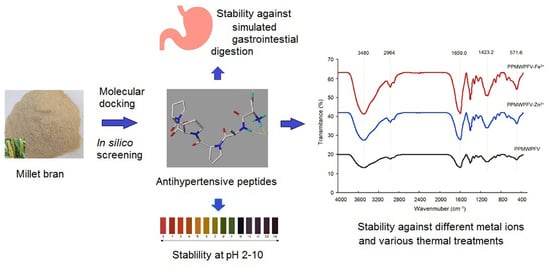
Two Novel Antihypertensive Peptides Identified in Millet Bran Glutelin-2 Hydrolysates: Purification, In Silico Characterization, Molecular Docking with ACE and Stability in Various Food Processing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Millet Bran Glutelin-2 Hydrolysates (MBGH)
2.3. ACE Inhibition and Definition of IC50 Value
2.4. Purification and Identification of ACE-Inhibitory Peptides from MBGH
2.5. In Silico Screening and Synthesis
2.6. In Silico Security Prediction
2.7. Molecular Docking
2.8. Stability Profiles under Different Processing Conditions
2.8.1. Thermal Stability Profiles
2.8.2. pH Stability Profiles
2.8.3. Effects of Different Metal Ions on Peptide Stability
2.9. Stability Profiles during Simulated Gastrointestinal Digestion
2.10. Data Analysis
3. Results and Discussion
3.1. Isolation of ACE-Inhibitory Peptides from MBGH
3.2. Peptide Identification from MBGH-E4 and In Silico Screening
3.3. In Silico Prediction of Physicochemical Properties and Potential Safety
3.4. Molecular Docking Analysis
3.5. Stability under Different Thermal Treatments and pH Values
3.6. Effects of Different Metal Ions on the Stability
3.6.1. Stability Profiles
3.6.2. Fourier-Infrared Spectroscopy Analysis
3.7. Resistance to Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J. Purification and identification of novel ACE inhibitory and ACE2 upregulating peptides from spent hen muscle proteins. Food Chem. 2021, 345, 128867. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, P.; Zapata, J.E.; Chamorro, V.C.; Fillería, S.F.G.; Tironi, V.A. Antioxidant and angiotensin I-converting enzyme (ACE) inhibitory peptides of rainbow trout (Oncorhynchus mykiss) viscera hydrolysates subjected to simulated gastrointestinal digestion and intestinal absorption. LWT-Food Sci. Technol. 2022, 154, 112834. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- WHO. Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 11 December 2021).
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Kim, K.-H.; Anderson, J.M. Enzyme kinetics, molecular docking, and in silico characterization of canary seed (Phalaris canariensis L.) peptides with ACE and pancreatic lipase inhibitory activity. J. Funct. Foods 2022, 88, 104892. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Chiozzi, R.Z.; Laganà, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef]
- Goh, N.-Y.; Razif, M.F.M.; Yap, Y.H.-Y.; Ng, C.L.; Fung, S.-Y. In silico analysis and characterization of medicinal mushroom cystathionine beta-synthase as an angiotensin converting enzyme (ACE) inhibitory protein. Comput. Biol. Chem. 2022, 96, 107620. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Int. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Bhaskar, B.; Ananthanarayana, L.; Jamdar, S. Purification, identification, and characterization of novel angiotensin I converting enzyme (ACE) inhibitory peptides from alcalase digested horse gram flour. LWT-Food Sci. Technol. 2019, 103, 155–161. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M.; Soleymanzadeh, N. The stability of antioxidant and ACE-inhibitory peptides as influenced by peptide sequences. LWT-Food Sci. Technol. 2020, 130, 109710. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Shi, P.Q.; Li, Y.; Zhuang, Y.L.; Zhang, Y.L.; Liu, L.; Wang, W. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions. LWT-Food Sci. Technol. 2021, 147, 111682. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Surasani, V.K.R. Influence of processing and pH on amino acid profile, morphology, electrophoretic pattern, bioactive potential and functional characteristics of alfalfa protein isolates. Food Chem. 2020, 333, 127503. [Google Scholar] [CrossRef]
- Yousaf, L.; Hou, D.; Liaqat, H.; Shen, Q. Millet: A review of its nutritional and functional changes during processing. Food Res. Int. 2021, 142, 110197. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Joshi, R.; Gupt, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yuan, J.; Gao, J.; Wu, Y. Antioxidant and anti-inflammatory potential of peptides derived from the in vitro gastrointestinal digestion of germinated and heat-treated foxtail millet (Setaria italica) proteins. J Agric. Food Chem. 2020, 68, 9415–9426. [Google Scholar] [CrossRef]
- Gu, H.; Gao, J.; Shen, Q.; Gao, D.; Wang, Q.; Tangyu, M.; Mao, X. Dipeptidyl peptidase-IV inhibitory activity of millet protein peptides and the related mechanisms revealed by molecular docking. LWT-Food Sci. Technol. 2020, 138, 110587. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, F.; Liu, Z.; Zhao, Q.; Xue, Y.; Shen, Q. Improvement of diabetes-induced metabolic syndrome by millet prolamin is associated with changes in serum metabolomics. Food Biosci. 2021, 44, 10143. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Li, Y.; Zhang, Y.L.; Ruan, X.H.; Zhang, R.G. Purification, characterization, synthesis, in vivo ACE inhibition and in vitro antihypertensive activity of bioactive peptides derived from palm kernel expeller glutelin-2 hydrolysates. J. Funct. Foods 2017, 28, 48–58. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, high-throughput assay for screening angiotensin I-converting enzyme inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Sun, L.P.; Zhuang, Y.L. Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: Inhibition kinetics and molecular docking. Food Funct. 2018, 9, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, J.S.; Singh, S.; Gautam, A.; Raghava, P.S. AHTPDB: A comprehensive platform for analysis and presentation of antihypertensive peptides. Nucleic Acids Res. 2015, 43, D956–D962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaide-Hidalgo, J.M.; Romero, M.; Duarte, J.; López-Huertas, E. Antihypertensive effects of virgin olive oil (unfiltered) low molecular weight peptides with ACE inhibitory activity in spontaneously hypertensive rats. Nutrients 2020, 12, 271. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, G.; Pallavi, K.; Kumardeep, C.; Ankur, G.; Rahul, K.; Raghava, G.P.S.; Lee, P.R. In silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, 73957. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Raghava, G.P.S. AlgPred: Prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006, 34, 202–209. [Google Scholar] [CrossRef]
- Chai, T.T.; Xiao, J.; Mohana, S.D.; Teoh, J.Y.; Eea, K.Y.; Nga, W.; Wong, F.C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef]
- Hao, L.; Gao, X.; Zhou, T.; Cao, J.; Sun, Y.; Dang, Y.; Pan, D. Angiotensin I-Converting Enzyme (ACE) inhibitory and antioxidant activity of umami peptides after in vitro gastrointestinal digestion. J. Agric. Food Chem. 2020, 68, 8232–8241. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Chen, Q.; Wu, Q.; He, Q. Screening and mechanisms of novel angiotensin-I-converting enzyme inhibitory peptides from rabbit meat proteins: A combined in silico and in vitro study. Food Chem. 2022, 370, 131070. [Google Scholar] [CrossRef]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and characterization of edible cricket peptides on hypertensive and glycemic In Vitro inhibition and their anti-inflammatory activity on RAW 264.7 macrophage cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Wang, X.; Zhuang, Y.L.; Li, Y.; Shi, P.Q.; Tian, H.L.; Li, X.T.; Chen, X. Isolation of novel ACE-inhibitory peptide from naked oat globulin hydrolysates in silico approach: Molecular docking, in vivo antihypertension and effects on renin and intracellular endothelin-1. J. Food Sci. 2020, 85, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, C.; Sun-Waterhouse, D.; Zhao, T.; Waterhouse, G.I.N.; Zhao, M.; Su, G. Identification of post-digestion angiotensin-I converting enzyme (ACE) inhibitory peptides from soybean protein Isolate: Their production conditions and in silico molecular docking with ACE. Food Chem. 2021, 345, 128855. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, D.; Li, B. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Wang, T.; Lin, S.; Cui, P.; Bao, Z.; Liu, K.; Jiang, P.; Zhu, N. Antarctic krill derived peptide as a nanocarrier of iron through the gastrointestinal tract. Food Biosci. 2020, 36, 100657. [Google Scholar] [CrossRef]
- Cui, P.; Sun, N.; Jiang, P.; Wang, D.; Lin, S. Optimised condition for preparing sea cucumber ovum hydrolysate-calcium complex and its structural analysis. Int. J. Food Sci. Technol. 2017, 52, 1914–1922. [Google Scholar] [CrossRef]
- De Angelis, M.; Siragusa, S.; Vacca, M.; Di Cagno, R.; Cristofori, F.; Schwarm, M.; Peizer, S.; Flügel, M.; Speckmann, B.; Francavilla, R.; et al. Selection of gut-resistant bacteria and constructioin of microbial consortia for improving gluten digestion under simulated gastrointestinal conditions. Nutrients 2021, 13, 992. [Google Scholar] [CrossRef]








| Peptide Sequence | PLLK | SGGRGGFGGG | NDFAGF | PPMWPFV |
|---|---|---|---|---|
| Mass (Da) | 469.66 | 807.97 | 669.76 | 873.18 |
| ALC (%) | 90 | 76 | 70 | 94 |
| SVMS | 0.99 | −0.10 | −0.94 | 0.97 |
| Prediction | AHT | Non-AHT | Non-AHT | AHT |
| Hydrophilicity | −0.15 | 0.08 | 0.00 | −1.24 |
| Amphiphilicity | 0.92 | 0.25 | 0.00 | 0.99 |
| Hydrophobicity | 0.53 | −0.03 | 0.05 | 0.22 |
| Isoelectric point | 9.11 | 10.11 | 3.80 | 5.88 |
| IC50 (μmol/L) | 549.87 | ND | ND | 364.62 |
| IC50 (μmol/L) after gastrointestinal digestion (μmol/L) | 591.57 | ND | ND | 397.83 |
| Toxicity | Non-Toxin | Non-Toxin | Non-Toxin | Non-Toxin |
| Allergenicity | ND | ND | ND | ND |
| Ligand | T-Score | C-Score | Hydrogen Bonds Number | Distance (Å) |
|---|---|---|---|---|
| PLLK | 11.76 | 5.00 | 2 | PRO508: 1.84, 1.92 |
| PPMWPFV | 9.93 | 5.00 | 8 | LYS368: 2.44; ASP377: 2.73; GLU376: 2.42; THR282: 2.37; LYS454: 2.04; ARG522: 2.73; PRO508: 2.04, 1.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, X.; Guo, M.; Yan, X.; Zhuang, Y.; Sun, Y.; Li, J. Two Novel Antihypertensive Peptides Identified in Millet Bran Glutelin-2 Hydrolysates: Purification, In Silico Characterization, Molecular Docking with ACE and Stability in Various Food Processing Conditions. Foods 2022, 11, 1355. https://doi.org/10.3390/foods11091355
Zheng Y, Wang X, Guo M, Yan X, Zhuang Y, Sun Y, Li J. Two Novel Antihypertensive Peptides Identified in Millet Bran Glutelin-2 Hydrolysates: Purification, In Silico Characterization, Molecular Docking with ACE and Stability in Various Food Processing Conditions. Foods. 2022; 11(9):1355. https://doi.org/10.3390/foods11091355
Chicago/Turabian StyleZheng, Yajun, Xueying Wang, Min Guo, Xiaoting Yan, Yongliang Zhuang, Yue Sun, and Junru Li. 2022. "Two Novel Antihypertensive Peptides Identified in Millet Bran Glutelin-2 Hydrolysates: Purification, In Silico Characterization, Molecular Docking with ACE and Stability in Various Food Processing Conditions" Foods 11, no. 9: 1355. https://doi.org/10.3390/foods11091355