-performance liquid chromatography–orbitrap mass spectrometry and chemometric strategies. J. Agric. Food Chem. 2019, 67, 1302–1311. [Google Scholar] [CrossRef]
- Song, X.; Jing, S.; Zhu, L.; Ma, C.; Song, T.; Wu, J.; Zhao, Q.; Zheng, F.; Chen, F. Untargeted and targeted metabolomics strategy for the classification of strong aroma-type baijiu (liquor) according to geographical origin using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Food Chem. 2020, 314, 126098. [Google Scholar] [CrossRef]
- Richter, R.; Basar, S.; Koch, A.; König, W.A. Three sesquiterpene hydrocarbons from the roots of Panax ginseng CA Meyer (Araliaceae). Phytochemistry 2005, 66, 2708–2713. [Google Scholar] [CrossRef]
- Praet, T.; Van Opstaele, F.; Steenackers, B.; De Vos, D.; Aerts, G.; De Cooman, L. Flavor activity of sesquiterpene oxidation products, formed upon lab-scale boiling of a hop essential oil–derived sesquiterpene hydrocarbon fraction (cv. Saaz). J. Am. Soc. Brew. Chem. 2016, 74, 65–76. [Google Scholar] [CrossRef]
- Campelo, P.H.; Alves Filho, E.G.; Silva, L.M.; de Brito, E.S.; Rodrigues, S.; Fernandes, F.A. Modulation of aroma and flavor using dielectric barrier discharge plasma technology in a juice rich in terpenes and sesquiterpenes. LWT 2020, 130, 109644. [Google Scholar] [CrossRef]




| Compound | RT A | RI B | American Ginseng | Yunpoong | Gumpoong | Cheonryang | Identification C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | ||||
| Alcohols | |||||||||||||||||||
| Methanol | 14.14 | 918 | 0.07 ± 0.03 | 0.11 ± 0.10 | 0.09 ± 0.03 | 0.13 ± 0.07 | 0.20 ± 0.15 | 0.18 ± 0.08 | 0.10 ± 0.00 | 0.21 ± 0.09 | 0.34 ± 0.04 | 0.21 ± 0.09 | 0.12 ± 0.02 | 0.09 ± 0.00 | 0.27 ± 0.13 | 0.20 ± 0.10 | 0.23 ± 0.09 | 0.16 ± 0.03 | MS, TI |
| Ethanol | 14.95 | 950 | nd D | nd | nd | nd | nd | nd | nd | 0.02 ± 0.03 | 0.06 ± 0.04 | 0.02 ± 0.03 | 0.03 ± 0.04 | 0.21 ± 0.16 | 0.06 ± 0.05 | 0.20 ± 0.04 | 0.22 ± 0.27 | 0.03 ± 0.03 | MS, TI |
| 1-Hexanol | 31.93 | 1372 | nd | nd | nd | 0.01 ± 0.03 | nd | nd | 0.02 ± 0.02 | 0.03 ± 0.04 | 0.13 ± 0.04 | 0.06 ± 0.06 | 0.09 ± 0.03 | 0.05 ± 0.01 | 0.20 ± 0.06 | 0.14 ± 0.02 | 0.11 ± 0.05 | 0.05 ± 0.02 | MS, TI, FI |
| 1-Octanol | 40.85 | 1577 | nd | nd | nd | 0.02 ± 0.03 | nd | nd | 0.12 ± 0.03 | 0.17 ± 0.09 | 0.13 ± 0.02 | 0.21 ± 0.05 | 0.18 ± 0.10 | 0.16 ± 0.03 | 0.34 ± 0.03 | 0.22 ± 0.03 | 0.25 ± 0.20 | 0.14 ± 0.12 | MS, TI, FI |
| 2,3-Butanediol | 42.10 | 1610 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.02 ± 0.03 | 0.05 ± 0.03 | nd | nd | nd | nd | MS, TI, FI |
| 1-Nonen-3-ol | 44.47 | 1668 | nd | nd | nd | nd | 0.01 ± 0.02 | nd | 0.01 ± 0.02 | nd | 0.02 ± 0.03 | 0.01 ± 0.02 | 0.01 ± 0.02 | nd | 0.08 ± 0.03 | nd | nd | 0.02 ± 0.03 | MS, TI |
| 2-Nonen-1-ol | 47.13 | 1739 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.02 ± 0.03 | nd | 0.03 ± 0.06 | 0.01 ± 0.01 | nd | 0.02 ± 0.04 | MS, TI, FI |
| Spathulenol | 62.23 | 2190 | nd | nd | nd | nd | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.05 ± 0.04 | 0.05 ± 0.09 | 0.03 ± 0.05 | 0.16 ± 0.07 | nd | 0.02 ± 0.03 | nd | nd | nd | nd | MS, TI |
| Aldehydes | |||||||||||||||||||
| Hexanal | 20.29 | 1111 | nd | nd | nd | nd | 0.05 ± 0.01 | 0.01 ± 0.01 | nd | nd | nd | nd | nd | nd | 0.02 ± 0.03 | nd | nd | nd | MS, TI |
| Octanal | 29.60 | 1322 | 0.20 ± 0.03 | 0.15 ± 0.07 | 0.06 ± 0.08 | 0.16 ± 0.07 | 0.28 ± 0.03 | 0.20 ± 0.03 | 0.02 ± 0.03 | nd | 0.01 ± 0.02 | nd | nd | nd | 0.10 ± 0.17 | nd | nd | nd | MS, TI, FI |
| 2-Nonenal | 41.04 | 1584 | 0.15 ± 0.05 | 0.03 ± 0.05 | 0.07 ± 0.08 | 0.07 ± 0.12 | 0.07 ± 0.13 | 0.08 ± 0.14 | nd | nd | nd | nd | nd | nd | 0.04 ± 0.07 | nd | nd | nd | MS, TI |
| (E,E)-2,4-Decadienal | 51.96 | 1872 | nd | nd | nd | nd | nd | nd | 0.02 ± 0.02 | 0.02 ± 0.04 | 0.14 ± 0.05 | 0.13 ± 0.04 | 0.06 ± 0.05 | nd | 0.08 ± 0.14 | 0.07 ± 0.01 | 0.04 ± 0.08 | 0.04 ± 0.06 | MS, TI, FI |
| (E)-4-Decenal | 57.16 | 2028 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.02 ± 0.04 | nd | 0.08 ± 0.07 | nd | nd | MS, TI |
| Alkenes | |||||||||||||||||||
| 1-Undecene | 21.69 | 1144 | nd | nd | nd | nd | nd | nd | 0.01 ± 0.02 | nd | 0.04 ± 0.01 | nd | nd | 0.01 ± 0.01 | 0.02 ± 0.03 | nd | 0.04 ± 0.04 | nd | MS, TI, FI |
| Ketones | |||||||||||||||||||
| 2-Nonanone | 34.09 | 1421 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.01 ± 0.01 | nd | 0.18 ± 0.13 | 0.02 ± 0.03 | MS, TI, FI |
| Furnas | |||||||||||||||||||
| 2-Pentyl furan | 26.38 | 1251 | nd | nd | nd | nd | nd | nd | nd | nd | 0.04 ± 0.01 | nd | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.04 ± 0.04 | 0.04 ± 0.00 | nd | 0.01 ± 0.01 | MS, TI, FI |
| Terpenes | |||||||||||||||||||
| α-Pinene | 17.69 | 1038 | 0.39 ± 0.17 | 0.14 ± 0.07 | 0.43 ± 0.32 | 0.58 ± 0.14 | 0.74 ± 0.19 | 0.75 ± 0.06 | 0.43 ± 0.07 | 0.53 ± 0.06 | 0.40 ± 0.11 | 0.71 ± 0.12 | 0.29 ± 0.06 | 0.22 ± 0.11 | 0.82 ± 0.26 | 0.57 ± 0.07 | 0.45 ± 0.04 | 0.48 ± 0.15 | MS, TI, FI |
| Camphene | 19.46 | 1087 | 0.07 ± 0.05 | 0.02 ± 0.03 | 0.10 ± 0.08 | 0.15 ± 0.04 | 0.09 ± 0.03 | 0.16 ± 0.02 | 0.08 ± 0.02 | 0.10 ± 0.03 | 0.07 ± 0.04 | 0.19 ± 0.06 | 0.07 ± 0.02 | 0.06 ± 0.03 | 0.18 ± 0.11 | 0.11 ± 0.05 | 0.12 ± 0.01 | 0.11 ± 0.04 | MS, TI |
| β-Pinene | 21.19 | 1130 | 0.29 ± 0.26 | 0.06 ± 0.08 | 0.29 ± 0.19 | 0.13 ± 0.06 | 2.21 ± 0.77 | 1.55 ± 0.09 | 1.35 ± 0.07 | 1.85 ± 0.26 | 0.19 ± 0.05 | 0.26 ± 0.04 | 0.44 ± 0.22 | 0.12 ± 0.03 | 0.37 ± 0.12 | 0.17 ± 0.02 | 0.25 ± 0.06 | 0.28 ± 0.06 | MS, TI, FI |
| Sabinene | 21.61 | 1142 | nd | nd | nd | nd | 0.01 ± 0.01 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.02 ± 0.02 | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI |
| 3-Carene | 22.93 | 1174 | 0.35 ± 0.25 | 0.16 ± 0.06 | 0.12 ± 0.10 | 0.13 ± 0.03 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI |
| β-Myrcene | 23.14 | 1176 | 0.07 ± 0.07 | 0.01 ± 0.01 | 0.05 0.01 | 0.04 ± 0.01 | 0.36 ± 0.11 | 0.33 ± 0.02 | 0.29 ± 0.04 | 0.28 ± 0.04 | 0.04 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.03 | 0.04 ± 0.00 | 0.08 ± 0.03 | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.04 ± 0.00 | MS, TI |
| D-Limonene | 25.27 | 1224 | 0.08 ± 0.05 | nd | 0.09 ± 0.08 | 0.11 ± 0.02 | 0.12 ± 0.04 | 0.17 ± 0.01 | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.04 | 0.14 ± 0.03 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.18 ± 0.09 | 0.08 ± 0.07 | 0.06 ± 0.05 | 0.03 ± 0.05 | MS, TI |
| Panaginsene | 35.25 | 1445 | 0.06 ± 0.05 | nd | 0.04 ± 0.05 | 0.11 ± 0.02 | 0.55 ± 0.09 | 0.56 ± 0.01 | 0.49 ± 0.07 | 0.65 ± 0.12 | 0.43 ± 0.10 | 0.55 ± 0.14 | 0.40 ± 0.06 | 0.30 ± 0.10 | 1.01 ± 0.21 | 0.61 ± 0.03 | 0.78 ± 0.32 | 0.88 ± 0.28 | MS, TI |
| Panaxene | 36.02 | 1465 | nd | nd | nd | nd | nd | 0.18 ± 0.15 | 0.09 ± 0.16 | 0.26 ± 0.11 | 0.05 ± 0.08 | 0.05 ± 0.09 | 0.03 ± 0.06 | nd | 0.08 ± 0.14 | nd | 0.04 ± 0.07 | 0.15 ± 0.14 | MS, TI |
| Ginsinsene | 36.55 | 1475 | 0.22 ± 0.09 | 0.05 ± 0.05 | 0.09 ± 0.054 | 0.30 ± 0.03 | 1.26 ± 0.18 | 1.28 ± 0.04 | 1.10 ± 0.14 | 1.44 ± 0.26 | 1.02 ± 0.21 | 1.29 ± 0.29 | 0.94 ± 0.09 | 0.69 ± 0.18 | 2.28 ± 0.52 | 1.33 ± 0.05 | 1.78 ± 0.81 | 1.91 ± 0.53 | MS, TI |
| Cedrene-V6 | 39.20 | 1538 | nd | nd | nd | nd | 0.49 ± 0.10 | 0.51 ± 0.02 | 0.46 ± 0.04 | 0.59 ± 0.11 | 0.39 ± 0.11 | 0.51 ± 0.19 | 0.36 ± 0.06 | 0.28 ± 0.08 | 0.87 ± 0.22 | 0.51 ± 0.02 | 0.64 ± 0.36 | 0.74 ± 0.24 | MS, TI |
| α-Isocomene | 40.18 | 1561 | 0.21 ± 0.12 | 0.02 ± 0.03 | 0.08 ± 0.14 | 0.20 ± 0.03 | 1.63 ± 0.33 | 1.58 ± 0.05 | 1.40 ± 0.13 | 1.78 ± 0.37 | 1.20 ± 0.26 | 1.68 ± 0.61 | 1.08 ± 0.16 | 0.89 ± 0.22 | 2.51 ± 0.63 | 1.53 ± 0.09 | 1.94 ± 1.05 | 2.15 ± 0.60 | MS, TI |
| β-Panasinsene | 40.59 | 1571 | 0.57 ± 0.24 | 0.21 ± 0.04 | 0.22 ± 0.14 | 0.72 ± 0.09 | 3.52 ± 0.53 | 3.39 ± 0.19 | 2.67 ± 0.28 | 3.47 ± 0.66 | 2.37 ± 0.48 | 3.05 ± 0.76 | 2.25 ± 0.33 | 1.71 ± 0.44 | 5.41 ± 1.00 | 3.16 ± 0.19 | 4.31 ± 2.06 | 4.54 ± 1.22 | MS, TI |
| Viridiflorol | 41.04 | 1584 | nd | nd | nd | 0.05 ± 0.09 | nd | 0.20 ± 0.18 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI, FI |
| β-Maaliene | 41.09 | 1585 | nd | nd | nd | 0.01 ± 0.03 | nd | nd | 0.16 ± 0.05 | 0.20 ± 0.03 | 0.21 ± 0.07 | 0.23 ± 0.08 | 0.09 ± 0.08 | 0.12 ± 0.02 | 0.17 ± 0.15 | 0.13 ± 0.06 | 0.08 ± 0.04 | 0.12 ± 0.06 | MS, TI |
| δ-Panasinsine | 41.80 | 1601 | nd | nd | nd | nd | 0.14 ± 0.03 | 0.19 ± 0.03 | 0.09 ± 0.08 | 0.20 ± 0.07 | 0.12 ± 0.02 | 0.15 ± 0.06 | 0.07 ± 0.06 | 0.02 ± 0.04 | nd | 0.05 ± 0.09 | 0.09 ± 0.15 | 0.08 ± 0.14 | MS, TI |
| α-Guaiene | 42.26 | 1613 | nd | nd | nd | nd | nd | nd | nd | 0.13 ± 0.12 | nd | nd | nd | nd | nd | nd | 0.05 ± 0.09 | 0.14 ± 0.07 | MS, TI, FI |
| Aristolene | 42.31 | 1614 | nd | 0.05 ± 0.05 | nd | 0.08 ± 0.07 | 0.18 ± 0.06 | 0.18 ± 0.01 | 0.15 ± 0.03 | 0.05 ± 0.09 | 0.13 ± 0.04 | 0.16 ± 0.06 | 0.09 ± 0.02 | 0.13 ± 0.07 | 0.16 ± 0.07 | 0.12 ± 0.01 | 0.08 ± 0.09 | nd | MS, TI |
| α-Bergamotene | 42.33 | 1615 | 0.02 ± 0.04 | nd | 0.04 ± 0.04 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI |
| β-Elemene | 42.82 | 1626 | 0.09 ± 0.03 | 0.04 ± 0.03 | 0.02 ± 0.03 | 0.13 ±0.01 | 1.19 ± 0.28 | 1.52 ± 0.10 | 1.10 ± 0.09 | 1.57 ± 0.41 | 0.72 ± 0.16 | 1.35 ± 0.66 | 0.71 ± 0.18 | 0.82 ± 0.25 | 1.07 ± 0.96 | 1.04 ± 0.16 | 1.43 ± 0.79 | 1.68 ± 0.47 | MS, TI, FI |
| Calarene | 43.34 | 1639 | 0.21 ± 0.22 | 0.38 ± 0.33 | 0.14 ± 0.16 | 0.67 ± 0.11 | 1.67 ± 0.49 | 1.40 ± 0.07 | 1.31 ± 0.21 | 1.36 ± 0.20 | 1.33 ± 0.29 | 1.59 ± 0.29 | 0.87 ± 0.10 | 0.94 ± 0.24 | 1.61 ± 0.59 | 1.19 ± 0.18 | 1.15 ± 0.53 | 1.15 ± 0.40 | MS, TI |
| Valerena-4,7(11)-dien | 43.97 | 1656 | nd | nd | nd | nd | 2.27 ± 0.107 | 1.49 ± 0.12 | 1.81 ± 0.38 | 1.59 ± 0.45 | 1.21 ± 0.30 | 2.73 ± 1.57 | 1.17 ± 0.19 | 1.16 ± 0.07 | 1.43 ± 0.60 | 0.90 ± 0.20 | 1.09 ± 0.58 | 1.16 ± 0.0 | MS, TI |
| β-Farnesene | 45.13 | 1683 | 7.20 ± 3.26 | 3.79 ± 1.27 | 4.97 ± 1.39 | 4.14 ± 0.62 | 2.43 ± 0.19 | 3.43 ± 0.26 | 1.91 ± 0.48 | 2.59 ± 1.01 | 0.39 ± 0.14 | 0.89 ± 0.30 | 0.86 ± 0.30 | 0.79 ± 0.04 | 0.60 ± 0.17 | 0.38 ± 0.04 | 0.42 ± 0.27 | 0.32 ± 0.09 | MS, TI, FI |
| α-Panasinsene | 45.55 | 1695 | 0.02 ± 0.04 | nd | 0.01 ± 0.02 | 0.12 ± 0.01 | 1.21 ± 0.33 | 0.68 ± 0.59 | 1.03 ± 0.09 | 1.15 ± 0.29 | 0.82 ± 0.17 | 1.32 ± 0.58 | 0.75 ± 0.13 | 0.62 ± 0.12 | 1.51 ± 0.44 | 0.90 ± 0.08 | 1.11 ± 0.60 | 1.26 ± 0.33 | MS, TI |
| α-Neoclovene | 45.78 | 1701 | 0.41 ± 0.19 | 0.12 ± 0.04 | 0.21 ± 0.02 | 0.61 ± 0.08 | 3.06 ± 0.48 | 3.27 ± 0.11 | 2.73 ± 0.34 | 3.56 ± 0.75 | 2.29 ± 0.52 | 3.00 ± 0.94 | 2.12 ± 0.35 | 1.65 ± 0.41 | 5.15 ± 1.24 | 2.93 ± 0.29 | 3.86 ± 1.965 | 4.39 ± 1.21 | MS, TI, FI |
| Caryophyllene | 46.80 | 1729 | nd | nd | nd | 0.04 ± 0.04 | 1.15 ± 0.33 | 1.43 ± 0.05 | 1.28 ± 0.11 | 1.48 ± 0.58 | 0.67 ± 0.19 | 1.11 ± 0.51 | 0.65 ± 0.20 | 0.64 ± 0.13 | 1.16 ± 0.51 | 0.93 ± 0.09 | 1.31 ± 0.66 | 1.61 ± 0.50 | MS, TI, FI |
| α-Bisabolene | 47.87 | 1760 | 0.23 ± 0.21 | 0.04 ± 0.07 | 0.24 ± 0.21 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI |
| β-Bisabolene | 47.91 | 1761 | 0.50 ± 0.86 | 1.07 ± 0.08 | 1.94 ± 0.72 | 2.09 ± 0.07 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI |
| β-Neoclovene | 47.95 | 1760 | nd | nd | nd | nd | 0.86 ± 0.12 | 0.96 ± 0.04 | 0.72 ± 0.09 | 0.99 ± 0.24 | 0.59 ± 0.15 | 0.77 ± 0.27 | 0.54 ± 0.10 | 0.41 ± 0.10 | 1.38 ± 0.29 | 0.75 ± 0.07 | 0.92 ± 0.52 | 1.13 ± 0.32 | MS, TI |
| α-Selinene | 48.54 | 1778 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.63 ± 0.32 | nd | nd | nd | nd | nd | nd | MS, TI |
| β-Selinene | 48.58 | 1777 | nd | nd | nd | nd | 0.36 ± 0.33 | 0.48 ± 0.42 | nd | nd | nd | nd | nd | 0.33 ± 0.10 | 0.82 ± 0.24 | 0.54 ± 0.05 | 0.39 ± 0.50 | 0.85 ± 0.25 | MS, TI |
| γ-Selinene | 48.58 | 1779 | nd | nd | nd | 0.05 ± 0.04 | nd | nd | 0.48 ± 0.06 | 0.73 ± 0.19 | 0.37 ± 0.08 | nd | 0.34 ± 0.08 | nd | nd | nd | nd | nd | MS, TI |
| Bicyclogermacrene | 48.93 | 1786 | nd | nd | nd | nd | 0.89 ± 0.55 | 0.59 ± 0.11 | 0.91 ± 0.31 | 0.55 ± 0.31 | 0.32 ± 0.12 | 1.07 ± 0.93 | 0.27 ± 0.09 | 0.44 ± 0.02 | 0.19 ± 0.13 | 0.21 ± 0.07 | 0.25 ± 0.07 | 0.29 ± 0.14 | MS, TI |
| α-Amorphene | 49.39 | 1797 | 0.02 ± 0.04 | 0.12 ± 0.22 | 0.19 ± 0.13 | 0.05 ± 0.02 | 0.05 ± 0.04 | nd | nd | 0.06 ± 0.02 | nd | nd | 0.01 ± 0.02 | 0.01 ± 0.03 | nd | nd | nd | 0.02 ± 0.03 | MS, TI |
| β-Sesquiphellandrene | 49.70 | 1809 | 0.11 ± 0.19 | 0.22 ± 0.02 | 0.48 ± 0.24 | 0.30 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | MS, TI, FI |
| Pacifigorgiol | 56.17 | 1997 | nd | nd | nd | nd | 0.05 ± 0.04 | nd | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.02 | 0.05 ± 0.06 | nd | 0.01 ± 0.01 | nd | nd | nd | nd | MS, TI |
| Caryophyllene oxide | 58.46 | 2069 | nd | nd | nd | nd | 0.17 ± 0.03 | 0.15 ± 0.01 | 0.04 ± 0.07 | 0.08 ± 0.08 | 0.05 ± 0.09 | 0.05 ± 0.08 | nd | nd | 0.25 ± 0.06 | 0.08 ± 0.07 | 0.04 ± 0.07 | 0.05 ± 0.09 | MS, TI, FI |
| Humulene oxide II | 60.23 | 2125 | nd | nd | nd | nd | nd | nd | nd | 0.12 ± 0.21 | 0.11 ± 0.19 | 0.27 ± 0.23 | 0.29 ± 0.05 | 0.04 ± 0.07 | 0.27 ± 0.47 | 0.27 ± 0.24 | nd | nd | MS, TI |
| Neointermedeol | 62.65 | 2201 | nd | nd | nd | nd | 0.12 ± 0.02 | 0.13 ± 0.00 | 0.09 ± 0.01 | 0.13 ± 0.07 | 0.05 ± 0.04 | 0.10 ± 0.04 | 0.02 ± 0.04 | nd | 0.15 ± 0.02 | 0.06 ± 0.01 | 0.08 ± 0.07 | 0.14 ± 0.05 | MS, TI |
| Ginsenol | 63.63 | 2234 | nd | nd | nd | nd | 0.23 ± 0.05 | 0.25 ± 0.04 | 0.11 ± 0.03 | 0.16 ± 0.08 | 0.11 ± 0.05 | 0.18 ± 0.05 | 0.05 ± 0.04 | 0.02 ± 0.02 | 0.31 ± 0.06 | 0.11 ± 0.02 | 0.10 ± 0.09 | 0.19 ± 0.05 | MS, TI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lee, J.-W.; Jo, I.-H.; Kwon, N.; Kim, D.; Chung, J.-W.; Bang, K.-H.; Sung, J. Volatile Compositions of Panax ginseng and Panax quinquifolium Grown for Different Cultivation Years. Foods 2023, 12, 136. https://doi.org/10.3390/foods12010136
Kim Y, Lee J-W, Jo I-H, Kwon N, Kim D, Chung J-W, Bang K-H, Sung J. Volatile Compositions of Panax ginseng and Panax quinquifolium Grown for Different Cultivation Years. Foods. 2023; 12(1):136. https://doi.org/10.3390/foods12010136
Chicago/Turabian StyleKim, Yejin, Jung-Woo Lee, Ick-Hyun Jo, Nayeong Kwon, Donghwi Kim, Jong-Wook Chung, Kyong-Hwan Bang, and Jeehye Sung. 2023. "Volatile Compositions of Panax ginseng and Panax quinquifolium Grown for Different Cultivation Years" Foods 12, no. 1: 136. https://doi.org/10.3390/foods12010136